Abstract
Myoporum sandwicense A. Gray (naio) is one of the characteristic trees of Hawaiian montane–subalpine mesic forests. In this study, lab-distilled oils of M. sandwicense leaves, wood, and twigs growing on the island of Hawaii, as well as industrially produced wood oils, were characterized by gas chromatography–mass spectrometry (GC-MS). The lab-distilled oils were screened for antimicrobial activity. M. sandwicense leaf essential oil was rich in β-caryophyllene (15.1%), α-humulene (12.8%), germacrene D (7.9%), bicyclogermacrene (12.5%), brigalow ketol (9.6%), and myoporone (16.8%), while the wood essential oils were dominated by α-bisabolol and trans-α-bisabolol oxide B. The sapwood oil was dominated by palmitic acid (35.5%), linoleic acid (19.7%), oleic acid (31.9%), and stearic acid (5.7%), whereas the oil from twigs was rich in tricosane (77.3%) and pentacosane (13.1%). M. sandwicense essential oils were screened for antimicrobial activity against a panel of potentially pathogenic bacteria and fungi. The leaf essential oil of M. sandwicense showed excellent antibacterial activity against S. pyogenes and antifungal activity against A. fumigatus. The wood essential oil showed notable activity against S. pyogenes, A. fumigatus, A. niger, and M. gypseum. The twig oil was remarkably active against mold species. This work is the first report we are aware of on the composition and antimicrobial properties of naio essential oils.
1. Introduction
Myoporum sandwicense A. Gray (Scrophulariaceae), commonly known as “Naio”, “false sandalwood”, or “bastard sandalwood”, is a notoriously polymorphic evergreen shrub or tree that can grow as a creeping, prostrate shrub or it may occur as a tree around 15 m tall [1]. Furthermore, there are several varieties of M. sandwicense (e.g., M. sandwicense var. sandwicense, M. sandwicense var. fauriei, M. sandwicense var. st.-johnii, and M. sandwicense var. wilder [1]. Myoporum sandwicense is one of the characteristic trees of Hawaiian montane–subalpine mesic forests where it occurs with Metrosideros polymorpha Gaudich. (ōhi’a), Acacia koa A. Gray (koa), Sapindus saponaria L. (a’e), Nestegis sandwicensis (A. Gray) O. Deg., I. Deg. & L.A.S. Johnson (olopua), and Sophora chrysophylla Seem. (māmane) [2]. On the island of Hawai’i, both M. sandwicense var. fauriei, and M. sandwicense var. st.-johnii, are found on the leeward slopes of Mauna Kea and Mauna Loa [1]. The fruits of M. sandwicense are important food sources for the Hawaiian thrush (omao, Phaeornis obscurus obscurus) [3] and were apparently the exclusive food source of the now-extinct Kona grosbeak (Chloridops kona) [4]. Myoporum sandwicense populations have been adversely affected by habitat damage by feral ungulates [5] and by an introduced insect. Klambothrips myopori Mound and Morris (Phlaeothripidae) is a potential threat to the tree form of naio [6].
We have been interested in examining the essential oils of tropical plants for potential cultivation for the fragrance industry [7,8]. The purpose of this work was to obtain the essential oils from the leaves, twigs, and wood of M. sandwicense growing on the island of Hawai’i, to chemically characterize the essential oils by gas chromatography–mass spectrometry, and to screen them for antimicrobial activity. This work is the first report we are aware of on the composition and antimicrobial properties of Naio essential oils.
