Abstract
In this work, silver nanoparticles (AgNPs) used in conductive inks were synthesized for implementation in printable and flexible electronics. The nanoparticles were obtained using silver nitrate as a precursor agent, sodium citrate as a reductive/protective agent and sodium borohydride as a reductive, whose concentrations were varied for optimization. The optical absorption, morphology, size-distribution, crystallinity and stability over time of the processed nanoparticles were determined upon the content of the chemical contents. The AgNPs-based inks were then tested as conductive wires drawn on different common flexible substrates to measure their electrical characteristics and demonstrate their relevance in printable electronics.
1. Introduction
The development of inks with efficient, robust and stable electrical conduction properties is at the heart of the challenges encountered in printed electronics, and more especially when these inks are implemented on flexible substrates, which requires an easy application and handling [1,2]. The fabrication of such liquid materials can be achieved using metallic nanoparticles obtained from a chemical synthesis involving the mixture of different precursors and reducing agents. The concentration of these chemicals can drastically affect the quality of the final product, as well as the kinetics of the reaction leading to the ink preparation. The nucleation and growth process of the nanoparticles are well described by the LaMer mechanism, where successive steps of supersaturation, nucleation and growth occur [3]. One of the most used conductive nanomaterials are silver nanoparticles (AgNPs) implemented as functional materials in printed electrical wires for numerous applications [4,5]. To improve the functionality and durability of such materials, it is essential to develop simple and cost-effective preparation methods, that enable the control of the physical properties of the formed AgNPs from their size and shape [6]. The synthesis of AgNPs can be carried out by various chemical processes. Wang, R. et al. [7] mentioned the relevance of a hybrid process to synthesize particles at nanoscale, using a microfluidic batch-cooling system to realize in situ redox sequential of metal ions and metal oxides. Electrochemical processes have also been reported to obtain AgNPs with sizes ranging from 5 to 50 nm. To activate the chemical reduction in metal precursors, it is also possible to use platinum electrodes with an argon atmosphere [8], as well as UV or double beam illumination systems, which can obtain nanoparticles ranging in size from 2 to 120 nm [9]. One of the simplest routes is the chemical reduction method, where the morphology and homogeneity of AgNPs can be set from the concentration of each reagent without requiring advanced instrumentation and complex environmental conditions during the synthesis process [10,11]. For the chemical reduction method, NPs ranging from 5 to 100 nm can be synthesized almost exclusively depending on the reducing agents used. Since the reaction conditions do not require sophisticated equipment or an inert atmosphere, the cost associated with this simple process is also reduced. An example of a simple chemical reduction method is the Turkevich method, where through the use of sodium citrate (C6H5Na3O7), silver nitrate (AgNO3) can be chemically reduced only needing heat to the temperature of the boiling point of the solution. Several modifications of this synthesis method have been already reported, where C6H5Na3O7 and sodium borohydride (NaBH4) were used, both as reducing agents and at low temperatures; at room temperature for the preparation of AgNO3 and C6H5Na3O7 solutions and at 0 °C for the addition of the NaBH4 solution and during the reaction time [12,13,14]. NaBH4 is a strong reductant and fine monodisperse AgNPs are formed with this agent, while weak reductants like C6H5Na3O7 lead to the formation of relatively large AgNPs [15,16]. The addition of C6H5Na3O7 at an optimal concentration could prevent the agglomeration of small particles into larger nanoclusters improving the concentration of available functional particles within the solution [17,18]. On the other hand, the use of NaBH4 as a reductant agent might offer the possibility of finalizing the optimal production of nanoparticles. This is because C6H5Na3O7, being a mild reductive agent, is less effective at donating electrons and tends to react more slowly with Ag+ ions. In contrast, NaBH4, as a strong reducing agent, donates electrons more easily, leading to faster and more dynamic reactions. However, the vigorous reaction could produce bigger particles [19,20]. Geng, S. et al. demonstrated the relevant use of C6H5Na3O7 and NaBH4 in a long process synthesis of AgNPs, where both were used as reductants through photoinduced processes by irradiation with a sodium lamp in a quartz beaker. These processes resulted in the growth of spherical silver seeds into nanoparticles. The growth occurred over an exposure time range from 0 to 8 h [21].
In this work, a parametric study was conducted to determine the optimal concentrations of reagents for the synthesis of efficient and stable AgNPs by the chemical reduction method. Our process was carried out using the common and easy-to-implement reagents: AgNO3, NaBH4 and C6H5Na3O7, which require short reaction times and avoid the need for heating. In this mixture, AgNO3 acts as a precursor agent, while NaBH4 acts as reducing agent and C6H5Na3O7 acts as reducing/protective agent. The size, crystallinity, stability and uniformity of the AgNPs prepared from various chemical concentrations were investigated prior to their testing as conductive wires on different common substrates such as bond and photographic papers. The most favorable conditions were found for AgNPs synthesized with 0.15 M AgNO3, 0.05 M NaBH4 and 0.03 M C6H5Na3O7, whose mechanical and electrical properties have the ability to operate a hand-printed circuit on a sheet of paper for activating a light-emitting diode.
2. Materials and Methods
Three sample sets were prepared for characterization and analysis. The reagents implemented in the three sample sets are NaBH4 (≥99%, Sigma Aldrich, St. Louis, MO, USA), C6H5Na3O7 (≥99%, Sigma Aldrich), AgNO3 (≥99%, Sigma Aldrich) and tridistilled water. In the first experimental set the C6H5Na3O7 and NaBH4 reagents were used as a reference to evaluate the reductive/protective effect of each of these. Solutions of C6H5Na3O7 0.05 M, NaBH4 0.05 M and AgNO3 0.05 M were prepared in tridistilled water. To start the reaction, in an ice bath (5 °C), the C6H5Na3O7 solution was added to a flask with 25 mL of water and then the AgNO3 solution was added drop by drop under magnetic stirring. The total reaction time was 30 min. The solution obtained was washed with ethanol and the particles were dispersed in water. The reaction between the NaBH4 and AgNO3 solutions was carried out in the same way. To distinguish the samples obtained, the reaction of C6H5Na3O7 with AgNO3 was named RA1 and the reaction of NaBH4 with AgNO3 was named RA2. The second set consisted of using AgNO3, NaBH4 and C6H5Na3O7 reacting together. To obtain the different samples, solutions of AgNO3 0.05 M and NaBH4 0.05 M were prepared and used as a matrix in each of the reactions performed, and the concentration of C6H5Na3O7 was varied from 0.01 M to 0.05 M, with a variation of 0.01 M in each sample. In this set of reactions, the experiment was started using a flask with 25 mL of water that was placed in an ice bath (5 °C) and one of the C6H5Na3O7 solutions were added. Under magnetic stirring, the AgNO3 solution was added drop by drop and then, the NaBH4 solution was added. It was allowed to react for 30 min. In this set of experiments, five samples were obtained and named SC1 to SC5 to distinguish the variation in citrate added at different concentrations (0.01 M to 0.05 M). Each of the products obtained was washed with ethanol and the particles were dispersed in water. Finally, in the third set, the reactions with C6H5Na3O7 0.03 M and NaBH4 0.05 M as matrix, and AgNO3 0.05 M (SN1), 0.10 M (SN2) and 0.15 M (SN3) were realized with the same conditions as those previously described in the second set. In Table 1, the different reactions in sample sets and the reagents involved at different concentrations are listed. The samples obtained were washed with ethanol and dispersed in water. The general chemical reaction of the experimental sets labeled ‘2’ and ‘3’ is described in Equation (1):
where [Ag(C6H5O7)]2− is a silver citrate complex formed from the addition of silver nitrate in sodium citrate solution.

