Self-Assembly of Block Copolymers to Prepare Advanced Materials with Hierarchical Functional Nanostructures
Abstract
1. Introduction
2. Topologies and Synthetic Methods of Block Copolymers
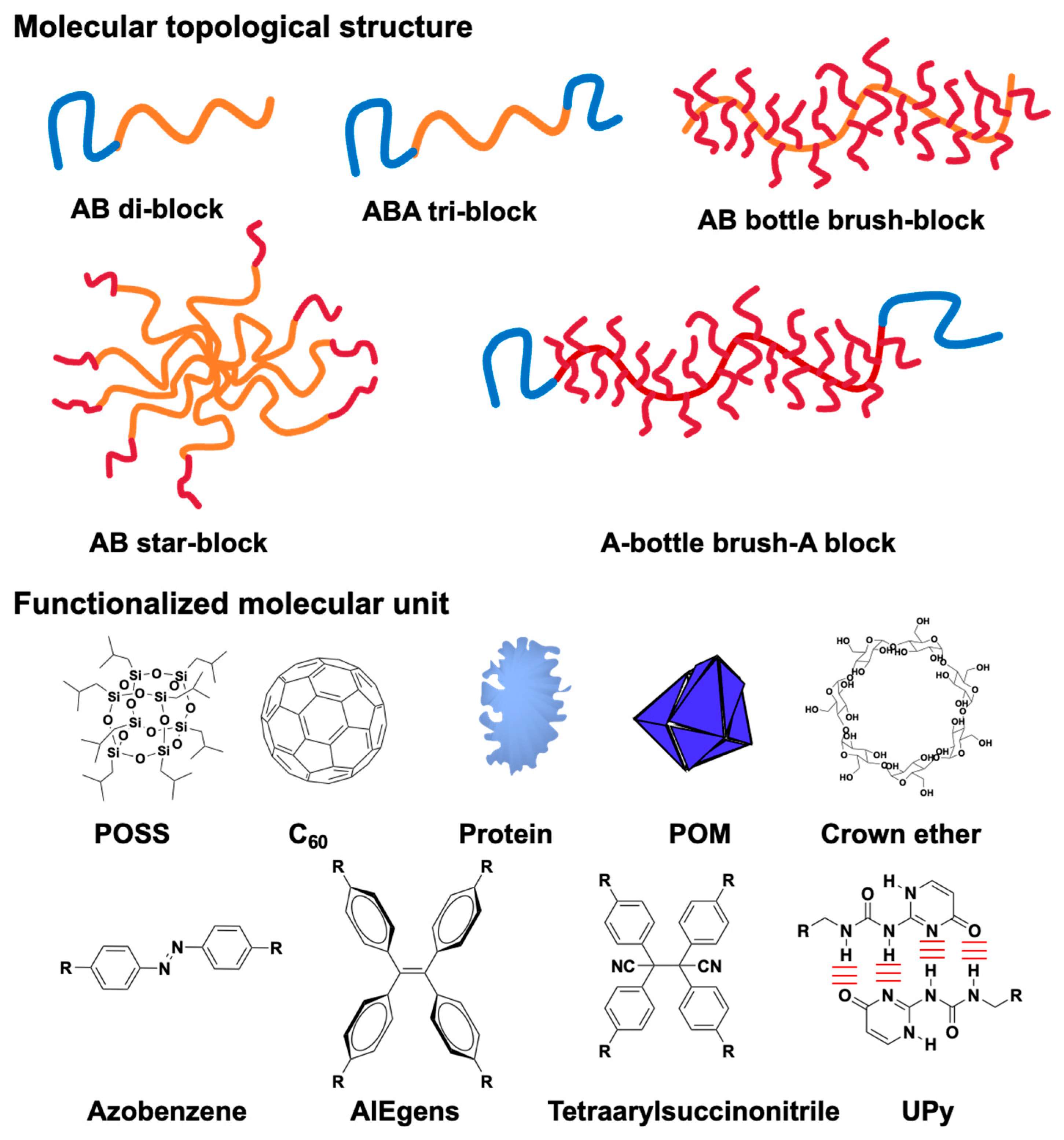
2.1. Topologies and Compositions of Block Copolymers
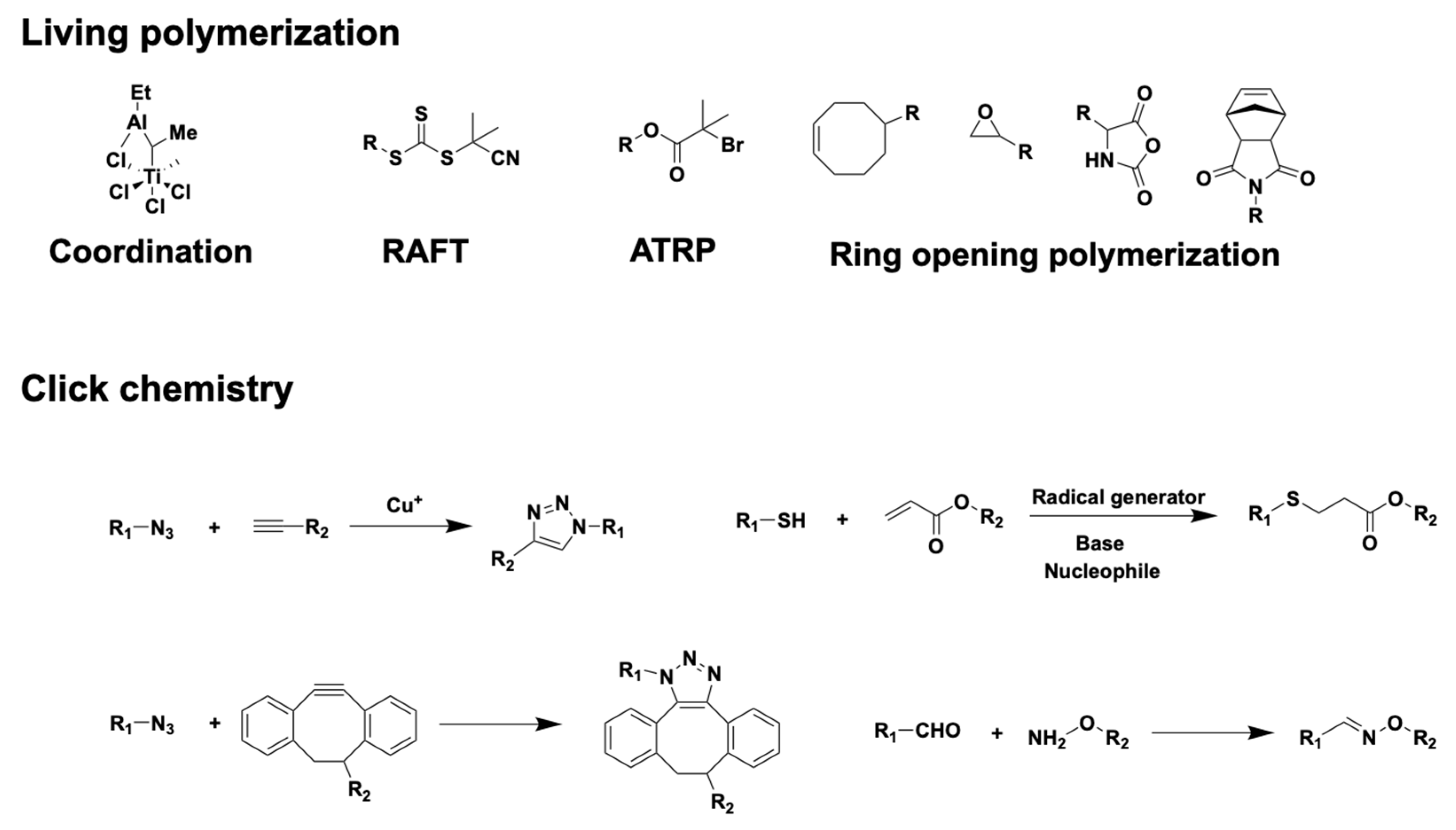
2.2. Common Chemical Synthesis Methods for Block Copolymers
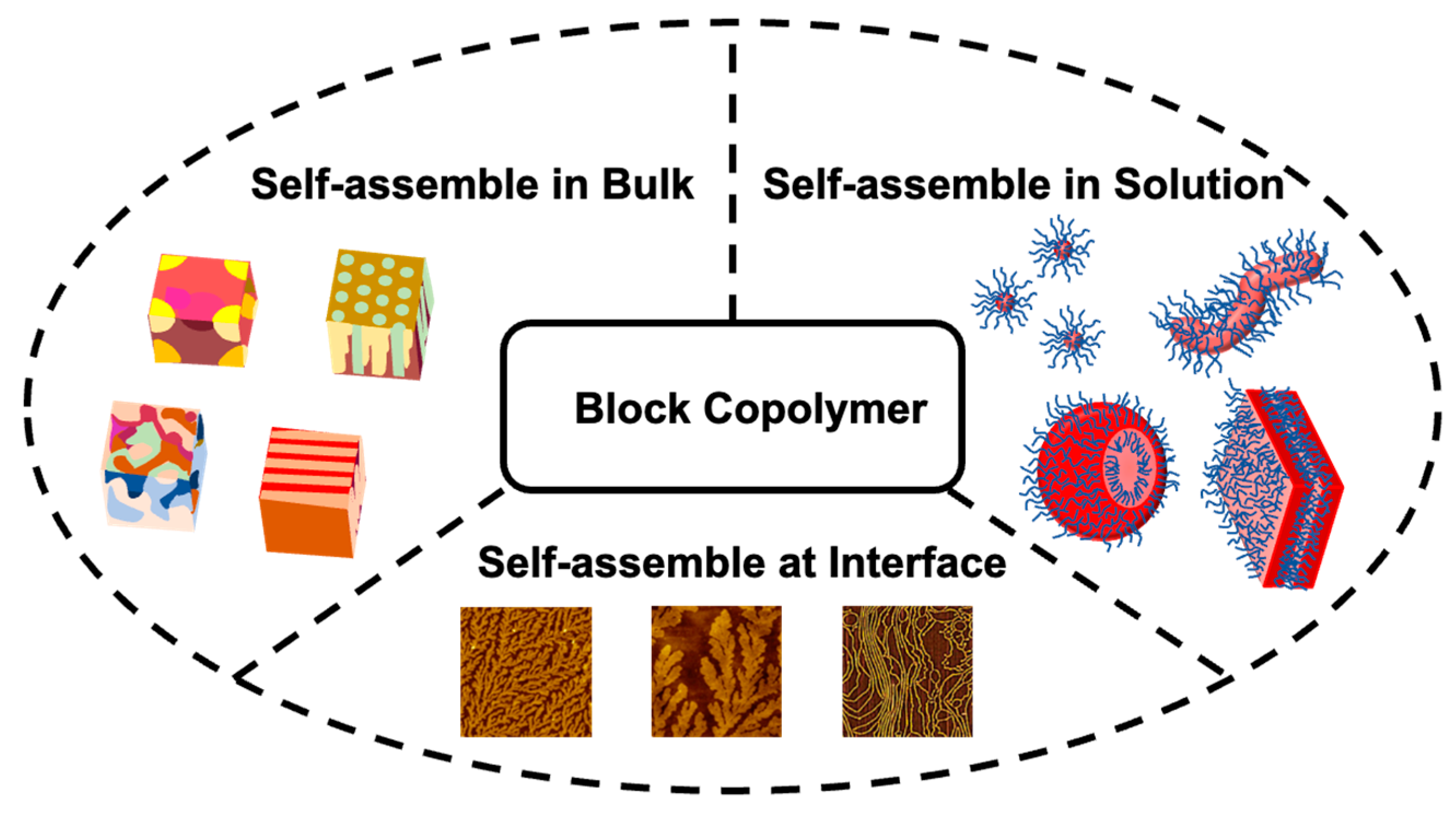
3. Block Copolymer Self-Assembly in Melt Bulk
3.1. Theory of Self-Assembly of Block Polymer Molecules
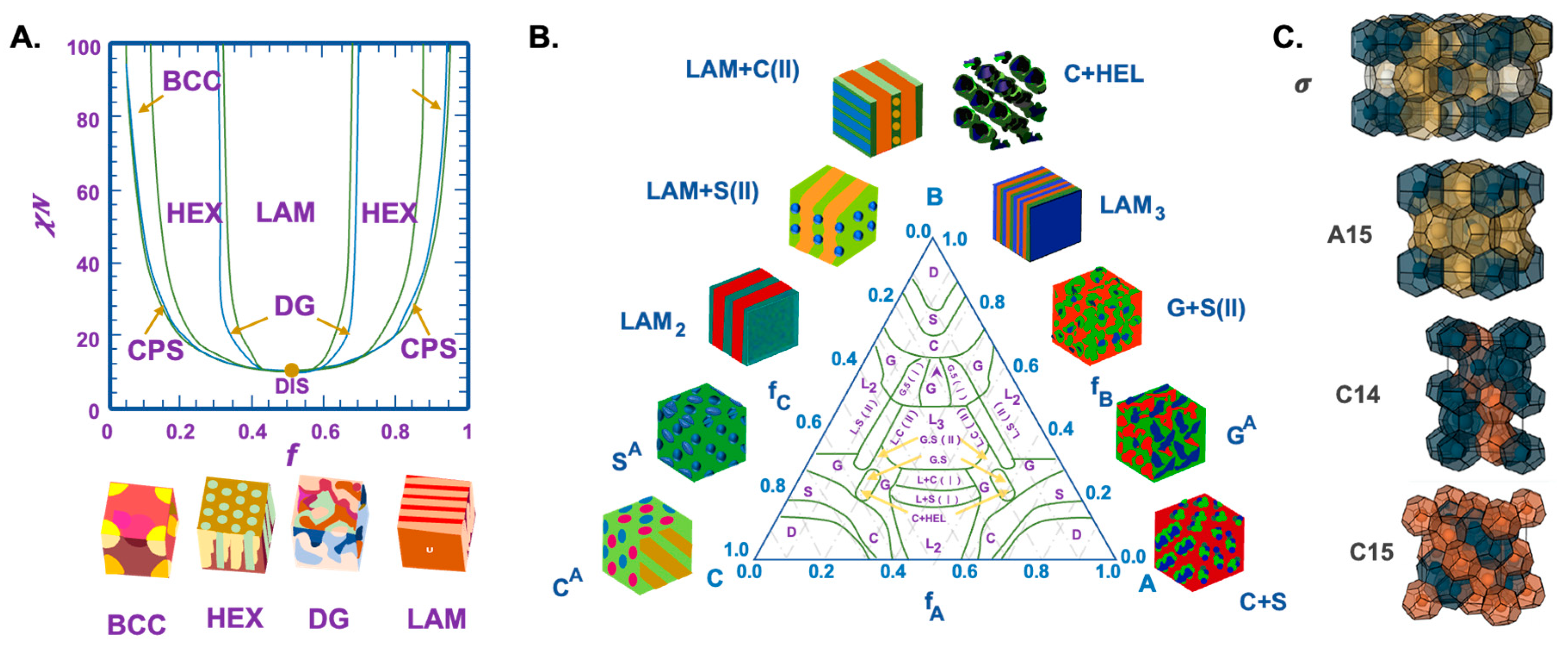
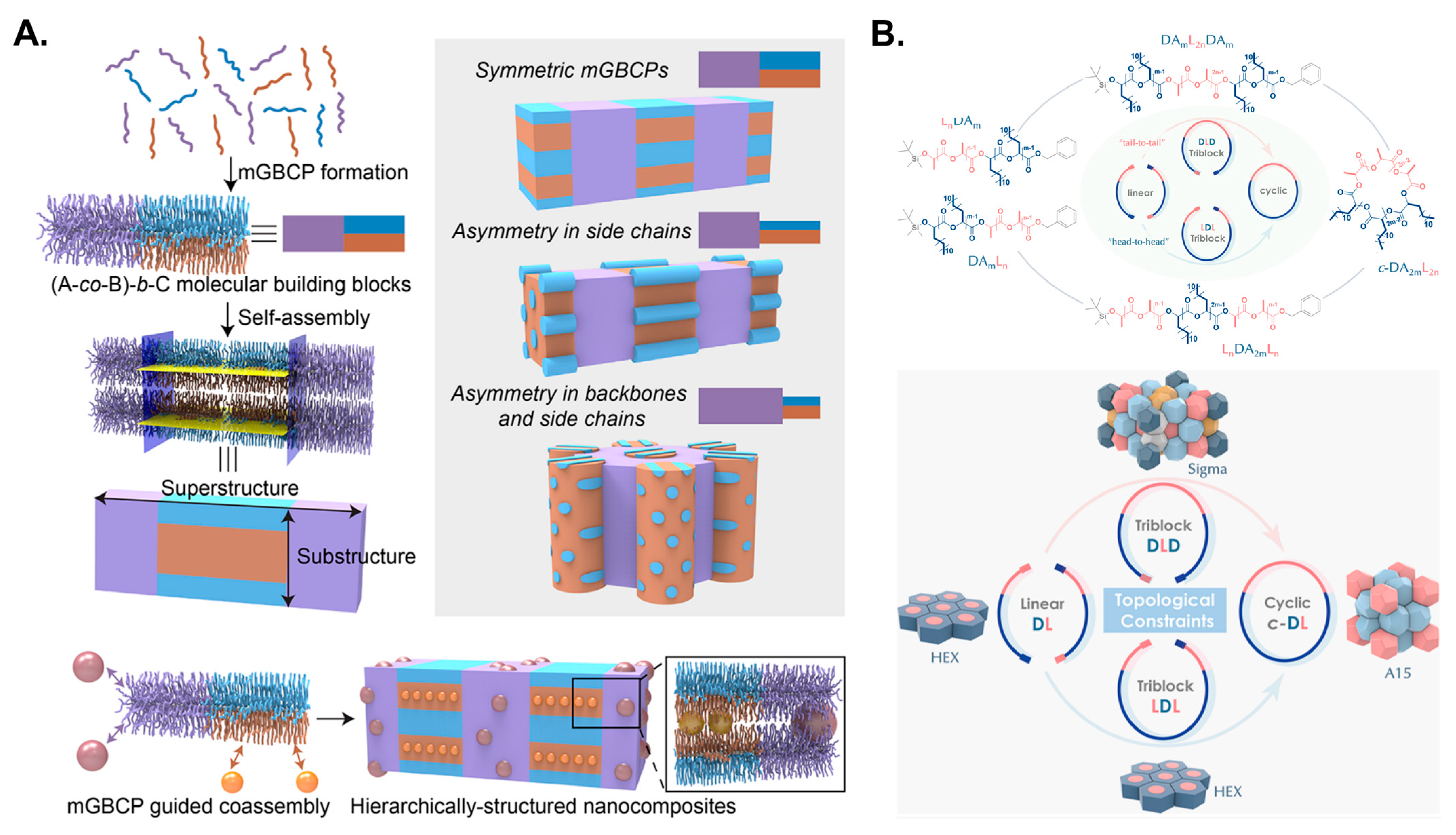
3.2. Examples Show the Assembly Structures of Block Copolymers with Different Topologies
3.3. Examples Show the Applications of Materials with Different Microphase Structure
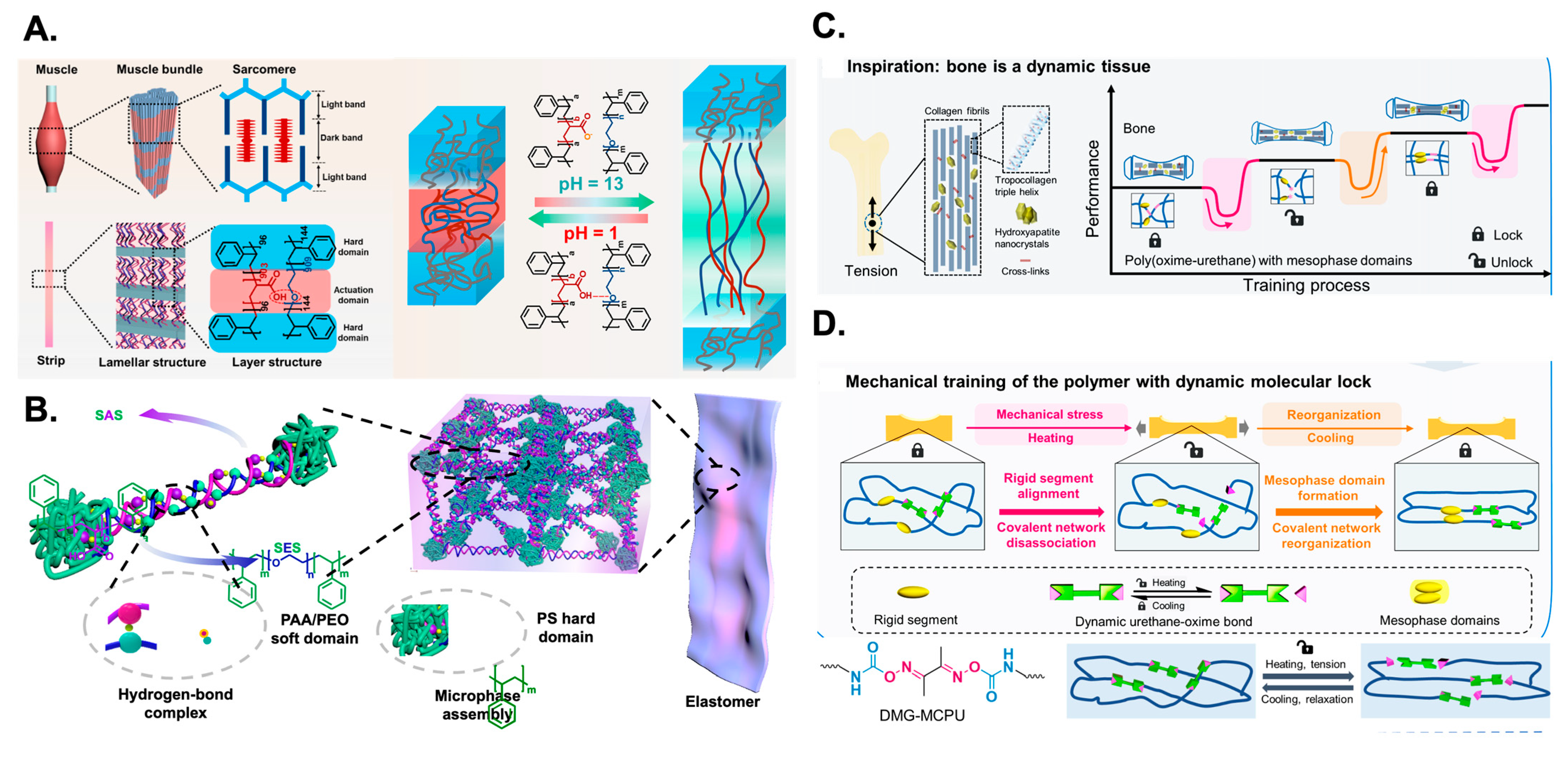
4. Block Copolymer Self-Assembly in Solution
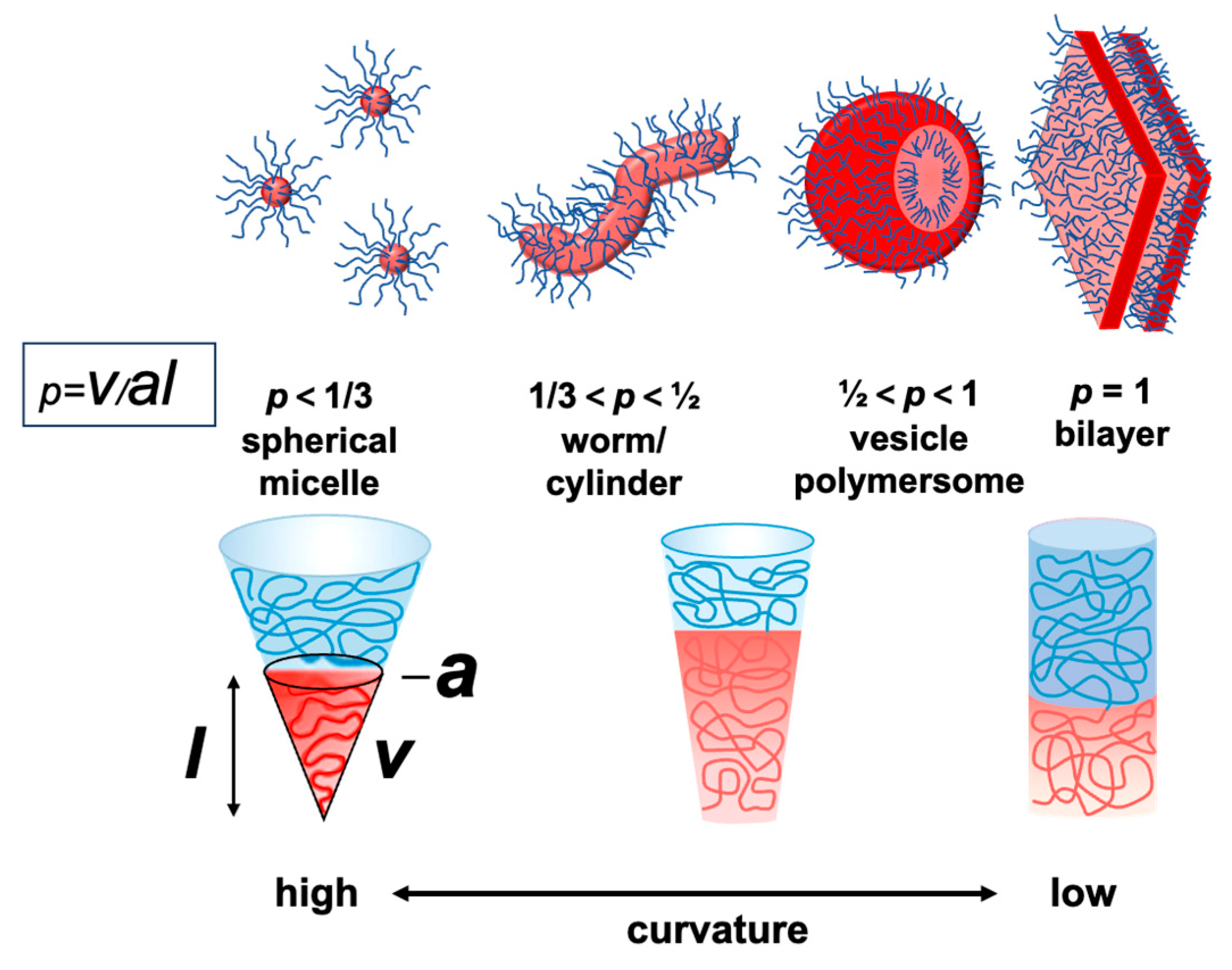
4.1. Theory of Block Copolymer Solution Assembly
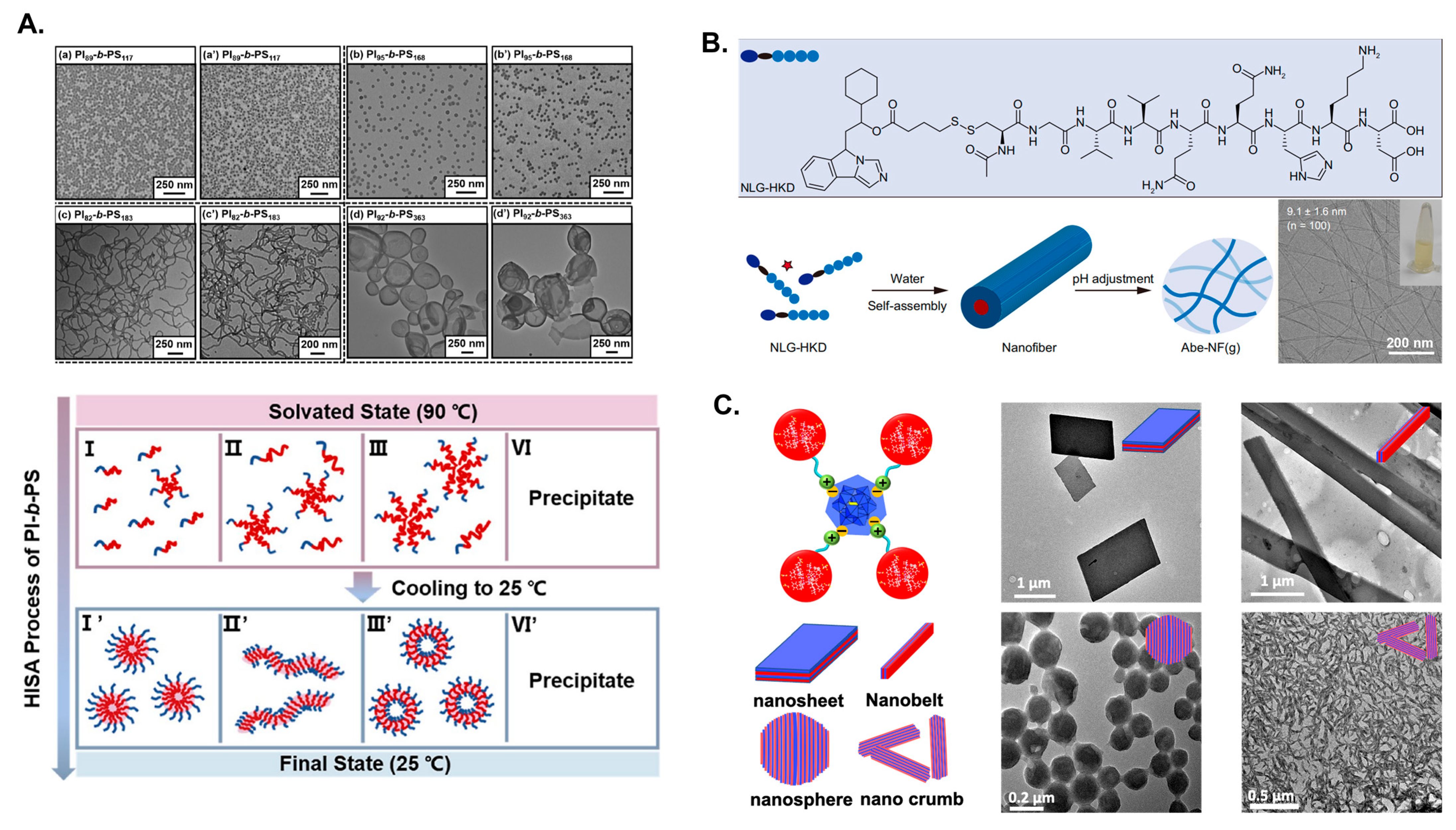
4.2. Examples Demonstrate Different Morphological Assembly of Block Copolymers in Solution
4.3. Examples Show the Application of Block Copolymers in Solution Assembly
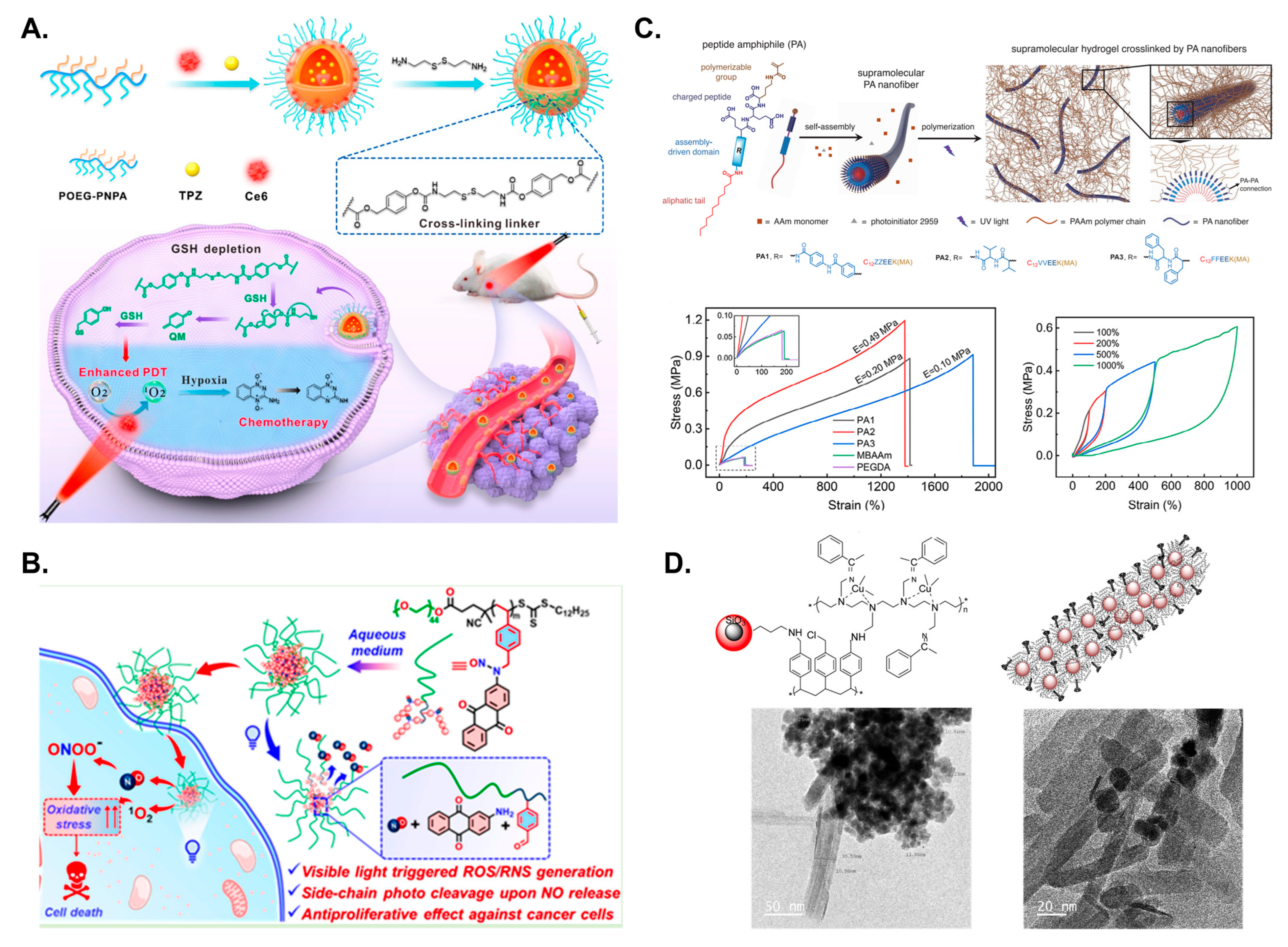
5. Block Copolymer Self-Assembly at Interface
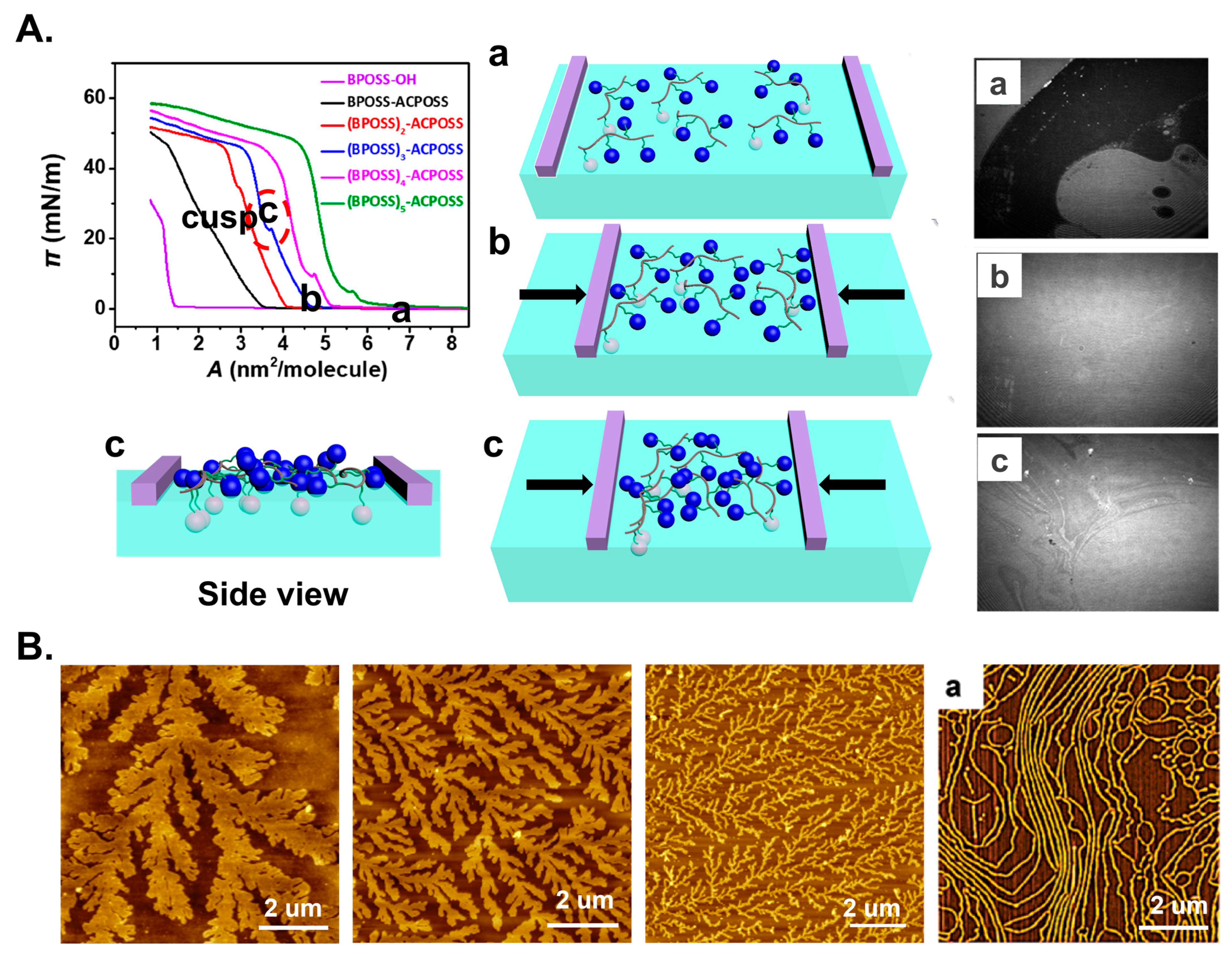
6. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, L.; Sha, Z.; Zheng, Y.; Zhu, R.; Yu, C.; Chen, Q.; Ran, R.; Cui, W. Bioinspired hydrogels thriving in harsh conditions: Where soft materials conquer hard challenges. Prog. Mater. Sci. 2025, 152, 101459. [Google Scholar] [CrossRef]
- Gu, X.; Yu, Y.; Zhong, S.; Zheng, M.; Zhang, M.; Wang, J.; Xu, Z.; Wan, Q.; Kundu, S.C.; Yang, M.; et al. Bismuth-nanosheet-armed pristine silk nanofiber dressing for multimodal pathogenic bacteria eradication and infected wound healing. Adv. Fiber Mater. 2025, 7, 1529. [Google Scholar] [CrossRef]
- Davies, K.E.; Nowak, K.J. Molecular mechanisms of muscular dystrophies: Old and new players. Nat. Rev. Mol. Cell Biol. 2006, 7, 762–773. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, L.; Zhao, J.; Zhang, H.; Zhao, X.; Liu, Y.; Bai, R.; Pan, H.; Yu, W.; Yan, X. Muscle-mimetic synergistic covalent and supramolecular polymers: Phototriggered formation leads to mechanical performance boost. J. Am. Chem. Soc. 2021, 143, 902–911. [Google Scholar] [CrossRef]
- Oveissi, F.; Fletcher, D.F.; Dehghani, F.; Naficy, S. Tough hydrogels for soft artificial muscles. Mater. Des. 2021, 203, 109609. [Google Scholar] [CrossRef]
- Mirvakili, S.M.; Hunter, I.W. Artificial muscles: Mechanisms, applications, and challenges. Adv. Mater. 2018, 30, 1704407. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Kotak, P.; Pagnotta, L.; Lamuta, C. The evolution of mechanical actuation: From conventional actuators to artificial muscles. Int. Mater. Rev. 2021, 67, 575–619. [Google Scholar] [CrossRef]
- Bai, L.; Liu, L.; Esquivel, M.; Tardy, B.L.; Huan, S.; Niu, X.; Liu, S.; Yang, G.; Fan, Y.; Rojas, O.J. Nanochitin: Chemistry, structure, assembly, and applications. Chem. Rev. 2022, 122, 11604. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Huang, J.; Khan, A.Q.; An, B.; Zhou, X.; Liu, Z.; Zhu, M. Spider silk-inspired artificial fibers. Adv. Sci. 2022, 9, e2103965. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Chen, P.-Y.; Lin, A.Y.-M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef]
- He, Y.; Li, R.; Tong, H.; Wang, W.; Zhang, J.; Li, J.; Zhao, D.; Lan, K. Emerging compositions in functional mesoporous materials. Chem. Soc. Rev. 2025, 54, 6927–6972. [Google Scholar] [CrossRef]