2. Materials and Methods
2.1. Essential Oil Extraction
The plant materials were collected in September of 2019 from cultivated trees in South Kona, east of Kealakekua, Hawai’i (19°30′48.31″ N, 155°48′49.53″ W, elev. 1421 m). Plant identification was verified by comparison with samples from the New York Botanical Garden Virtual Herbarium (https://sweetgum.nybg.org/science/vh/specimen-list/?SummaryData=Myoporum%20sandwicense; accessed on 9 January 2023) Each plant part (leaves, twigs, wood) was shredded and steam distilled for 4–7 h using a Clevenger-type apparatus. Twenty-three M. sandwicense volatile oils (N1-N23) produced in industrial settings were obtained from the collection of the Aromatic Plant Research Center (APRC, Lehi, UT, USA) in 2021. Wood industrial distillation settings included a steam flow of 100 L/h, pressure of 1–3 psi, steam flow increase rate of 0.15 L/h, hydrolyte temp of 55 °C, and duration of 3–5 days.
2.2. Gas Chromatography–Mass Spectrometry
M. sandwicense essential oils were analyzed by gas chromatography–mass spectrometry (GC-MS) using a Shimadzu GCMS-QP2010 Ultra operated in the electron impact (EI) mode (electron energy = 70 eV), scan range = 40–400 atomic mass units, scan rate = 3.0 scans/s, and GC-MS solution software v. 4.20 (Shimadzu Scientific Instruments, Columbia, MD, USA). The GC column was a ZB-5 ms fused silica capillary column (Phenomenex, Torrance, CA, USA) with a (5% phenyl)-polymethylsiloxane stationary phase and a film thickness of 0.25 μm. The carrier gas was helium, with a column head pressure of 552 kPa and a flow rate of 1.37 mL/min. The injector temperature was 260 °C and the ion source temperature was 260 °C. The GC oven temperature was programmed for 50 °C initial temperature; temperature increased at a rate of 2 °C/min to 260 °C. A 5% w/v solution of the sample in CH2Cl2 was prepared, and 0.1 μL was injected with a splitting mode (30:1). Identification of the oil components was based on their retention indices, determined by reference to a homologous series of n-alkanes, and by comparison of their mass spectral fragmentation patterns with those reported in the databases [9,10,11,12].
2.3. Hierarchical Cluster Analysis
M. sandwicense wood oils obtained from trusted industrial suppliers were used in the cluster analysis. The essential oil compositions were treated as operational taxonomic units (OTUs). The percentages of the major components (α-bisabolol, α-bisabolol oxide B, unidentified (1699), unidentified (1666), β-bisabolene, fokienol, dendrolasin, 6-epi-α-bisabolol oxide B, (E)-α-bisabolene, (E)-β-farnesene, himachal-2-en-7β-ol, (E)-nerolidol, limona ketone, and β-sesquiphellandrene) were used to determine the chemical associations between the essential oils using agglomerative hierarchical cluster (AHC) analysis using XLSTAT Premium, version 2018.5.53172 (Addinsoft, Paris, France). Dissimilarity was determined using Euclidean distance, and clustering was defined using Ward’s method.
2.4. Antimicrobial Screening
M. sandwicense essential oils were screened for antimicrobial activity against Gram-positive bacteria (Bacillus cereus (ATCC No. 14579), Cutibacterium acnes (ATCC No. 11827), Staphylococcus aureus (ATCC No. 29213), Staphylococcus epidermidis (ATCC No. 12228), Streptococcus pyogenes (ATCC No. 19615), and Streptococcus pneumoniae (ATCC No. 49136)), Gram-negative bacteria (Escherichia coli (ATCC No. 25922), Pseudomonas aeruginosa (ATCC No. 27853), Serratia marcescens (ATCC No. 14756), Helicobacter pylori (ATCC No. 51111), and Salmonella enterica subsp. enterica serovar Typhimurium (ATCC No. 14028)), molds (Aspergillus niger (ATCC No. 16888), Aspergillus fumigatus (ATCC No. 96918), Microsporum canis (ATCC No. 11621), Microsporum gypseum (ATCC No. 24102), and Trichophyton mentagrophytes (ATCC No. 18748)), and yeasts (Cryptococcus neoformans (ATCC No. 32045) and Candida albicans (ATCC No. 18804)) using the microbroth dilution technique, as previously reported [13].