Table 1.
Description of the different reactions and reagents involved in the three sets of samples 1.
To produce conductive AgNPs-based inks, the AgNPs obtained from the third experimental set (labeled ‘SN’) were mixed with ethanol and centrifugated at 1000 rpm for 30 min. The solution was then deposited onto corning glass and heated at 60 °C for 30 min in order to evaporate the solvent. The conductive inks were deposited and dried at RT and at 60 °C on commercial acetate, bond paper, photographic paper, opaline paper and PET substrates.
Both size and morphology of the formed AgNPs were investigated using a Tescan Vega TS5136 SB Scanning Electron Microscope (SEM) (TESCAN, Kohoutovice, Czech Republic) operated at 25 kV and equipped with an SE detector, as well as through Dynamic Light Scattering (DLS), also implemented to evaluate the stability of the AgNPs through zeta potential measurements, using a Zetasizer Nano-ZS90 apparatus (Malvern Panalytical Ltd., Singapore). The chemical composition and crystallinity of the nanoparticles were determined by θ-2θ X-ray diffraction using a D8 Bruker AXS instrument (Bruker AXS GmbH, Karlsruhe, Germany) with the Cu-Kα radiation source (λ = 0.15406 nm) and an Energy Dispersive X-ray Spectroscope (EDS) operated at a voltage of 40 kV and a current of 25 mA.
The compactness and uniformity of the AgNPs were evaluated from their UV-Vis optical absorbance using a Cary 100 UV-Vis spectrometer (Agilent Technologies, Inc., Santa Clara, CA, USA) operated between 320 and 800 nm, as well as through AFM mapping, using a Veeco Multimode (DI) atomic force microscope (Veeco Instruments Inc., New York, NY, USA). All electrical tests and current-to-voltage measurements aiming to demonstrate the functionality of the conductive inks of the third experimental set were conducted using a Keithley-4200 Semiconductor (Keithley Instrumen, Cleveland, OH, USA) Characterization System under air ambient and at room temperature (RT).
3. Results and Discussion
3.1. Geometrical Characterization of Silver Nanoparticles
The size and uniformity in size of the AgNPs synthesized upon the chemical recipes listed in Table 1 were investigated by SEM. As presented in Figure 1, large and relatively isolated silver particles of quasi-spherical shapes are found in samples RA1 and RA2, while the AgNPs observed in SC1 to SC5 samples are all clustered in large and multibranched bundles. The surface morphology of these structures reveals the presence of small agglomerated spherical grains, corresponding to irregular forms of micrometric lengths. These results are consistent with the ones available in the literature, where similar morphologies have been reported for AgNPs synthesized using C6H5Na3O7 as a reducing/protective agent [22,23,24,25].
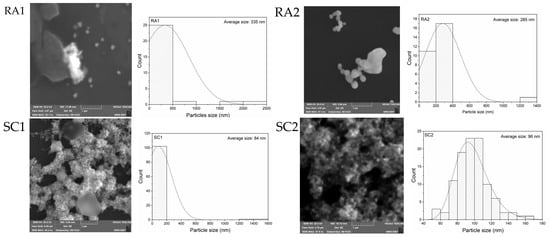
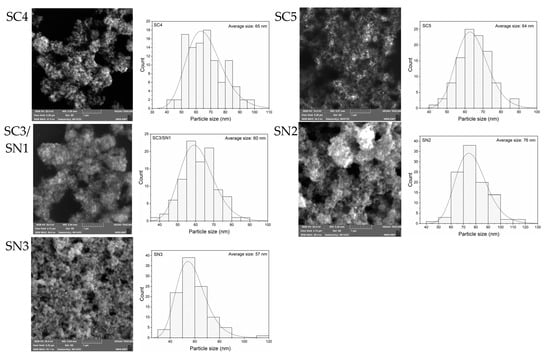
Figure 1.
SEM images and size-distribution of AgNPs synthesized from the concentrations reported in Table 1.
The mean diameter of AgNPs measured from SEM analyses were conducted using the ImageJ (version 1.54d) freeware and reported in the second column of Table 2 [26]. The samples containing no NaBH4 (RA1) or no C6H5Na3O7 (RA2) are found to have large dimensions, around 300 nm, whereas the other ones (SC1, SC2, SC3/SN1, SC4, SC5, SN2, SN3) have quite similar diameters, between 60 and 80 nm. The uniformity in size of the AgNPs is depicted from the widths at FHWM of the size-distribution histograms. The values reported in the third column of Table 2 reveal an acceptable homogeneity in the size of AgNPs in the SC and SN sample series, with standard deviations between 10 and 15% around the average diameter of the nanoparticles. The small variations in the AgNPs dimensions are attributed to the use of different AgNO3:C6H5Na3O7:NaBH4 molar ratios [3,27].

Table 2.
Particle size (DLS and SEM), PDI and zeta potential measured in each sample.
The data recorded by DLS are given in the fourth, fifth and sixth columns of Table 2, each referring to the size of AgNPs, and the PDI and zeta potential associated with their uniformity in size, respectively. Figures of particle size distributions are in the Supplementary Material. These values are in agreement with those obtained from our SEM analyses. For PDI ≤ 0.1, the colloidal solution is considered as highly monodisperse; between 0.1 and 0.4, it is moderately polydisperse; and above 0.4, it is identified as highly polydisperse [28]. This result confirms that SC and SN sample series of the AgNPs synthesized through the implemented chemical recipes of Table 1 have homogeneous dimensions fluctuating between 50 and 100 nm.