- Zeng, H.; Liang, K.; Jiang, L.; Zhao, D.; Kong, B. Electrochemical sensing mechanisms and interfacial design strategies of mesoporous nanochannel membranes in biosensing applications. Acc. Chem. Res. 2025, 58, 732–745. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Huang, H.; Wu, H.; Zhang, Y.; Feng, X. Polyhedral polymeric microparticles with interwoven 1 nm gyroid pores for precise adsorption and nanoconfined degradation. ACS Nano 2025, 19, 8926–8938. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wu, Y.; Zhou, F. Tuning surface functions by hydrophilic/hydrophobic polymer brushes. ACS Nano 2025, 19, 11576. [Google Scholar] [CrossRef] [PubMed]
- Putranto, A.F.; Fleury, G.; Wulandari, C.; Muslihati, A.; Amrillah, Y.T.; Yuliarto, B.; Kogelschatz, M.; Nugroho, F.A.A.; Wasisto, H.S.; Zelsmann, M. Block copolymer self-assembly for biological and chemical sensing. ACS Appl. Polym. Mater. 2024, 6, 14970. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Progress in the self-assembly of organic/inorganic polyhedral oligomeric silsesquioxane (POSS) hybrids. Soft Matter 2022, 18, 5535–5561. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, R.; Yan, X.-Y.; Guo, Q.-Y.; Huang, J.; Shan, W.; Liu, Y.; Liu, T.; Huang, M.; Cheng, S.Z.D. The role of architectural engineering in macromolecular self-assemblies via non-covalent interactions: A molecular LEGO approach. Prog. Polym. Sci. 2020, 103, 101230. [Google Scholar] [CrossRef]
- Chowdhury, S.; Toth, I.; Stephenson, R.J. Dendrimers in vaccine delivery: Recent progress and advances. Biomaterials 2022, 280, 121303. [Google Scholar] [CrossRef]
- Adnan Raza, M.; Firdous, A.; Gupta, U.; Patil, U.; Singh, A.; Ajazuddin. Emerging trends in designing the dendrimer-based drug delivery for antineoplastic therapies. ACS Appl. Bio Mater. 2025, 8, 5462–5495. [Google Scholar] [CrossRef]
- Zhang, D.; Dashtimoghadam, E.; Fahimipour, F.; Hu, X.; Li, Q.; Bersenev, E.A.; Ivanov, D.A.; Vatankhah-Varnoosfaderani, M.; Sheiko, S.S. Tissue-adaptive materials with independently regulated modulus and transition temperature. Adv. Mater. 2020, 32, e2005314. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Cheng, S.Z.D. The deconstruction of supramolecular structures based on modular precise macromolecules. Macromol. Chem. Phys. 2018, 219, 1700390. [Google Scholar] [CrossRef]
- Dong, X.-H.; Hsu, C.-H.; Li, Y.; Liu, H.; Wang, J.; Huang, M.; Yue, K.; Sun, H.-J.; Wang, C.-L.; Yu, X.; et al. Supramolecular crystals and crystallization with nanosized motifs of giant molecules. Adv. Polym. Sci. 2016, 276, 183–213. [Google Scholar]
- Zhang, W.-B.; Yu, X.; Wang, C.-L.; Sun, H.-J.; Hsieh, I.F.; Li, Y.; Dong, X.-H.; Yue, K.; Van Horn, R.; Cheng, S.Z.D. Molecular nanoparticles are unique elements for macromolecular science: From “nanoatoms” to giant molecules. Macromolecules 2014, 47, 1221–1239. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Yang, Z.; Shao, Y.; Li, G.; Dong, X.-H. Influence of regio-configuration and molecular geometry on the assembly behavior of giant molecules. Macromolecules 2024, 57, 7499–7507. [Google Scholar] [CrossRef]
- Feng, F.; Guo, D.; Shao, Y.; Yan, X.; Yue, K.; Pan, Z.; Li, X.; Xiao, D.; Jin, L.; Zhang, W.B.; et al. Thickness control of 2D nanosheets assembled from precise side-chain giant molecules. Chem. Sci. 2021, 12, 5216–5223. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Z.; Xing, H.; Zhang, Y.; Liu, T.; Zhang, Y.; Ma, D. Multifunctional polyrotaxane hydrogel with dynamic molecular anchors and microenvironment-activatable cationization properties for healing drug-resistant bacterial-infected chronic skin wounds. ACS Appl. Mater. Interfaces 2025, 17, 49357–49373. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, X.; Li, Z.; Zhao, F.; Liu, G.; Zeng, Y. New family of multistimuli-responsive acrylamide-based homopolymers: Synthesis, responsive behavior, and application in controlled release. Macromolecules 2025, 58, 5208. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, Y.; Xu, L.; Wang, S.; Li, Z.; Liu, Y.; Kwok, R.T.K.; Sun, J.; Lam, J.W.Y.; He, G.; et al. Aggregation-induced emission luminogen based wearable visible-light penetrator for deep photodynamic therapy. ACS Nano 2024, 18, 29930. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Kwok, R.T.K.; Qiu, Z.; Zhao, Z.; Tang, B.Z. A Holistic perspective on living aggregate. J. Am. Chem. Soc. 2024, 146, 5030. [Google Scholar] [CrossRef]
- Gao, M.; Hao, X.; Li, Y.; Tang, Y.; Yang, W.; Zhang, K.; Lu, Y.; Zhou, X. Engineering supramolecular composite hydrogels via “pH-induced ureidopyimidinone (UPy) tautomerization” strategy. Adv. Funct. Mater. 2025, 35, 2505578. [Google Scholar] [CrossRef]
- Bai, C.; Kang, J.; Wang, Y.Q. Kirigami-inspired light-responsive conical spiral actuators with large contraction ratio using liquid crystal elastomer fiber. ACS Appl. Mater. Interfaces 2025, 17, 14488–14498. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wilborn, A.M.; Lemaire, B.; Trigka, F.; Stricker, F.; Weible, A.H.; Li, S.; Bennett, R.K.A.; Cheung, T.C.; Grinthal, A.; et al. Programming liquid crystal elastomers for multistep ambidirectional deformability. Science 2024, 386, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; He, W.; Yao, X. Mucus-Iinspired supramolecular adhesives: Exploring the synergy between dynamic networks and functional liquids. ACS Nano 2025, 19, 14540–14556. [Google Scholar] [CrossRef]
- Addonizio, C.J.; Braegelman, A.S.; Schmidt, C.R.; Ollier, R.C.; Antwi, A.A.; Su, B.; Farshad, M.; Whitmer, J.K.; Webber, M.J. Impact of guest orientation in host-guest supramolecular hydrogels. ACS Macro Lett. 2025, 14, 8–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, L.; Zhang, J.; Zhong, M. pH-Responsive hyperbranched polymer scaffolds for polymer and peptide conjugation through molecular recognition: Synthesis and self-Assembly. Biomacromolecules 2025, 26, 2960–2970. [Google Scholar] [CrossRef]
- Ando, S.; Ito, K. Recent progress and future perspective in slide-ring based polymeric materials. Macromolecules 2025, 58, 2157–2177. [Google Scholar] [CrossRef]
- Sathi Devi, L.; Gigliobianco, M.R.; Gabrielli, S.; Agas, D.; Sabbieti, M.G.; Morelli, M.B.; Amantini, C.; Casadidio, C.; Di Martino, P.; Censi, R. Localized cancer treatment using thiol-ene hydrogels for dual drug delivery. Biomacromolecules 2025, 26, 3234–3254. [Google Scholar] [CrossRef]
- Liao, J.; Wang, W.; Xu, X.; Jian, H.; Yang, S. Interfacial behavior of giant amphiphiles composed of azobenzene and polyhedral oligomeric silsesquioxane. Langmuir 2022, 38, 1611–1620. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, W.; Zhang, C.; Zhu, L.; Yang, S. Photo-responsive behaviors of hydrogen-bonded polymer complex fibers containing azobenzene functional groups. Adv. Fiber Mater. 2021, 3, 172–179. [Google Scholar] [CrossRef]
- Hu, Y.; Barbier, L.; Li, Z.; Ji, X.; Le Blay, H.; Hourdet, D.; Sanson, N.; Lam, J.W.Y.; Marcellan, A.; Tang, B.Z. Hydrophilicity-hydrophobicity transformation, thermoresponsive morphomechanics, and crack multifurcation revealed by AIEgens in mechanically strong hydrogels. Adv. Mater. 2021, 33, e2101500. [Google Scholar] [CrossRef]
- Horst, M.; Bhat, V.; Xia, Y. Polymer mechanochemistry: Where is it going? J. Am. Chem. Soc. 2025, 147, 30529–30544. [Google Scholar] [CrossRef] [PubMed]