All bacteria were cultured on tryptic soy agar (Sigma-Aldrich, St. Louis, MO, USA) except for H. pylori and Streptococcus pneumoniae, which were grown on tryptic soy agar supplemented with 7% (v/v) defibrillated whole sheep blood (Cleveland Scientific, Ohio, USA) under micro-aerophilic conditions for 3 days [14]. All fungi were cultured on yeast malt agar (Sigma-Aldrich, St. Louis, MO, USA). For bacteria, a 50-μL volume of 1% (w/v) solution of the samples in DMSO was diluted in 50 μL of cation-adjusted Mueller–Hinton broth (CAMHB) (Sigma-Aldrich, St. Louis, MO, USA). The sample solutions were then serially diluted (1:1) in fresh CAMHB to obtain concentrations of 2500, 1250, 625, 313, 156, 78, 39, and 20 μg/mL. The microbes were harvested from a fresh culture and added to each well at a concentration of approximately 1.5 × 108 colony-forming unit (CFU)/mL for bacteria, and 7.5 × 107 CFU/mL for fungi. The 96-well microdilution plates for bacteria were incubated at 37 °C, and the fungi were incubated at 35 °C for 24 h. The minimum inhibitory concentration (MIC) was determined as the lowest concentration with no turbidity. Gentamicin (Sigma-Aldrich, St. Louis, MO, USA) was used as a positive antibiotic control, and DMSO was used as the negative control (50 μL DMSO diluted in 50 μL broth medium, and then serially diluted, as above). For fungi, the above-mentioned method was implemented using yeast nitrogen base growth medium (Sigma-Aldrich, St. Louis, MO, USA) and Amphotericin B (Sigma-Aldrich, St. Louis, MO, USA) as a positive antifungal control.
3. Results and Discussion
3.1. Essential Oil Composition
Steam distillation of the leaves of M. sandwicense produced a yield of 0.11%. Analysis of the leaf essential oil by GC-MS showed the oil to be rich in the sesquiterpene hydrocarbons β-caryophyllene (15.1%), α-humulene (12.8%), germacrene D (7.9%), bicyclogermacrene (12.5%), and the furanoterpenoids brigalow ketol (9.6%) and myoporone (16.8%) (Table 1).

Table 1.
Essential oil composition of Myoporum sandwicense leaf essential oil.
Two samples of M. sandwicense wood were steam-distilled in a lab setting and analyzed by GC-MS (Table 2). The average oil yield was 0.34%. The oil was yellow to golden in color and had a woody, sweet, slightly spicy, and sandalwood-like aroma. Twenty-three industrially produced M. sandwicense wood oils (N1–N23) were analyzed by GC-MS (Table 3). Both lab-distilled and industrially distilled M. sandwicense wood essential oils were dominated by α-bisabolol and trans-α-bisabolol oxide B. Based on M. sandwicense essential oil compositions, a hierarchical cluster analysis of the oils from this work was carried out. The dissimilarity index was very small, indicating no significant differences in the essential oil compositions of the tested samples (Figure 1).

Table 2.
Essential oil compositions of lab-distilled Myoporum sandwicense wood essential oils.

Table 3.
Essential oil compositions (%) of industrially-distilled Myoporum sandwicense wood essential oils.
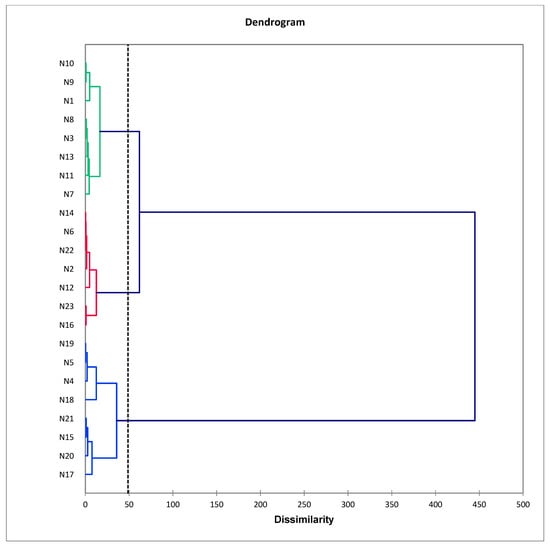
Figure 1.