The qualitative agreement between the main sizes measured by SEM after spreading the AgNPs on a solid silicon wafer substrate, and when dispersed in water for DLS measurements, suggests that AgNPs are relatively unaffected chemically by the environment. This feature results in a relatively high stability over time, which can be evaluated and compared between the different samples from the zeta potential measurements given in the sixth column of Table 2. According to the standards, chemical potentials greater than +30 mV or lower than −30 mV indicate stable and poor reactive materials, thus avoiding the tendency of small nanoparticles to growth or cluster in bigger nanoparticles [29,30,31]. The negative zeta potentials found around −24 mV for RA1 and RA2 are also compatible with the greater size of silver particles or complex, reported in the second column of Table 2, since the smaller curvature of their outer surface reduces the number of chemical dangling bounds. Similar potentials have been reported in the literature [32,33], where the negative zeta potential is attributed to charge stabilization, resulting from the adsorption of anions surrounding AgNPs and forming a monolayer [32,34]. Table 2 also shows the pH of the prepared samples; it is expected that the pH will be influenced by variations in reagent concentrations. As can be seen, if the suspension is more alkaline, the particles tend to acquire a more negative zeta potential. For series SC and SN, it can be observed that the pH is in the range of 7.5 and 7.7. In contrast, for samples RA1 and RA2, the alkalinity is influenced by the concentration of AgNO3, which is slightly acidic compared to sodium citrate and sodium borohydride, this tends to maintain the pH around 7. This influences the stability of the solution and is consistent with the zeta potential measurements.
Greater stability is found for AgNPs prepared from the mixture of the three reagents: AgNO3, NaBH4 and C6H5Na3O7, with zeta potentials lower than −30 mV measured in the samples SC1, SC2, SC3/SN1, SC4, SC5, SN2 and SN3. All these AgNPs can be considered as stable. Nevertheless, as the zeta potential of sample SN3 was the best (−32.2 mV), the chemical recipe with 0.05 M of NaBH4, 0.03 M of C6H5Na3O7 and 0.15 of AgNO3 is optimal.
3.2. Chemical Composition Analyses
The composition and crystallinity of the AgNPs have been investigated by XRD and EDS analyses. Such characterization techniques can also be relevant for the detection of contaminants, as well as the presence of residues coming from incomplete chemical reactions, for this reason this section considers the SN series only. In Figure 2, the diffraction patterns of the SN series samples are shown and compared to the tabulated data of Ag. The spectra exhibit diffraction peaks at 38°, 44°, 64°, 77° and 81° corresponding to the Ag planes labeled (111), (200), (220), (311) and (222), respectively. These reflections correspond to the crystalline structure of silver face-centered cubic (fcc) symmetry [35,36,37]. No other peaks related to impurities or residues were observed, suggesting negligible sample contamination. By comparing the data, it is observed that the increase of AgNO3 concentration increases the intensity of the Ag diffraction peaks, indicating the occurrence of a more efficient reduction in Ag+ for AgNPs prepared with the highest AgNO3 content. The thinning of these diffraction peaks also suggests that the formed AgNPs have a higher crystallinity in SN3.
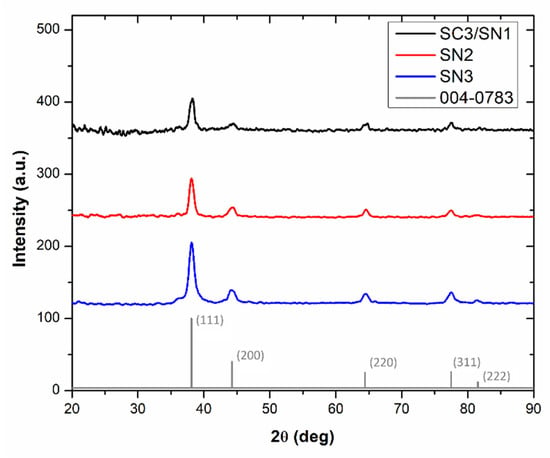
Figure 2.
XRD patterns of the formed AgNPs.
EDS experiments were also conducted on the SN sample series. The data were collected from a surface of 1 × 1 µm2 to quantify the chemical composition of the medium. An example of EDS measurement is shown for the SN3 sample in Figure 3a, where the characteristic peaks of Ag at Lα1 = 2.98 keV and Lβ1 = 3.15 keV are clearly seen. In Figure 3a, other energy peaks around 0.25, 0.50 and 1.00 keV are attributed to C, N, O and Na, respectively. The additional peak at Kα = 1.739 keV is related to the silicon substrate where the AgNPs have been deposited for EDS measurements.
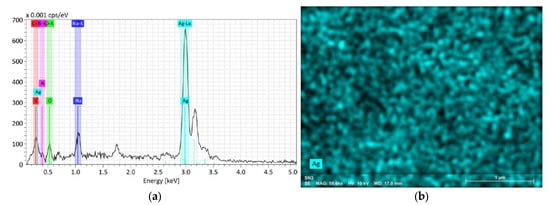
Figure 3.
EDS composition analysis of SN3 sample (a) with surface mapping of Ag (b).
The relative percentages of Ag, N, C, O and Na found in the SN series sample are reported in Table 3, giving the wt.% and atomic concentrations of each species found in the samples. All are exhibiting a high concentration of Ag (over 80%) denoting the efficiency of the AgNPs synthesis process. The other elements are associated with chemical residues coming from the AgNO3:C6H5Na3O7:NaBH4 mixture. Indeed, once the reducing agents are dissolved in water, they can form various ionic species such as silver ions (Ag+), borohydride ions (BH4−), sodium ions (Na+), citrate (C6H5O73−), silver citrate complexes, [Ag(C6H5O7)]2− or nitrate NO3−, that do not participate in the AgNPs synthesis or act as byproducts [38,39,40,41].

Table 3.
Elemental quantification (wt.% and at.%) by EDS.
The high concentration of Ag found at the surface of the Si substrate on which the AgNPs have been deposited was investigated by EDX mapping, as depicted in Figure 3b, showing the uniform spatial distribution of AgNPs.
3.3. AgNPs Ink Density and Surface Morphology
The aqueous colloidal solutions containing AgNPs, used as functionalized inks, have been studied by UV-Vis spectroscopy, where the activation of surface plasmon resonance (SPR) effects can be correlated to the concentration of AgNPs [42,43,44,45,46,47]. The recorded UV-Vis spectra are presented in Figure 4. The effects of C6H5Na3O7 (RA1) and NaBH4 (RA2) are shown in Figure 4a. These measurements indicate a strong attenuation of the broad absorption band around 430 nm in RA2 compared to RA1, as well as a spectral broadening compatible with the higher PDI of the RA2 solution (given in the 5th column of Table 2) [48]. Nevertheless, both samples have poor absorbance capacities in UV-Vis, indicating the presence of a low concentration of AgNPs in these aqueous solutions. Furthermore, it is possible to obtain a silver complex instead of nanoparticles in the sample RA1, considering the synthesis conditions [23,49]. The non-activation (or activation) at low formation rates of the Ag nanoclustering in RA1 and RA2 is consistent with the expected for recipes using only C6H5Na3O7 or NaBH4 [10,49,50].