- Rutten, M.G.T.A.; Raffaelli, C.; Grillaud, M.O.; Bellan, R.; Ellenbroek, W.G.; Dankers, P.Y.W. Controlling the physical properties in hybrid hydrogel networks via tunable supramolecular interactions. Macromolecules 2025, 58, 6077–6087. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, B.; Wu, X.; Fang, X.; Liang, X.; Peng, Z.; Wang, G. Expanding the scope of self-assembly: Heat-induced self-assembly of block copolymers with high solids. Macromolecules 2025, 58, 4985–5000. [Google Scholar] [CrossRef]
- Qiao, L.; Zhou, M.; Shi, G.; Cui, Z.; Zhang, X.; Fu, P.; Liu, M.; Qiao, X.; He, Y.; Pang, X. Ultrafast visible-light-induced ATRP in aqueous media with carbon quantum dots as the catalyst and its application for 3D printing. J. Am. Chem. Soc. 2022, 144, 9817–9826. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Zhou, Y.; Ge, C.; Li, X.; Hou, M.; Wei, Y.; Chen, Y.; Leong, K.W.; Yin, L. Inhaled immunoantimicrobials for the treatment of chronic obstructive pulmonary disease. Sci. Adv. 2024, 10, eabd7904. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Xia, W.; Li, J.; Yang, H.; Yang, S.; Chen, E.Q. Liquid crystal promoted self-assembly of statistical copolymers into diverse nanostructures with precise dimensions. J. Am. Chem. Soc. 2024, 146, 31221–31229. [Google Scholar] [CrossRef]
- Heble, A.Y.; Chen, C.L. Access to advanced functional materials through postmodification of biomimetic assemblies via click chemistry. Biomacromolecules 2024, 25, 1391–1407. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Bhat, G.A.; Darensbourg, D.J. Post-polymerization functionalization of aliphatic polycarbonates using click chemistry. Polym. Chem. 2024, 15, 1803–1820. [Google Scholar] [CrossRef]
- Ghosal, K.; Bhattacharyya, S.K.; Mishra, V.; Zuilhof, H. Click chemistry for biofunctional polymers: From observing to steering cell behavior. Chem. Rev. 2024, 124, 13216–13300. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.-H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Abetz, V.; Simon, P.F.W. Phase behaviour and morphologies of block copolymers. Adv. Polym. Sci. 2005, 189, 125–212. [Google Scholar]
- Lynd, N.A.; Meuler, A.J.; Hillmyer, M.A. Polydispersity and block copolymer self-assembly. Prog. Polym. Sci. 2008, 33, 875–893. [Google Scholar] [CrossRef]
- Matsen, M.W.; Thompson, R.B. Equilibrium behavior of symmetric ABA triblock copolymer melts. J. Chem. Phys. 1999, 111, 7139–7146. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Lu, S.; Feng, X.; Wang, G.; Li, W. SCFT-guided experimental fabrication of double-diamond structures in A1B/A2B block copolymer binary blends. Macromolecules 2025, 58, 9776–9785. [Google Scholar] [CrossRef]
- Yamanaka, R.; Sugawara-Narutaki, A.; Takahashi, R. Microphase separation and gelation through polymerization-induced self-assembly using star polyethylene glycols. ACS Macro Lett. 2024, 13, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nandan, B. Fascinating morphologies and hybrid nanostructures via block copolymer/nanoparticle self-assembly. Macromolecules 2025, 58, 2807–2828. [Google Scholar] [CrossRef]
- Santra, S.; Cui, S.; Bates, F.S.; Lodge, T.P. Phase behavior of frustrated ABC bottlebrush block terpolymers. Macromolecules 2025, 58, 2807–2828. [Google Scholar] [CrossRef]
- Park, S.J.; Bates, F.S.; Dorfman, K.D. Alternating gyroid in block polymer blends. ACS Macro Lett. 2022, 11, 643–650. [Google Scholar] [CrossRef]
- Willis, J.D.; Matsen, M.W. Bicontinuous microemulsion region in blends of A+B homopolymers with an AB diblock copolymer. Macromolecules 2025, 58, 6981–6990. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Chang, T.-W.; Nishimura, T.; Lai, Y.-C.; Su, C.-J.; Wang, J.; Isono, T.; Satoh, T.; Chen, H.-L. Accessing Frank–Kasper phases via blending of architecturally distinct and sustainable sugar-based block co-oligomers. Macromolecules 2025, 58, 8686–8697. [Google Scholar] [CrossRef]