Agglomerative hierarchical cluster (AHC) analysis of Myoporum sandwicense essential oil compositions.
Steam distillation of a sample of sapwood and a sample of twigs did not produce a separable essential oil. The aqueous distillate (hydrosol) was extracted with dichloromethane, however, to provide vanishingly small quantities of volatiles that could be analyzed by GC-MS (Table 4). The sapwood essential oil was dominated by fatty acids, palmitic acid (35.5%), linoleic acid (19.7%), oleic acid (31.9%, and stearic acid (5.7%), and derivatives of fatty acids. The essential oil from the twigs was rich in n-alkanes, tricosane (77.3%), and pentacosane (13.1%), probably reflecting a waxy coating on the twigs.

Table 4.
Volatile components from the sapwood and twigs of Myoporum sandwicense.
There have been several previous investigations on essential oils of Myoporum species reported in the literature. Furanosesquiterpenoids have generally been the dominant constituents (see Table 5).

Table 5.
Major components of Myoporum essential oils reported in the literature.
3.2. Antimicrobial Activity
M. sandwicense essential oils were screened for antimicrobial activity against a panel of potentially pathogenic bacteria and fungi (Table 6). The leaf essential oil of M. sandwicense showed excellent antibacterial activity against S. pyogenes (MIC = 78 μg/mL) and antifungal activity against A. fumigatus (MIC = 39 μg/mL). In fact, these two organisms were the most susceptible in our panel. The major components in the leaf essential oil: β-caryophyllene, α-humulene, bicyclogermacrene, and myoporone, may be responsible for the antimicrobial activity. Both β-caryophyllene and α-humulene have shown antimicrobial activity [21]. In earlier investigation, the essential oil of Centella asiatica, also rich in β-caryophyllene, α-humulene, and bicyclogermacrene, showed antibacterial activity [22]. Myoporone has shown antibacterial activity [20,23]. The wood essential oil of M. sandwicense, which was dominated by α-bisabolol and α-bisabolol oxide B, also showed notable activity against S. pyogenes and A. fumigatus (MIC = 78 μg/mL for each) as well as A. niger and M. gypseum (MIC = 39 μg/mL for each). α-Bisabolol has shown marginal antibacterial activity [24], but good antifungal activity [25,26]. In addition, α-bisabolol has been shown to potentiate the antibacterial activities of several antibiotics [27,28].

Table 6.
Antimicrobial activities, MIC a (μg/mL), of essential oils of Myoporum sandwicense.
The antifungal activity of the essential oil from the twigs against the mold species was surprising. The twig essential oil was composed of 95.4% n-alkanes. Yin and co-workers have examined the antifungal activity of cuticular wax from Asian pear fruit (Pyrus bretchneideri) and concluded that long-chain alkanes have antifungal activity [29]. The flower SFE-CO2 extract of black elderberry, rich in ethyl palmitate, n-pentacosane, n-tricosane, and n-heneicosane, showed antifungal activity [30]. In contrast, the alkane-rich wood essential oil of agarwood (Aquilaria sinensis) showed only marginal antifungal activity [31]. Similarly, n-alkane-rich fractions from a hexane extract of Cupressus lusitanica leaves (>90% alkanes) showed no antifungal activity against a panel of dermatophytes [32]. It is likely that synergistic interactions with the minor components account for the observed antifungal activity of the twig essential oil. The non-polar fraction of surface wax from Asian pear fruits, dominated by long-chain n-alkanes showed inhibition of germination of Alternaria alternata [29]. Likewise, sorghum leaf wax, rich in long-chain alkanes, suppressed the growth of A. alternata [33]. Several long-chain n-alkanes have been screened for inhibition of mycelia and conidial germination of A. alternata and were found to be inactive [34]. Since alkanes do not have functional groups, they are unlikely to interact with biological targets involved with fungal metabolic pathways (e.g., glyoxylate cycle, pyrimidine biosynthesis, cytochrome P450 enzymes, iron metabolism, heme biosynthesis, and acetate metabolism), signal transduction pathways (e.g., MAP kinase, PDK1, and calcium signaling), or gene expression [35]. They are, however, non-polar compounds and would be expected to interact with fungal membranes and disturb membrane integrity, affecting membrane permeability. Thus, n-alkanes may act synergistically with other components in the essential oil, allowing these components to diffuse into the fungal cells.