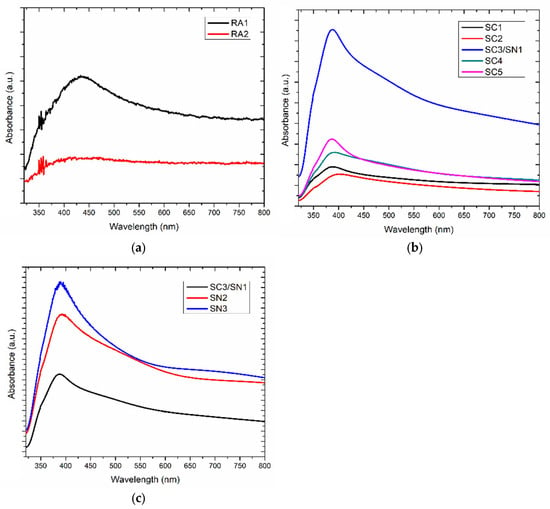
Figure 4.
UV-Vis spectra of the AgNPs formed in the RA (a), SC (b) and SN (c) samples series, respectively.
The UV-Vis spectra of samples SC1 to SC5 and SN1 to SN3 are shown in Figure 4b and Figure 4c, respectively. For these solutions containing AgNPs synthesized from a mixture of AgNO3:C6H5Na3O7:NaBH4, a greater optical absorbance is reported between 350 and 550 nm, in agreement with the data reported for AgNPs in the literature [21]. The more pronounced peak around 400 nm is attributed to the presence of AgNPs, dropped from the colloidal solutions. The position in wavelength and width of this absorption band may be sensitive to the size and the PDI of the AgNPs [48,51,52,53,54], as well as to the density of deposited AgNPs. The more efficient synthesis of AgNPs observed when mixing the C6H5Na3O7 solution with NaBH4 is related to the pH increase, which slows down the degradation of BH4− ions and favors electron exchanges for the Ag+ → Ag° reduction [55]. The greater optical absorbance is measured for the SN3 sample, suggesting optimal and homogeneous surface covering by the AgNPs-based layer.
In addition to the broad absorption band at 400 nm observed in Figure 4, different absorption baseline elevations, as well as secondary spectral contributions between 500 and 600 nm are reported. These features are both related to the formation of AgNPs aggregates and the variation in size of the AgNPs [56,57]. The observed variations indicate the occurrence of different agglomeration rates resulting from the different chemical recipes and the physical properties of the synthesized AgNPs (See Supplementary Material). As suggested from the PDI, SEM and XRD analyses, this feature is not surprising since the stability, concentration and morphology of the AgNPs may significantly vary upon the concentration of reagents. The optical variations reported on Figure 4 (and consistent with previous works) are associated with the spectral contribution of both isolated and clustered AgNPs to the optical absorbance of the dried solution.
The surface roughness and layer compactness of the SN3 sample were investigated by AFM. As illustrated on the surface mapping of Figure 5, the topography of the layer formed by the deposited AgNPs is relatively uniform, showing surface roughness of the 2.5 × 2.5 µm2 AFM profile. The root mean square (Rq) and the average roughness (Ra) values are found to be 65 nm and 53 nm, respectively. It is also observed that most of the AgNPs are in contact with each other, suggesting efficient current conduction through percolation.
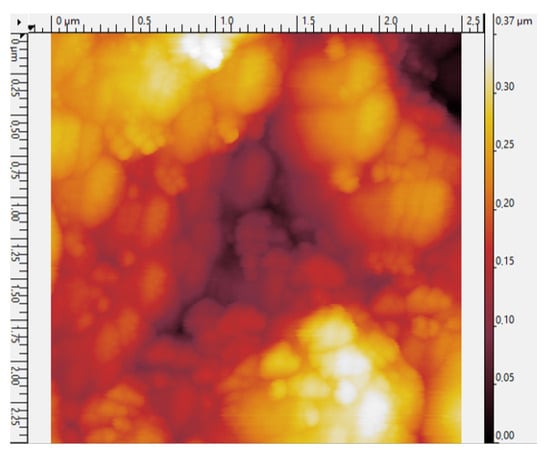
Figure 5.
Surface roughness AFM 2D image.
3.4. Electrical Measurements
Based on the previous morphological, composition and quality analyses, it has been established that concentrations of 0.15 M AgNO3, 0.05 M NaBH4 and 0.03 M C6H5Na3O7 (SN3) offer the best stability and homogeneity to the AgNPs. The preparation time required for the synthesis of these AgNPs is around 30 min, which makes the chosen fabrication process suitable for large-scale production. The more uniform size of AgNPs found in SN3 should also facilitate their interconnection and improve electrical conduction [58,59,60,61]. This feature has been reported by Mustafa et al. for silver nanoparticles investigated by electron microscopy and attributed to the better way they can interconnect upon their size and size-uniformity [62]. The AgNPs obtained from the chemical recipes of SN samples were added in ethanol, deposited onto corning glass and heated to evaporate the solvent. To prepare samples suitable for electrical measurements, a drop of the solution was placed on the different substrates, forming a circle of approximately 3 mm in diameter, and then dried at room temperature and 60 °C. A small amount of silver contact paint (SPI-Paint) was then deposited around the edge of the evaporated drop, to serve as electrodes. Figure 6 shows the current-to-voltage (I-V) curves that have been recorded for applied voltages between −10 V and +10 V. In the case of sample SN3, the compliance current of the Keithley system was reached at ±0.6V.
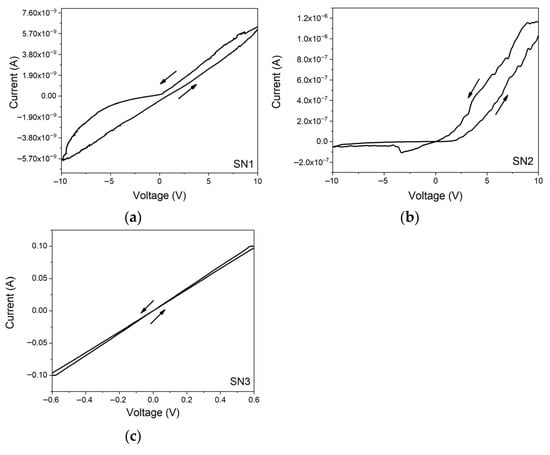
Figure 6.
I-V curves of the samples (a) SN1, (b) SN2 and (c) SN3 deposited on corning glass.
In Figure 6, the I-V curves of SN1 and SN2 samples are nonsymmetric and non-linear which is incompatible with the ohmic behavior required for efficient electrical condition. In addition, high hysteresis effects are observed for both samples as a consequence of possible structural irreversible changes. In contrast, it is observed that the I-V curve is symmetric and linear for the SN3 sample, as expected for ohmic contact [63]. The electrical resistances obtained from Ohm’s law are 1.63 × 109 Ω, 7.71 × 106 Ω and 5.78 Ω for samples SN1, SN2 and SN3, respectively. For samples SN1 and SN2, it was considered the most linear region of the curves. These values are compatible with the resistances reported between 400 Ω and 2000 Ω in the literature for AgNPs obtained through different synthesis methods [63,64,65,66]. Hence, the low electrical resistance of the SN3 sample makes the AgNPs prepared according to this chemical recipe an excellent candidate for the functionalization of conductive ink.