- Chen, H.; Dong, Q.; Qiang, Y.; Peng, L.; Huang, X.; Li, W. Mechanisms of multiple reentrant transitions between Frank-Kasper and classical spherical phases in AB-type dendron-like copolymer. Macromolecules 2025, 58, 10192–10202. [Google Scholar] [CrossRef]
- Dorfman, K.D. Frank–Kasper phases in block polymers. Macromolecules 2021, 54, 10251–10270. [Google Scholar] [CrossRef]
- Ma, Z.; Tan, R.; Gan, Z.; Zhou, D.; Yang, Y.; Zhang, W.; Dong, X.-H. Modulation of the complex spherical packings through rationally doping a discrete homopolymer into a discrete block copolymer: A quantitative study. Macromolecules 2022, 55, 4331. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block copolymers—Designer soft materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Nishimura, T.; Cheng, C.-J.; Oishi, Y.; Li, F.; Borsali, R.; Yamamoto, T.; Tajima, K.; Chen, H.-L.; Satoh, T.; Isono, T. POSS–oligosaccharide hybrid materials as a versatile platform for constructing unique spherical phases. Macromolecules 2025, 58, 7094–7103. [Google Scholar] [CrossRef]
- Xue, Y.; Song, Q.; Liu, Y.; Smith, D.; Li, W.; Zhong, M. Hierarchically structured nanocomposites via mixed-graft block copolymer templating: Achieving controlled nanostructure and functionality. J. Am. Chem. Soc. 2024, 146, 567–577. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Gan, Z.; Ma, Z.; Shi, A.-C.; Dong, X.-H. Synergistic impact of intra- and interchain dispersity on block copolymer self-assembly. Macromolecules 2025, 58, 3860–3871. [Google Scholar] [CrossRef]
- Wang, W.J.; Jian, H.X.; Huang, H.; Feng, F.F.; Meng, Q.Y.; Li, Y.L.; Chen, G.; Yang, S.G. Structure, humidity adaptivity, and elasticity of hydrogen-bonded complexes formed by self-assembly of a triblock copolymer and a homopolymer. Polym. Chem. 2024, 15, 4710–4720. [Google Scholar] [CrossRef]
- Wang, W.-J.; Zhang, C.-H.; Huang, H.; Liu, Z.-X.; Jian, H.-X.; Yang, S.-G. Combining microphase separation and hydrogen-bonding complexation to construct elastomer. Acta Polym. Sin. 2023, 54, 487–495. [Google Scholar]
- Wang, W.; Xu, X.; Zhang, C.; Huang, H.; Zhu, L.; Yue, K.; Zhu, M.; Yang, S. Skeletal muscle fibers inspired polymeric actuator by assembly of triblock polymers. Adv. Sci. 2022, 9, e2105764. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Lv, Z.; Cao, L.; Ren, J.; Shao, Z.; Ling, S. Silk fibroin nacre. Adv. Fiber Mater. 2022, 4, 1191–1208. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, S.; Cao, L.; Lv, Z.; Ren, J.; Shao, Z.; Yao, Y.; Ling, S. Natural silk spinning-inspired meso-assembly-processing engineering strategy for fabricating soft tissue-mimicking biomaterials. Adv. Funct. Mater. 2022, 32, 2200267. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Huang, H.; Xue, B.; Yang, S. Ambient environment adaptive elastomer constructed by microphase separation and segment complexation of triblock copolymers. ACS Appl. Mater. Interfaces 2023, 15, 22426–22434. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, Q.; Guo, Y.; Sun, L.; Neisiany, R.E.; Guo, X.; Huang, H.; Yang, L.; You, Z. Bone-inspired stress-gaining elastomer enabled by dynamic molecular locking. Sci. Adv. 2024, 10, eadk5177. [Google Scholar] [CrossRef] [PubMed]
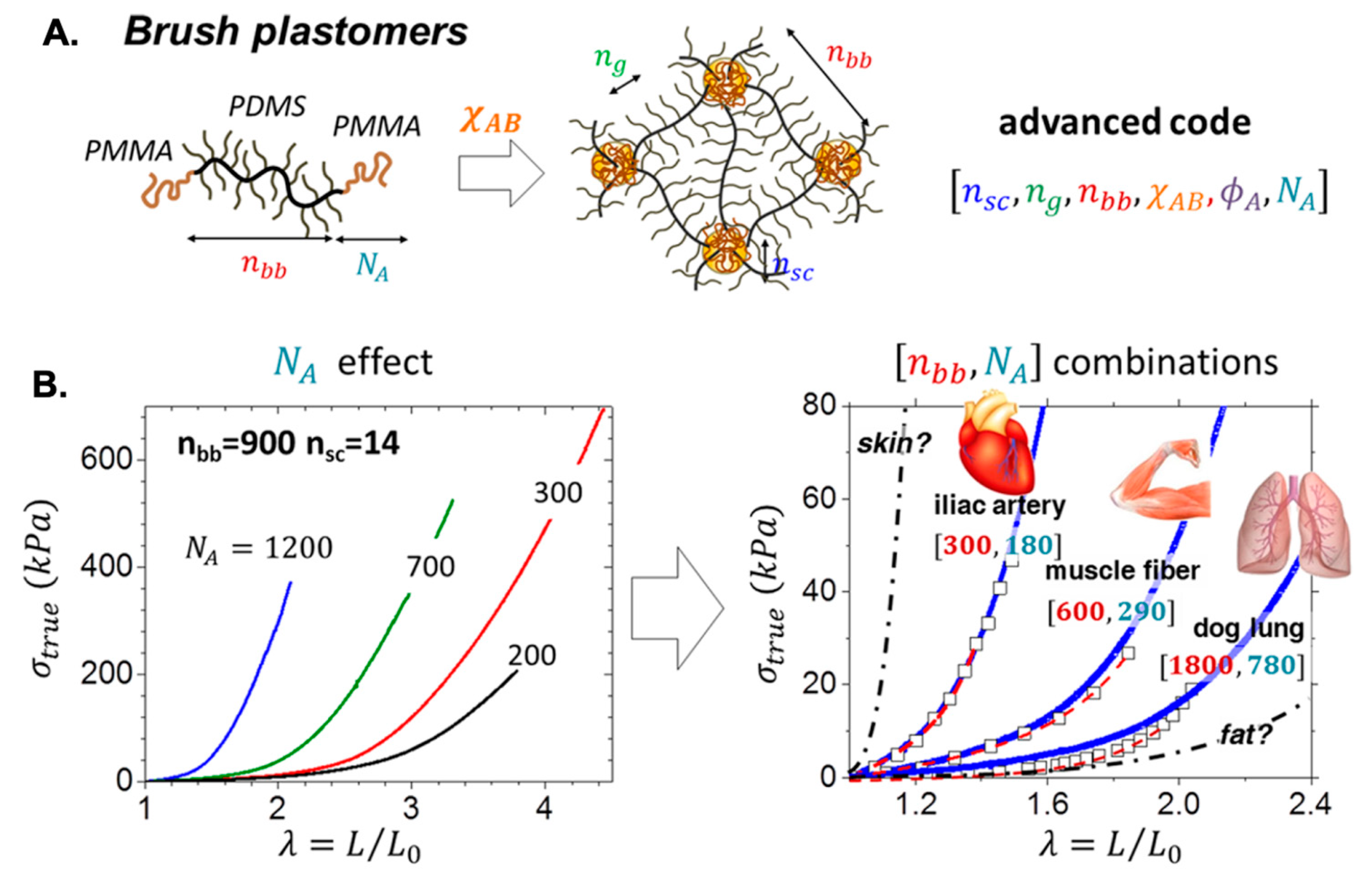
- Vatankhah-Varnosfaderani, M.; Keith, A.N.; Cong, Y.; Liang, H.; Rosenthal, M.; Sztucki, M.; Clair, C.; Magonov, S.; Ivanov, D.A.; Dobrynin, A.V.; et al. Chameleon-like elastomers with molecularly encoded strain-adaptive stiffening and coloration. Science 2018, 359, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Vatankhah-Varnosfaderani, M.; Karimkhani, V.; Keith, A.N.; Leibfarth, F.A.; Martinez, M.R.; Matyjaszewski, K.; Sheiko, S.S. Understanding the synthesis of linear–bottlebrush–linear block copolymers: Toward plastomers with well-defined mechanical properties. Macromolecules 2020, 53, 8324–8332. [Google Scholar] [CrossRef]