4. Conclusions
This work is the first report on the chemical composition and antimicrobial properties of Myoporum sandwicense A. Gray (naio) essential oils. The leaf essential oil was made of β-caryophyllene, α-humulene, germacrene D, bicyclogermacrene, brigalow ketol, and myoporone, while the wood essential oil was dominated by α-bisabolol and trans-α-bisabolol oxide B. Palmitic acid, linoleic acid, oleic acid, and stearic acid were the major components of the sapwood oil, whereas the oil from twigs was rich in tricosane and pentacosane. The leaf essential oil of M. sandwicense showed excellent antibacterial activity against Streptococcus pyogenes and antifungal activity against A. fumigatus. The wood essential oil showed notable activity against S. pyogenes, A. fumigatus, A. niger, and M. gypseum. The twig oil was remarkably active against mold species.
Author Contributions
Conceptualization, A.S. and P.S.; methodology, N.S.D. and P.S.; software, W.N.S.; formal analysis, N.S.D. and P.S.; investigation, N.S.D. and P.S.; data curation, P.S. and W.N.S.; writing—original draft preparation, W.N.S. and N.S.D.; writing—review and editing, N.S.D., P.S., A.S. and W.N.S.; supervision, W.N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within this article.
Acknowledgments
The authors would like to thank Willie Rice for plant identification and Greg Hendrickson for valuable discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Webster, G.L. The Polynesian Species of Myoporum. Pac. Sci. 1951, 5, 52–77. [Google Scholar]
- Lowe, S.; Ball, D.L.; Reeves, M.K.; Amidon, F.; Miller, S.E. Hawaiʻi: Mesic Forests. Ref. Modul. Earth Syst. Environ. Sci. 2019, 1–27. [Google Scholar] [CrossRef]
- van Riper, C., III; Scott, J.M. Observations on the Distribution, Diet, and Breeding of the Hawaiian Thrush. Condor 1979, 81, 65–71. [Google Scholar]
- Olson, S.L. A Hard Nut to Crack: Rapid Evolution in the Kona Grosbeak of Hawaii for a Locally Abundant Food Source (Drepanidini: Chloridops kona). Wilson J. Ornithol. 2014, 126, 1–8. [Google Scholar] [CrossRef]
- Scowcroft, P.G.; Sakai, H.F. Impact of Feral Herbivores on Mamane Forests of Mauna Kea, Hawaii: Bark Stripping and Diameter Class Structure. J. Range Manag. 1983, 36, 495–498. [Google Scholar] [CrossRef]
- Conant, P.; Hauff, R.; Loope, L.; King, C. Forest and Forestry Insect Pests in Hawai’i: Past, Present, and Future. In Proceedings of the 7th Meeting of IUFRO Working Party 7.03.04 Diseases and Insects in Forest Nurseries, Hilo, HI, USA, 13–17 July 2009; Cram, M.M., Ed.; pp. 16–38. [Google Scholar]
- Vargas Suarez, A.; Satyal, P.; Setzer, W.N. Volatile Components of the Wood of Spanish Cedar, Cedrela Odorata, from Costa Rica. Am. J. Essent. Oils Nat. Prod. 2018, 6, 27–30. [Google Scholar]
- Vargas Suarez, A.; Satyal, P.; Setzer, W.N. The Wood Essential Oil Composition of Swietenia Macrophylla from Guanacaste, Costa Rica. Am. J. Essent. Oils Nat. Prod. 2019, 7, 14–16. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mondello, L. FFNSC 3; Shimadzu Scientific Instruments: Columbia, ML, USA, 2016. [Google Scholar]