To evaluate the capability of these AgNPs on other substrates, they were tested on acetate, bond paper, photographic paper, opaline paper and PET substrates. The samples were dried in two sets of samples at RT and 60 °C, according to the data and drying times provided in Table 4. The electrical resistance was measured five times in three different samples for each substrate, and the error rate was calculated from their averaging.

Table 4.
Calculated resistance of samples SN3 on different flexible substrates.
The calculated resistance is reported in Table 4. For samples prepared at RT the drying time was variable, since the surface is different for each substrate and considering the natural capillarity of the paper [67,68,69]. Figure 7 shows the representative I-V curves measured for these samples. It is observed that the protection current of the Keithley system is reached at very low voltages. In addition, very small hysteresis and low resistances are found in all the samples. This indicates that despite noticeable variability in the rheological properties of the tested substrates, the AgNPs conductive ink based on the SN3 sample has an acceptable stability and is compatible with the different natural and synthetic polymeric substrates. At drying temperature of 60 °C (Figure 8), the drying time was set to 5 min on PET and acetate substrates, exhibiting electrical resistance of 4.00 and 2.86 Ω, respectively. Oliveira et al. reported similar resistance values for AgNPs synthesized for 4 h at 100 °C and AgNPs-based inks dried at 100 °C for 20 min [47]. Our values are consistent with previous works [70,71,72,73,74,75,76,77,78], as shown in the comparison tables in the Supplementary Material.
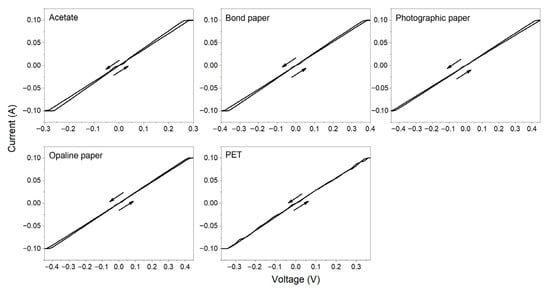
Figure 7.
I-V curves of the SN3 conductive AgNPs ink deposited on different substrates at RT.
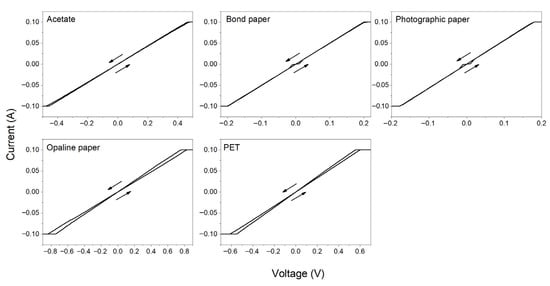
Figure 8.
I-V curve of conductive AgNPs ink deposited on different substrates at 60 °C.
Satisfactory preparation conditions resulting in ohmic contact for silver nanoparticles of conductive sample SN3 were tested on other substrates such as filter paper. To this end, these AgNPs were deposited onto filter paper using a conventional fountain pen [79], and a basic circuit was realized by handwriting. Perpendicular lines were drawn and (Figure 9a) dried at RT in ambient air. The electrical resistance of these wires was measured to be 10.18 Ω. Two 5 cm long traces were connected to an LED (Figure 9b), which was then successfully switched on and off, as shown in the image in Figure 9c, where the blue circle indicates the emitted white light. Moreover, to test the performance of printed tracks in photographic paper, a basic circuit was fabricated by adding a resistor and using the same previous conditions, as shown Figure 10. The circuit successfully turns on the LED device after being folded and under bending.

Figure 9.
(a) Conductive ink based on AgNPs hand-written on filter paper, (b) prototype of a conductive track and its functionality on filter paper with LED off and (c) LED on.
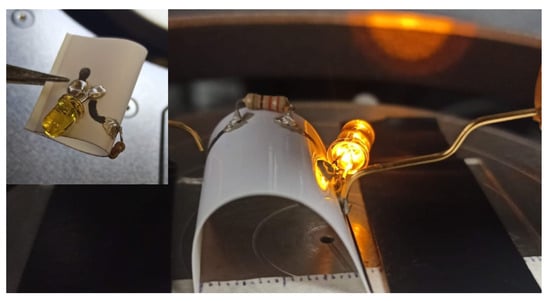
Figure 10.
Basic circuit performance under bending using conductive AgNPs ink in photographic paper.
The versatility of the conductive nanoparticles obtained by the chemical reduction method allows them to be used in the preparation of solvent-based eco-friendly inks while retaining their conductive properties on flexible substrates under several bending cycles [80].
4. Conclusions
In this work, a simple chemical recipe was developed to synthesize silver nanoparticles for functionalizing printing inks. Based on a parametric study in which the concentrations of three chemical reagents were varied, the solution prepared from 0.15 M AgNO3, 0.05 M NaBH4 and 0.03 M C6H5Na3O7 was found to produce AgNPs with optimal properties for printed electronics applications. This systematic analysis was conducted for different reagent concentrations to determine the size, uniformity and stability of the AgNPs produced, while ensuring minimal residual chemical elements.
The resulting AgNPs were then tested on various substrates to demonstrate their ability to ensure effective electrical connections. These experiments were successfully conducted on corning glass, flexible filters and photographic paper substrates, using AgNPs-based ink pen-drawn electrical wires connecting a switchable LED. This simple, inexpensive chemical method, which requires relatively short drying times, is proving relevant for applications at larger scale in printed electronics and inkjet printing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nanomanufacturing5040019/s1, Table S1: Summary of parameters for commercial conductive inks; Table S2: Summary of parameters for published conductive inks; Figure S1: Particle size distributions of all samples by DLS.
Author Contributions
Conceptualization, S.C. and M.A.D.; methodology, S.C., D.B. and M.A.D.; writing—original draft preparation, S.C.; writing—review and editing, S.C., D.B. and M.A.D.; visualization, S.C. and M.A.D.; supervision, D.B. and M.A.D.; project administration, D.B. and M.A.D.; funding acquisition, D.B. and M.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Groupe de Travail Québec-Mexique 2023–2025 du Ministère des Relations Internationales et de la Francophonie (MRIF), the DGQM-AMEXCID (Mexico), and the Fondo Sectorial de Investigación para la Educación (grant number A1-S-7888).