- Vatankhah-Varnosfaderani, M.; Daniel, W.F.M.; Everhart, M.H.; Pandya, A.A.; Liang, H.; Matyjaszewski, K.; Dobrynin, A.V.; Sheiko, S.S. Mimicking biological stress-strain behaviour with synthetic elastomers. Nature 2017, 549, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Keith, A.N.; Clair, C.; Lallam, A.; Bersenev, E.A.; Ivanov, D.A.; Tian, Y.; Dobrynin, A.V.; Sheiko, S.S. Independently tuning elastomer softness and firmness by incorporating side chain mixtures into bottlebrush network strands. Macromolecules 2020, 53, 9306–9312. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Dobrynin, A.V. Architectural code for rubber elasticity: From supersoft to superfirm materials. Macromolecules 2019, 52, 7531–7546. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Stroujkova, A.; Vatankhah-Varnosfaderani, M.; Sheiko, S.S. Coarse-grained artificial intelligence for design of brush networks. ACS Macro Lett. 2023, 12, 1510–1516. [Google Scholar] [CrossRef]
- Sinsinbar, G.; Bindra, A.K.; Liu, S.; Chia, T.W.; Yoong Eng, E.C.; Loo, S.Y.; Lam, J.H.; Schultheis, K.; Nallani, M. Amphiphilic block copolymer nanostructures as a tunable delivery platform: Perspective and framework for the future drug product development. Biomacromolecules 2024, 25, 541–563. [Google Scholar] [CrossRef]
- Kerai, S.D.; Takano, S.; Zeng, P.; Mullner, M. Self-Assembly of bottlebrush-linear, rod-coil copolymers into discoidal nanoparticles. ACS Macro Lett. 2025, 14, 834–840. [Google Scholar] [CrossRef]
- Green, K.A.; Kulkarni, A.S.; Jankoski, P.E.; Newton, T.B.; Derbigny, B.; Clemons, T.D.; Watkins, D.L.; Morgan, S.E. Biocompatible glycopolymer-PLA amphiphilic hybrid block copolymers with unique self-assembly, uptake, and degradation properties. Biomacromolecules 2024, 25, 6681–6692. [Google Scholar] [CrossRef]
- Duijs, H.; Kumar, M.; Dhiman, S.; Su, L. Harnessing competitive interactions to regulate supramolecular “micelle-droplet-fiber” transition and reversibility in water. J. Am. Chem. Soc. 2024, 146, 29759–29766. [Google Scholar] [CrossRef]
- Li, X.; Luo, Z.; Fielding, L.A. Pyrene-decorated thermally responsive luminescent nanoparticles: The effect of copolymer micelle morphology. Macromolecules 2025, 58, 5995–6004. [Google Scholar] [CrossRef]
- Nunes, S.P. Block copolymer membranes for aqueous solution applications. Macromolecules 2016, 49, 2905–2916. [Google Scholar] [CrossRef]
- Baek, J.; Song, N.; Yoo, B.; Lee, D.; Kim, B.S. Precisely programmable degradation and drug release profiles in triblock copolyether hydrogels with cleavable acetal pendants. J. Am. Chem. Soc. 2024, 146, 13836–13845. [Google Scholar] [CrossRef]
- Wang, X.; Shen, M.; Ma, M.; Zhang, H.; Shi, C.; Lu, H.; He, W.; Chen, Y. Rational construct of extracellular matrix mimics via peptide-co-assembling nanofibers for efficient bone regeneration. Adv. Fiber Mater. 2025, 7, 1093. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Zheng, F.; Wang, L.; Xu, W.; Li, Q.; Zhan, B.; Salvado Correia, J.; SomuncuoĞlu, B.; Da Vela, S.; Papadakis, C.M.; et al. Thermogels based on block versus gradient terpolymers: Differences in the nano- and macro-scale. Macromolecules 2025, 58, 9122–9139. [Google Scholar] [CrossRef]
- He, H.; Xie, S.; Du, J.; Ma, M.; Shi, Y.; Chen, S.; Wang, X. Nanotoroid self-weaving from nanofibers assembled by a radially symmetrical POSS-based dendrimer. ACS Macro Lett. 2025, 14, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hou, W.; Zhang, Q.; Qiao, H.; Lin, M.; Shen, Z.; Pang, X.; Sui, K. Hierarchical self-Assembly of injectable alginate supramolecular nanofibril hydrogels for hemostasis in vivo. Adv. Fiber Mater. 2024, 6, 489. [Google Scholar] [CrossRef]