- NIST17. NIST17; National Institute of Standards and Technology: Gaithersburg, ML, USA, 2017. [Google Scholar]
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils; University of Alabama in Huntsville: Huntsville, AL, USA, 2015. [Google Scholar]
- Sahm, D.H.; Washington, J.A. Antibacterial Susceptibility Tests: Dilution Methods. In Manual of Clinical Microbiology; Balows, A., Hausler, W.J., Herrmann, K.L., Isenberg, H.D., Eds.; American Society for Microbiology: Washington, DC, USA, 1991. [Google Scholar]
- Blanchard, T.G.; Nedrud, J.G. Laboratory Maintenance of Helicobacter Species. Curr. Protoc. Microbiol. 2006, 8B.1.1–8B.1.13. [Google Scholar] [CrossRef]
- Menut, C.; Cabalion, P.; Hnawia, E.; Agnaniet, H.; Waikedre, J.; Fruchier, A. Two New Furanosesquiterpenes from Myoporum crassifolium from New Caledonia. Flavour. Fragr. J. 2005, 20, 621–625. [Google Scholar] [CrossRef]
- Grant, H.G.; Russell-Maynard, C.A.; Sutherland, M.D. Terpenoid Chemistry. XXVII. Further Iridoid Constituents, (1R)- and (1S)-Acetoxymyodesert-3-Ene, from Myoporum deserti (Myoporaceae). Aust. J. Chem. 1985, 38, 325–336. [Google Scholar] [CrossRef]
- Allen, J.G.; Seawright, A.A.; Hrdlicka, J. The Toxicity of Myoporum tetrandrum (Boobialla) and Myoporaceous Furanoid Essential Oils for Ruminants. Aust. Vet. J. 1978, 54, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.M.; Omer, E.A.E. Seasonal Variation in the Volatile Oil of Myoporum laetum Leaves. Med. Aromat. Plant Sci. Biotechnol. 2009, 3, 50–51. [Google Scholar]
- Métra, P.L.; Sutherland, M.D. Further Skeletal Variety in the Toxic Furanosesquiterpene Ketones in the Myoporum Genus. Tetrahedron Lett. 1983, 24, 1749–1752. [Google Scholar] [CrossRef]
- Zaleta-Pinet, D.; McCluskey, A.; Hall, S.; Brophy, J.; Ashhurst-Smith, C.; Sakoff, J.; Van Altena, I. The Use of the Toxic Plant Myoporum montanum in a Traditional Australian Aboriginal Medicine. Aust. J. Chem. 2016, 69, 161–168. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Noletto, J.A.; Vogler, B.; Setzer, W.N. Abaco Bush Medicine: Chemical Composition of the Essential Oils of Four Aromatic Medicinal Plants from Abaco Island, Bahamas. J. Herbs Spices Med. Plants 2006, 12, 43–65. [Google Scholar] [CrossRef]
- Oyedeji, O.A.; Afolayan, A.J. Chemical Composition and Antibacterial Activity of the Essential Oil of Centella asiatica Growing in South Africa. Pharm. Biol. 2005, 43, 249–252. [Google Scholar] [CrossRef]
- Dong, L.M.; Huang, L.L.; Dai, H.; Xu, Q.L.; Ouyang, J.K.; Jia, X.C.; Gu, W.X.; Tan, J.W. Anti-MRSA Sesquiterpenes from the Semi-Mangrove Plant Myoporum bontioides A. Gray. Mar. Drugs 2018, 16, 438. [Google Scholar] [CrossRef]