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Instituto de Física de la Universidad Autónoma de Puebla and the INRS for the characterizations performed using their research facilities. All the authors are also grateful to the UNESCO MATECSS chair for initiating this international collaborative work between INRS and BUAP in 2018. The authors would like to thank the technical staff of INRS: C. Chabanier, C. Harnagea and P. Soucis for their help in SEM/EDS and AFM characterizations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Williams, D.F.; Hemmati, S. An Overview of Silver Nanowire Polyol Synthesis Using Millifluidic Flow Reactors for Continuous Transparent Conductive Film Manufacturing by Direct Ink Writing. Nanomanufacturing 2025, 5, 7. [Google Scholar] [CrossRef]
- Ibrahim, N.; Akindoyo, J.O.; Mariatti, M. Recent development in silver-based ink for flexible electronics. J. Sci. Adv. Mater. Devices 2022, 7, 100395. [Google Scholar] [CrossRef]
- Huang, Q.; Shen, W.; Xu, Q.; Tan, R.; Song, W. Properties of polyacrylic acid-coated silver nanoparticle ink for inkjet printing conductive tracks on paper with high conductivity. Mater. Chem. Phys. 2014, 147, 550–556. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Aroche, A.F.; Schuck, A.; Lamberty, P.; Peter, C.R.; Hasenkamps, W.; Rocha, T.L.A.C. Silver nanoparticle conductive inks: Synthesis, characterization, and fabrication of inkjet-printed flexible electrodes. Sci. Rep. 2020, 10, 8878. [Google Scholar] [CrossRef]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, 6687290. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X. Synthesis and electrical properties of uniform silver nanoparticles for electronic applications. J. Mater. Sci. 2009, 44, 1076–1081. [Google Scholar] [CrossRef]
- Wang, R.; Yang, W.; Song, Y.; Shen, X.; Zhong, X.; Song, Y. A General Strategy for Nanohybrids Synthesis via Coupled Competitive Reactions Controlled in a Hybrid Process. Sci. Rep. 2015, 5, 9189. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, Q.; Dai, W.; Chen, J.L.; Mao, G.; Peng, Y.-K. Conductive Coatings on PDMS, PMMA, and Glass: Comparative Study of Graphene, Graphene Oxide, and Silver Nanoparticle Composites. Electrochem 2024, 5, 380–392. [Google Scholar] [CrossRef]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Ahmad Yasin, H.K.; Pratap, A. Silver Nanoparticles (AgNPs): Comprehensive Insights into Bio/Synthesis, Key Influencing Factors, Multifaceted Applications, and Toxicity—A 2024 Update. CS Omega 2025, 10, 7549–7582. [Google Scholar] [CrossRef] [PubMed]
- Velgosova, O.; Mačák, L.; Čižmárová, E.; Mára, V. Influence of Reagents on the Synthesis Process and Shape of Silver Nanoparticles. Materials 2022, 15, 6829. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Marinescu, L.; Ficai, D.; Ficai, A.; Oprea, O.; Nicoara, A.I.; Vasile, B.S.; Boanta, L.; Marin, A.; Andronescu, E.; Holban, A.-M. Comparative Antimicrobial Activity of Silver Nanoparticles Obtained by Wet Chemical Reduction and Solvothermal Methods. Int. J. Mol. Sci. 2022, 23, 5982. [Google Scholar] [CrossRef] [PubMed]
- Budlayan, M.L.; Lagare-Oracion, J.P.; Dela Rosa, L.; Rodriguez, M.J.; Capangpangan, R.Y.; Manigo, J.; Alguno, A.; Austria, E.; Arco, S.; Patricio, J. A Facile Route in Crontolling the Optical Absorbance of Polyvinylpyrrolidone-Capped Silver Nanoparticles Via Chemical Reduction Technique. IOP Conf. Ser. Mater. Sci. Eng. 2020, 925, 012050. [Google Scholar] [CrossRef]
- Velgosova, O.; Mačák, L.; Lisnichuk, M.; Vojtko, M. Synthesis and Analysis of Polymorphic Silver Nanoparticles and Their Incorporation into the Polymer Matrix. Polymers 2022, 14, 2666. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Das, S.S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, M.S.; Nayak, A.K. Bactericidal activity of silver nanoparticles: A mechanical review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Kumar, M.; Reddy, G.B. Effect of atmospheric exposure on the growth of citrate-capped silver nanoparticles. Phys. E Low Dimens. Syst. Nanostruc. 2010, 42, 1940–1943. [Google Scholar] [CrossRef]
- Affonso de Oliveira, J.F.; Borba Cardoso, M. Partial aggregation of silver nanoparticles induced by capping and reducing agents competition. Langmuir 2013, 30, 4879–4886. [Google Scholar] [CrossRef]
- La Spina, R.; Mehn, D.; Fumagalli, F.; Holland, M.; Reniero, F.; Rossi, F.; Gilliland, D. Synthesis of Citrate-Stabilized Silver Nanoparticles Modified by Thermal and pH Preconditioned Tannic Acid. Nanomaterials 2020, 10, 2031. [Google Scholar] [CrossRef]
- Dong, X.; Ji, X.; Jing, J.; Li, M.; Li, J.; Yang, W. Synthesis of Triangular Silver Nanoprisms by Stepwise Reduction of Sodium Borohydride and Trisodium Citrate. J. Phys. Chem. C 2010, 114, 2070–2074. [Google Scholar] [CrossRef]
- Zielińska, A.; Skwarek, E.; Zaleska, A.; Gazda, M.; Hupka, J. Preparation of silver nanoparticles with controlled particle size. Procedia Chem. 2009, 1, 1560–1566. [Google Scholar] [CrossRef]
- Geng, S.; Yu, Z.; Zhang, R.; Fan, B.; Wang, Q.; Guang, J.; Wang, S.; Zhang, X.; Hou, C.; Wang, C.; et al. Transformation of silver nanospheres into triangular nanoplates through a photoinduced process. J. Saudi Chem. Soc. 2023, 27, 101610. [Google Scholar] [CrossRef]
- Vaseen, M.; Akhter, Z.; Li, W.; Yarali, E.; Anthopoulos, T.D.; Shamim, A. High-conductivity screen-printable silver nanowire ink for optically transparent flexible radio frequency electronics. Flex. Print. Electron. 2022, 7, 044001. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size-and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Monowar, T.; Rahman, S.; Bhore, S.J.; Raju, G.; Sathasivam, K.V. Silver Nanoparticles Synthesized by Using the Endophytic Bacterium Pantoea ananatis are Promising Antimicrobial Agents agains Multidrug Resistant Bacteria. Molecules 2018, 23, 322. [Google Scholar] [CrossRef] [PubMed]
- Kora, A.J.; Manjusha, R.; Arunachalam, J. Superior bactericidal activity of SDS capped silver nanoparticles: Synthesis and characterization. Mater. Sci. Eng. 2009, 29, 2104–2109. [Google Scholar] [CrossRef]
- ImageJ. Image Processing and Analysis in Java. Available online: https://imagej.net/ij/ (accessed on 30 April 2024).