- Zhu, B.; Cai, Y.; Zhou, L.; Zhao, L.; Chen, J.; Shan, X.; Sun, X.; You, Q.; Gong, X.; Zhang, W.; et al. Injectable supramolecular hydrogel co-loading abemaciclib/NLG919 for neoadjuvant immunotherapy of triple-negative breast cancer. Nat. Commun. 2025, 16, 687. [Google Scholar] [CrossRef] [PubMed]
- Vleugels, M.E.J.; de Korver, E.; Hendrikse, S.I.S.; Kardas, S.; Dhiman, S.; de Waal, B.F.M.; Schoenmakers, S.M.C.; Wijker, S.; De Geest, B.G.; Surin, M.; et al. Antibody-recruiting surfaces using adaptive multicomponent supramolecular copolymers. Biomacromolecules 2025, 26, 2971–2985. [Google Scholar] [CrossRef]
- Feng, F.; Xiao, D.; Yang, F.; Liu, H.; Qu, M.; Wang, W. Self-assembled nanostructures of noncovalent giant amphiphilic molecules composed of hydrophobic isobutyl BPOSS and hydrophilic POM in different cosolvents. Langmuir 2025, 41, 5371–5378. [Google Scholar] [CrossRef] [PubMed]
- Higaki, Y.; Eguchi, Y.; Masuda, T.; Mitsunobu, Y. Lyotropic microphase separation in aqueous solutions of double sulfobetaine diblock copolymers induced by charge separation length mismatch. Macromolecules 2025, 26, 2971–2985. [Google Scholar] [CrossRef]
- Zhang, C.H.; Wang, W.J.; Zhang, P.F.; Yang, S.G. Thermodynamic analysis of hydrogen-bonded polymer complexation with isothermal titration calorimetry. Polymer 2022, 256, 125196. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Zhang, P.; Yang, S. Hydrogen-bonding polymer complexation: Coacervation interfered with gelation. Giant 2023, 14, 100166. [Google Scholar] [CrossRef]
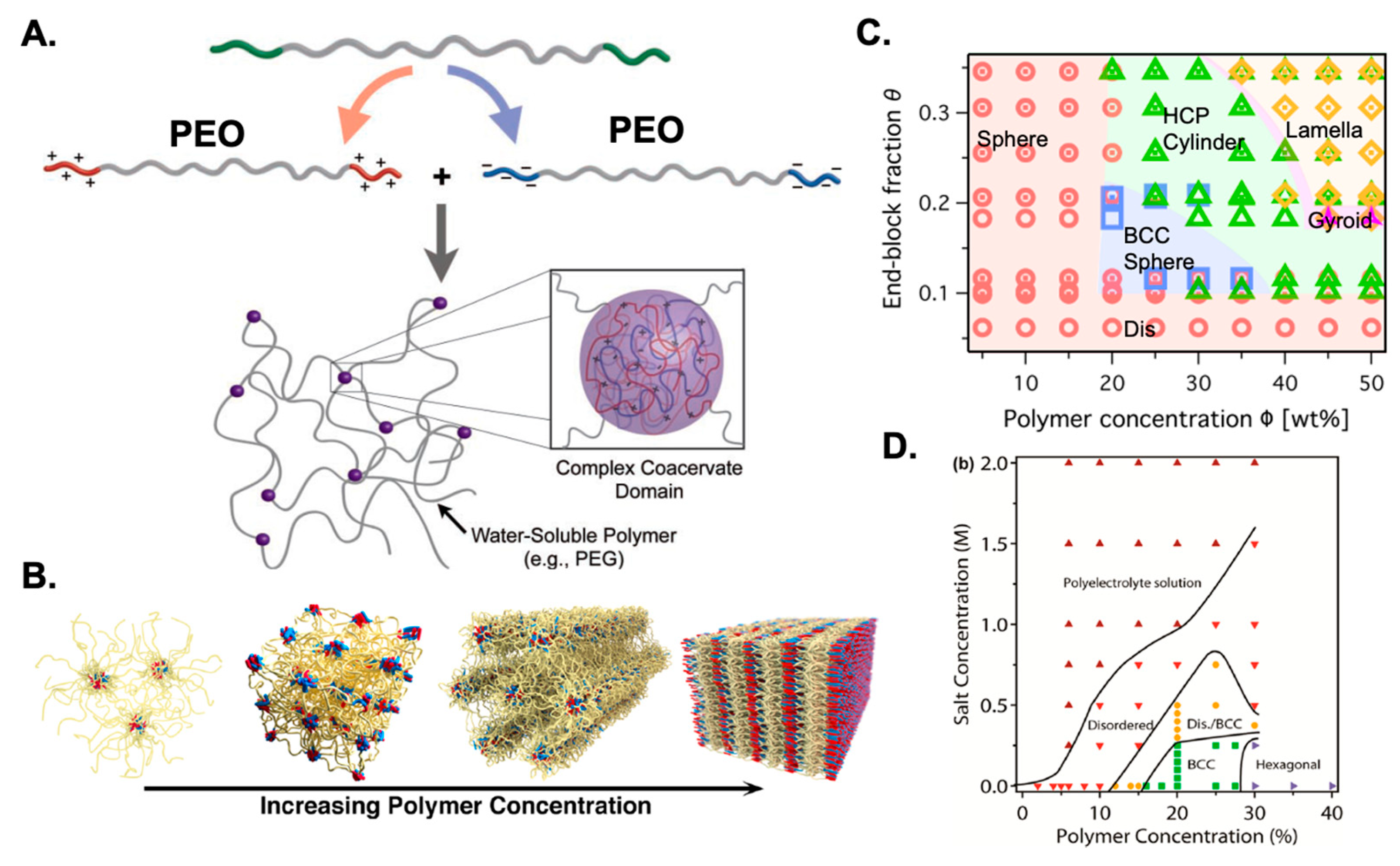
- Chen, X.; Du, Z.; Hu, Y.; Sun, N.; Ren, B. Aggregation and rheology of a triblock supra-amphiphilic polymer prepared by ionic self-assembly of a double-hydrophilic polyelectrolyte with an oppositely charged surfactant in aqueous solution. Macromolecules 2021, 54, 5498–5508. [Google Scholar] [CrossRef]
- Krogstad, D.V.; Lynd, N.A.; Choi, S.-H.; Spruell, J.M.; Hawker, C.J.; Kramer, E.J.; Tirrell, M.V. Effects of polymer and salt concentration on the structure and properties of triblock copolymer coacervate hydrogels. Macromolecules 2013, 46, 1512–1518. [Google Scholar] [CrossRef]
- Srivastava, S.; Andreev, M.; Levi, A.E.; Goldfeld, D.J.; Mao, J.; Heller, W.T.; Prabhu, V.M.; de Pablo, J.J.; Tirrell, M.V. Gel phase formation in dilute triblock copolyelectrolyte complexes. Nat. Commun. 2017, 8, 14131. [Google Scholar] [CrossRef]
- Hunt, J.N.; Feldman, K.E.; Lynd, N.A.; Deek, J.; Campos, L.M.; Spruell, J.M.; Hernandez, B.M.; Kramer, E.J.; Hawker, C.J. Tunable, high modulus hydrogels driven by ionic coacervation. Adv. Mater. 2011, 23, 2327. [Google Scholar] [CrossRef]
- Srivastava, S.; Levi, A.E.; Goldfeld, D.J.; Tirrell, M.V. Structure, morphology, and rheology of polyelectrolyte complex hydrogels formed by self-Assembly of oppositely charged triblock polyelectrolytes. Macromolecules 2020, 53, 5763–5774. [Google Scholar] [CrossRef]
- Krogstad, D.V.; Lynd, N.A.; Miyajima, D.; Gopez, J.; Hawker, C.J.; Kramer, E.J.; Tirrell, M.V. Structural evolution of polyelectrolyte complex core micelles and ordered-phase bulk materials. Macromolecules 2014, 47, 8026–8032. [Google Scholar] [CrossRef]
- Mirkin, C.A.; Langer, R.; Mrksich, M.; Margolin, A.A.; Petrosko, S.H.; Artzi, N. Blueprints for better drugs: The structural revolution in nanomedicine. ACS Nano 2025, 19, 18889–18901. [Google Scholar] [CrossRef]