- Wanner, J.; Schmidt, E.; Bail, S.; Jirovetz, L.; Buchbauer, G.; Gochev, V.; Girova, T.; Atanasova, T.; Stoyanova, A. Chemical Composition and Antibacterial Activity of Selected Essential Oils and Some of Their Main Compounds. Nat. Prod. Commun. 2010, 5, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- van Zyl, R.L.; Seatlholo, S.T.; van Vuuren, S.F.; Viljoen, A.M. The Biological Activities of 20 Nature Identical Essential Oil Constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Romagnoli, C.; Baldisserotto, A.; Malisardi, G.; Vicentini, C.B.; Mares, D.; Andreotti, E.; Vertuani, S.; Manfredini, S. A Multi-Target Approach toward the Development of Novel Candidates for Antidermatophytic Activity: Ultrastructural Evidence on α-Bisabolol-Treated Microsporum Gypseum. Molecules 2015, 20, 11765–11776. [Google Scholar] [CrossRef] [PubMed]
- Brehm-Stecher, B.F.; Johnson, E.A. Sensitization of Staphylococcus Aureus and Escherichia Coli to Antibiotics by the Sesquiterpenoids Nerolidol, Farnesol, Bisabolol, and Apritone. Antimicrob. Agents Chemother. 2003, 47, 3357–3360. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.D.S.; de Freitas, T.S.; da Cruz, R.P.; Costa, M.D.S.; Pereira, R.L.S.; Quintans-Júnior, L.J.; Andrade, T.D.A.; Menezes, P.D.P.; de Sousa, B.M.H.; Nunes, P.S.; et al. Evaluation of the Antibacterial and Modulatory Potential of α-Bisabolol, β-Cyclodextrin and α-Bisabolol/β-Cyclodextrin Complex. Biomed. Pharmacother. 2017, 92, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Bi, Y.; Chen, S.; Li, Y.; Wang, Y.; Ge, Y.; Ding, B.; Li, Y.; Zhang, Z. Chemical Composition and Antifungal Activity of Cuticular Wax Isolated from Asian Pear Fruit (Cv. Pingguoli). Sci. Hortic. 2011, 129, 577–582. [Google Scholar] [CrossRef]
- Schoss, K.; Kočevar Glavač, N.; Dolenc Koce, J.; Anžlovar, S. Supercritical CO2 Plant Extracts Show Antifungal Activities against Crop-Borne Fungi. Molecules 2022, 27, 1132. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, X.M.; Wei, J.H.; Xue, J.; Yang, Y.; Liang, L.; Li, X.J.; Guo, Q.M.; Xua, Y.H.; Gao, Z.H. Compositions and Antifungal Activities of Essential Oils from Agarwood of Aquilaria sinensis (Lour.) Gilg Induced by Lasiodiplodia theobromae (Pat.) Griffon. & Maubl. J. Braz. Chem. Soc. 2014, 25, 20–26. [Google Scholar] [CrossRef]
- Kuiate, J.R.; Bessière, J.M.; Zollo, P.H.A.; Kuate, S.P. Chemical Composition and Antidermatophytic Properties of Volatile Fractions of Hexanic Extract from Leaves of Cupressus lusitanica Mill. from Cameroon. J. Ethnopharmacol. 2006, 103, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, X.; Yao, L.; Xu, D.; Li, Y.; Zhang, X.; Li, Z.; Xiao, Q.; Ni, Y.; Guo, Y. Chemical profiles of cuticular waxes on various organs of Sorghum bicolor and their antifungal activities. Plant Physiol. Biochem. 2020, 155, 596–604. [Google Scholar] [CrossRef]
- Prakash, J.; Arora, N.K. Novel metabolites from Bacillus safensis and their antifungal property against Alternaria alternata. Antonie Leeuwenhoek 2021, 114, 1245–1258. [Google Scholar] [CrossRef]
- Ivanov, M.; Ćirić, A.; Stojković, D. Emerging antifungal targets and strategies. Int. J. Mol. Sci. 2022, 23, 2756. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).