- Wu, W.; Wang, Z.; Jiang, P.; Tang, Z. Effect of Electroplating Variables on Electrodeposition of Ni Rich Ni-Ir Alloys from Citrate Aqueous Solutions. J. Electrochem. Soc. 2017, 164, D985. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Gupta, V.; Trivedi, P. Chapter 15—In vitro and in vivo characterization of pharmaceutical topical nanocarriers containing anticancer drugs for skin cancer treatment. In Lipid Nanocarriers for Drug Targeting; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 563–627. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Chapter 10—Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Advances in Pharmaceutical Product Development and Research; Takade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 369–400. [Google Scholar] [CrossRef]
- Vokhidova, N.R.; Rashidova, S.S. The influence of synthesis conditions on the film morphology of chitosan-stabilized silver nanoparticles. Polym. Bull. 2022, 79, 3419–3436. [Google Scholar] [CrossRef]
- Shikha, S.; Dureja, S.; Sapra, R.; Babu, J.; Haridas, V.; Pattanayek, S.K. Interaction of borohydride stabilized silver nanoparticles with sulfer-containing organophosphates. RSC Adv. 2021, 11, 32286. [Google Scholar] [CrossRef]
- Gakiya-Teruya, M.; Palomino-Marcelo, L.; Rodriguez-Reyes, J.C.F. Synthesis of Highly Concentrated Suspensions of Silver Nanoparticles by Two Versions of the Chemical Reduction Method. Methods Protoc. 2019, 2, 3. [Google Scholar] [CrossRef]
- Stewart, A.; Murray, S.; Bell, S.E.J. Simple preparation of positively charged silver nanoparticles for detection of anions by surface-enhanced Raman spectroscopy. Analyst 2015, 140, 2988–2994. [Google Scholar] [CrossRef] [PubMed]
- Ider, M.; Abderrafi, K.; Eddahbi, A.; Ouaskit, S.; Kassiba, A. Silver Metallic Nanoparticles with Surface Plasmon Resonance: Synthesis and Characterizations. J. Clust. Sci. 2017, 28, 1051–1069. [Google Scholar] [CrossRef]
- Abd El-Aziz, S.M.; Farahat, E.A. The Activity of Vossia cuspidata Polysaccharides-Derived Monometallic CuO, Ag, Au, and Trimetallic CuO-Ag-Au Na noparticles Against Cancer, Inflammation, and Wound Healing. J. Inorg. Organomet. Polym. Mater. 2023, 33, 853–865. [Google Scholar] [CrossRef]
- Chen, B.; Lei, Z.; Zhao, M.; Si, P. Facile macro fabrication of ultra-fine, ultra-long silver nanowire and growth mechanism. Synth. Met. 2023, 292, 117244. [Google Scholar] [CrossRef]
- Kutus, B.; Dudás, C.; Friesen, S.; Peintler, G.; Pálinkó, I.; Sipos, P.; Buchner, R. Equilibria and Dynamics of Sodium Citrate Aqueous Solutions: The Hydration of Citrate and Formation of the Na3Cit0 Ion Aggregate. J. Phys. Chem. B 2020, 124, 9604–9614. [Google Scholar] [CrossRef]
- Lo, C.-t.F.; Karan, K.; Davis, B.R. Kinetic Studies of Reaction between Sodium Borohydride and Methanol, Water, and Their Mixtures. Ind. Eng. Chem. Res. 2007, 46, 5478–5484. [Google Scholar] [CrossRef]
- Arrad, M.; Aliyeva, M.; Martins, M.A.R.; Thomsen, K.; Pinho, S.P. Thermodynamic description of aqueous solutions of silver nitrate: Experimental and modeling. Fluid Phase Equilib. 2025, 597, 114459. [Google Scholar] [CrossRef]
- Janz, G.J.; Lakshminarayanan, G.R.; Klotzkin, M.P.; Mayer, G.E. Diffusion of Silver Nitrate in Concentrated Aqueous Solutions. J. Phys. Chem. 1966, 70, 536–539. [Google Scholar] [CrossRef]
- Liu, J.-f.; Yu, S.-j.; Yin, Y.-g.; Chao, J.-b. Methods for separation, identification, characterization and quantification of silver nanoparticles. Trends. Analyt. Chem. 2012, 33, 95–106. [Google Scholar] [CrossRef]
- Desai, R.; Mankad, V.; Gupta, S.K.; Jha, P.K. Size Distribution of Silver Nanoparticles: UV-Visible Spectroscopic Assessment. Nanosci. Nanotechnol. Lett. 2012, 4, 30–35. [Google Scholar] [CrossRef]
- Patil, R.B.; Chougale, A.D. Analytical methods for the identification and characterization of silver nanoparticles: A brief review. Mater. Today Proc. 2021, 47, 5520–5532. [Google Scholar] [CrossRef]
- Semchuk, O.Y.; Biliuk, A.A.; Havryliuk, O.O.; Biliuk, A.I. Kinetic theory of electroconductivity of metal nanoparticles in the condition of surface plasmon resonance. Appl. Surf. Sci. Adv. 2021, 3, 100057. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-Triviño, D.; Saldarriaga, J.; Pérez, V.Z. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater. Res. 2019, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.E.F.; Pereira, A.C.; Asevedo Campos de Resende, M.; Franco Ferreira, L. Synthesis of a silver nanoparticles ink for fabrication of reference electrodes. Talanta Open 2022, 5, 100085. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Yang, A.; Qian, H.; Chen, H.; Anker, J.N. One-pot hydrothermal synthesis of silver nanowires via citrate reduction. J. Colloid Interface Sci. 2010, 352, 285–291. [Google Scholar] [CrossRef]
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Krzyzowska, M.; Celichowski, G.; Grobelny, J. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanopart. Res. 2017, 19, 273. [Google Scholar] [CrossRef]
- Piñero, S.; Camero, S.; Blanco, S. Silver nanoparticles: Influence of the temperature synthesis on the particles morphology. J. Phys. Conf. Ser. 2017, 786, 012020. [Google Scholar] [CrossRef]
- Song, K.C.; Lee, S.M.; Park, T.S.; Lee, B.S. Preparation of colloidal silver nanoparticles by chemical reduction method. Korean J. Chem. Eng. 2009, 26, 153–155. [Google Scholar] [CrossRef]
- Smitha, S.L.; Nissamudeen, K.M.; Philip, D.; Gopchandran, K.G. Studies on surface plasmon resonance and photoluminescence of silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 71, 186–190. [Google Scholar] [CrossRef]
- Krukowski, S.; Karasiewicz, M.; Kolodziejski, W. Convenient UV-spectrophotometric determination of citrates in aqueous solutions with applications in the pharmaceutical analysis of oral electrolyte formulations. J. Food Drug Anal. 2017, 25, 717–722. [Google Scholar] [CrossRef]