- Cheng, G.; Tao, S.; Liu, S.; Wang, P.; Zhang, C.; Liu, J.; Hao, C.; Wang, S.; Guo, D.; Xu, B. Glutathione-responsive polymersome with continuous glutathione depletion for enhanced photodynamic therapy and hypoxia-activated chemotherapy. ACS Macro Lett. 2024, 13, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Ghosh, S.; Maity, T.; Behera, P.P.; Mukherjee, A.; De, P. Photocleavable visible light-triggered anthraquinone-derived water-soluble block copolymer for peroxynitrite generation in cancer therapy. ACS Macro Lett. 2024, 13, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, S.; Yuan, Y.; Li, C. Hierarchical engineering of amphiphilic peptides nanofibrous crosslinkers toward mechanically robust, functionally customable, and sustainable supramolecular hydrogels. Adv. Mater. 2025, 37, e2503324. [Google Scholar] [CrossRef]
- Cho, Y.; Fincher, C.D.; Lamour, G.; Christoff-Tempesta, T.; Zuo, X.; Chiang, Y.M.; Ortony, J.H. Reversible self-assembly of small molecules for recyclable solid-state battery electrolytes. Nat. Chem. 2025. [Google Scholar] [CrossRef]
- Rathod, P.B.; Kumar, K.S.A.; Athawale, A.A.; Pandey, A.K. Polymeric nanoassembly of imine functionalized magnetite for loading copper salts to catalyze Henry and A3-coupling reactions. React. Funct. Polym. 2021, 161, 104868. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Feng, F.; Shao, Y.; Jian, H.; Liu, H.; Dong, X.H.; Ge, A.; Yang, S. Interfacial behaviors of giant amphiphilic molecules composed of hydrophobic isobutyl POSS and hydrophilic POSS bearing carboxylic acid groups at the air-water interface. Langmuir 2023, 39, 16854–16862. [Google Scholar] [CrossRef]
- Wang, W.-J.; Xu, X.; Shao, Y.; Liao, J.-W.; Jian, H.-X.; Xue, B.; Yang, S.-G. Fractal growth of giant amphiphiles in langmuir-blodgett films. Chin. J. Polym. Sci. 2022, 40, 556–566. [Google Scholar] [CrossRef]
- Cheyne, R.B.; Moffitt, M.G. Self-assembly of polystyrene-block-poly(ethylene oxide) copolymers at the air-water interface: Is dewetting the genesis of surface aggregate formation? Langmuir 2006, 22, 8387–8396. [Google Scholar] [CrossRef]
- Glagola, C.P.; Miceli, L.M.; Milchak, M.A.; Halle, E.H.; Logan, J.L. Polystyrene-poly(ethylene oxide) diblock copolymer: The effect of polystyrene and spreading concentration at the air/water interface. Langmuir 2012, 28, 5048. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shao, Y.; Wang, W.; Zhu, L.; Liu, H.; Yang, S. Fluorinated polyhedral oligomeric silsesquioxanes end-capped poly(ethylene oxide) giant surfactants: Precise synthesis and interfacial behaviors. Polymer 2020, 186, 122055. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Y.; Wang, W.; Liu, H.; Zhang, W.; Yang, S. Morphological variation of an LB film of giant amphiphiles composed of poly(ethylene oxide) and hydrophobically modified POSS. Langmuir 2021, 37, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, Y.; Feng, F.; Wang, W. Self-Assembly of Block Copolymers to Prepare Advanced Materials with Hierarchical Functional Nanostructures. Nanomanufacturing 2025, 5, 18. https://doi.org/10.3390/nanomanufacturing5040018
Liu Y, Liu Y, Feng F, Wang W. Self-Assembly of Block Copolymers to Prepare Advanced Materials with Hierarchical Functional Nanostructures. Nanomanufacturing. 2025; 5(4):18. https://doi.org/10.3390/nanomanufacturing5040018
Chicago/Turabian StyleLiu, Yanzhen, Yang Liu, Fengfeng Feng, and Weijie Wang. 2025. "Self-Assembly of Block Copolymers to Prepare Advanced Materials with Hierarchical Functional Nanostructures" Nanomanufacturing 5, no. 4: 18. https://doi.org/10.3390/nanomanufacturing5040018
APA StyleLiu, Y., Liu, Y., Feng, F., & Wang, W. (2025). Self-Assembly of Block Copolymers to Prepare Advanced Materials with Hierarchical Functional Nanostructures. Nanomanufacturing, 5(4), 18. https://doi.org/10.3390/nanomanufacturing5040018