- Indana, M.K.; Gangapuram, B.R.; Dadigala, R.; Bandi, R.; Guttena, V. A novel green synthesis and characterization of silver nanoparticles using gum tragacanth and evaluation of their potential catalytic reduction activities with methylene blue and Congo red. J. Anal. Sci. Technol. 2016, 7, 1. [Google Scholar] [CrossRef]
- Dutta, A.; Paul, A.; Chattopadhyay, A. The effect of temperature on the aggregation kinetics of partially bare gold nanoparticles. RSC Adv. 2016, 6, 82138–82149. [Google Scholar] [CrossRef]
- Afshinnia, K.; Baalousha, M. Effect of phosphate buffer on aggregation kinetics of citrate-coated silver nanoparticles induced by monovalent and divalent electrolytes. Sci. Total Environ. 2017, 581–582, 268–276. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, X.; Huang, Q.; Xu, Q.; Song, W. Preparation of solid silver nanoparticles for inkjet printed flexible electronics with high conductivity. Nanoscale 2014, 6, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Grabill, C.N.; Freppon, D.; Hettinger, M.; Kuebler, S.M. Nanoscale morphology of electrolessly deposited silver metal. Appl. Surf. Sci. 2019, 466, 230–243. [Google Scholar] [CrossRef]
- Ceran, Ö.B.; Şimşek, B.; Doruk, S.; Uygunoğlu, T.; Şara, O.N. Effects of dispersed and powdered silver nanoparticles on the mechanical, thermal, electrical and durability properties of cementitious composites. Constr. Build Mater. 2019, 222, 152–167. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.L.T.; Hu, Y.; Zhu, P.; Sun, R. Facile synthesis of monodisperse silver nanoparticles for screen printing conductive inks. J. Mater. Sci. Mater. Electron. 2017, 28, 16939–16947. [Google Scholar] [CrossRef]
- Mustafa, F.; Razwan, M.; Shabbir, S. Microstructure and Resistivity Analysis of Silver Nanoparticle-Based Crystalline Conductive Films Synthesized using PEG Surfactant. Processes 2019, 7, 245. [Google Scholar] [CrossRef]
- Bhadra, J.; Al-Thani, N.J.; Karmakar, S.; Madi, N.K. Photo-reduced route of polyaniline nanofiber synthesis with embedded silver nanoparticles. Arab. J. Chem. 2019, 12, 4848–4860. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Ren, X.-L.; Zheng, M.-L.; Jin, F.; Liu, J.; Dong, X.-Z.; Zhao, Z.-S.; Duan, X.-M. Plasmon-enhanced nanosoldering of silver nanoparticles for high-conductive nanowires electrodes. Opto Electron. Adv. 2021, 4, 200101. [Google Scholar] [CrossRef]
- Chettri, P.; Tripathi, A.; Tiwari, A. Effect of silver nanoparticles on electrical and magnetic properties of reduced graphene oxide. Mater. Res. Bull. 2022, 150, 111752. [Google Scholar] [CrossRef]
- Männl, U.; van den Berg, C.; Magunje, B.; Härting, M.; Britton, D.T.; Jones, S.; van Staden, M.J.; Scriba, M.R. Nanoparticle composites for printed electronics. Nanotechnology 2014, 25, 094004. [Google Scholar] [CrossRef]
- Dominguez, M.; Sosa, J. Copper phthalocyanine buffer interlayer film incorporated in paper substrates for printed circuit boards and dielectric applications in flexible electronics. Solid State Electron. 2020, 172, 107898. [Google Scholar] [CrossRef]
- Pedrosa, J.F.S.; Alves, L.; Neto, C.P.; Rasteiro, M.G.; Ferreira, P.J.T. Assessment of the Performance of Cationic Cellulose Derivatives as Calcium Carbonate Flocculant for Papermaking. Polymers 2022, 14, 3309. [Google Scholar] [CrossRef]
- Jasmee, S.; Omar, G.; Masripan, N.A.B.; Kamarolzaman, A.A.; Ashikin, A.S.; Che Ani, F. Hydrophobicity performance of polyethylene terephthalate (PET) and thermoplastic polyurethane (TPU) with thermal effect. Mater. Res. Express 2018, 5, 096304. [Google Scholar] [CrossRef]
- Silver Nanoparticle Ink. Available online: https://www.sigmaaldrich.com/CA/en/product/aldrich/798738?srsltid=AfmBOorU4Qo-Lq6LYVYUdyRBCASjh46s6iT7Jj-nLsIsV03ecwJaft_mYiI (accessed on 16 September 2025).
- Conductive Ink Silver-Based. Available online: https://caig.com/product/circuitwritertm-pen-cw100p/ (accessed on 16 September 2025).
- Conductive Silver Printing Ink. Available online: https://www.sigmaaldrich.com/CA/en/product/aldrich/791881?srsltid=AfmBOooBnLce0N3RCWVodZQ0rdRv-DEKRzAB6ECxZkZokcgSZkGzcM381t8 (accessed on 16 September 2025).
- Silver Nanoparticles Ink for Inkjet Printing. Available online: https://www.sigmaaldrich.com/CA/en/product/aldrich/901083?srsltid=AfmBOoqEq5L0qXHyS3piA9rWbZg4E6dXMqJy3k6cfspZPSBTUZeb-KwsL8 (accessed on 16 September 2025).
- Silver Nanoparticles Ink. Available online: https://www.sigmaaldrich.com/CA/en/product/aldrich/796042?srsltid=AfmBOoohu5ieGSeMrTuBnczdD7twtahYUc5J_YE2cTzh5McU-7PcXUYMGQ (accessed on 16 September 2025).
- Gao, H.; Liao, Y.Q.; Wang, Y.Y.; Zhu, J.; Liu, P.; Ding, R.; Guo, D.M.; Wang, F.; Song, F.; Wang, Y. Conductive Superhydrophobic Smart Coatings Based on Speherical Silver Nanoparticles and Waterbone Polyurethane for Flexible and Wearable. ACS Appl. Mater. Interfaces 2024, 16, 65553–65564. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Ahn, B.Y.; Adams, J.J.; Duoss, E.B.; Bernhard, J.T.; Lewis, J.A. Pen-on-Paper Flexible Electronics. Adv. Mater. 2011, 23, 3426–3430. [Google Scholar] [CrossRef] [PubMed]
- Naderi-Samani, E.; Razavi, R.S.; Nekouee, K.; Naderi-Samani, H. Synthesis of silver nanoparticles for use in conductive inks by Chemical reduction method. Heliyon 2023, 9, e20548. [Google Scholar] [CrossRef]
- Iram, N.; Khan, S.N.; Ahmed, M.; Mir, A.; Anwar, N.; Naeem, M.; Nguyen, V.H.; Pham, P.V. Synthesis and characterizations of silver nanoparticles-based conductive ink for high-frequency electronics. Phys. Scr. 2024, 99, 0589a8. [Google Scholar] [CrossRef]
- Jung, H.J.; Jong-Man, K.; Jaworski, J. Progression in the Fountain Pen Approach: From 2D Writing to 3D Free-Form Micro/Nanofabrication. Small 2017, 13, 1600137. [Google Scholar] [CrossRef]
- Ceron, S.; Barba, D.; Dominguez, M.A. Solution-Processable and Eco-Friendly Functionalization of Conductive Silver Nanoparticles Inks for Printable Electronics. Electron. Mater. 2024, 5, 45–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).