Tuning Optical and Photoelectrochemical Properties of TiO2/WOx Heterostructures by Reactive Sputtering: Thickness-Dependent Insights
Abstract
1. Introduction
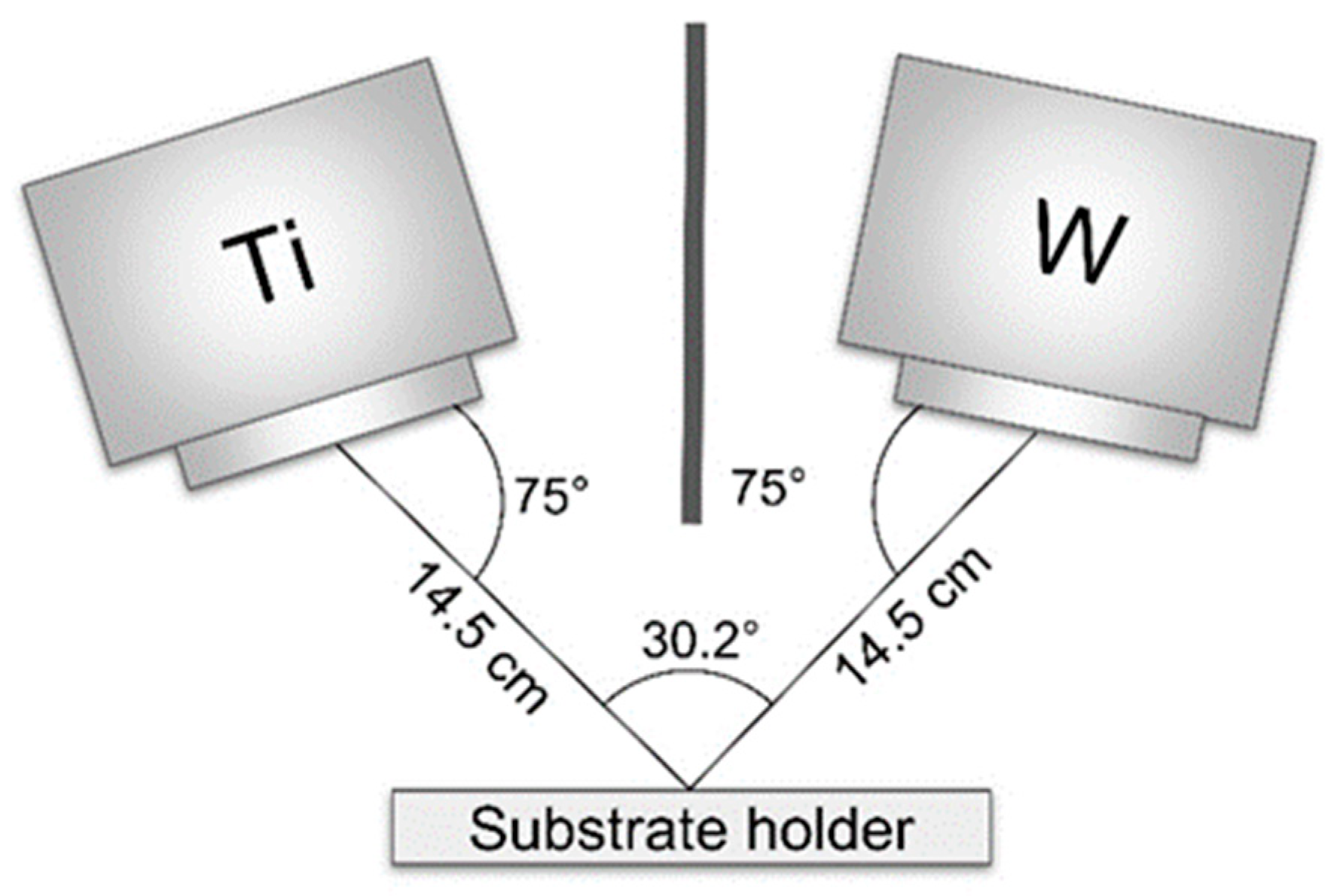
2. Materials and Methods
3. Results and Discussion
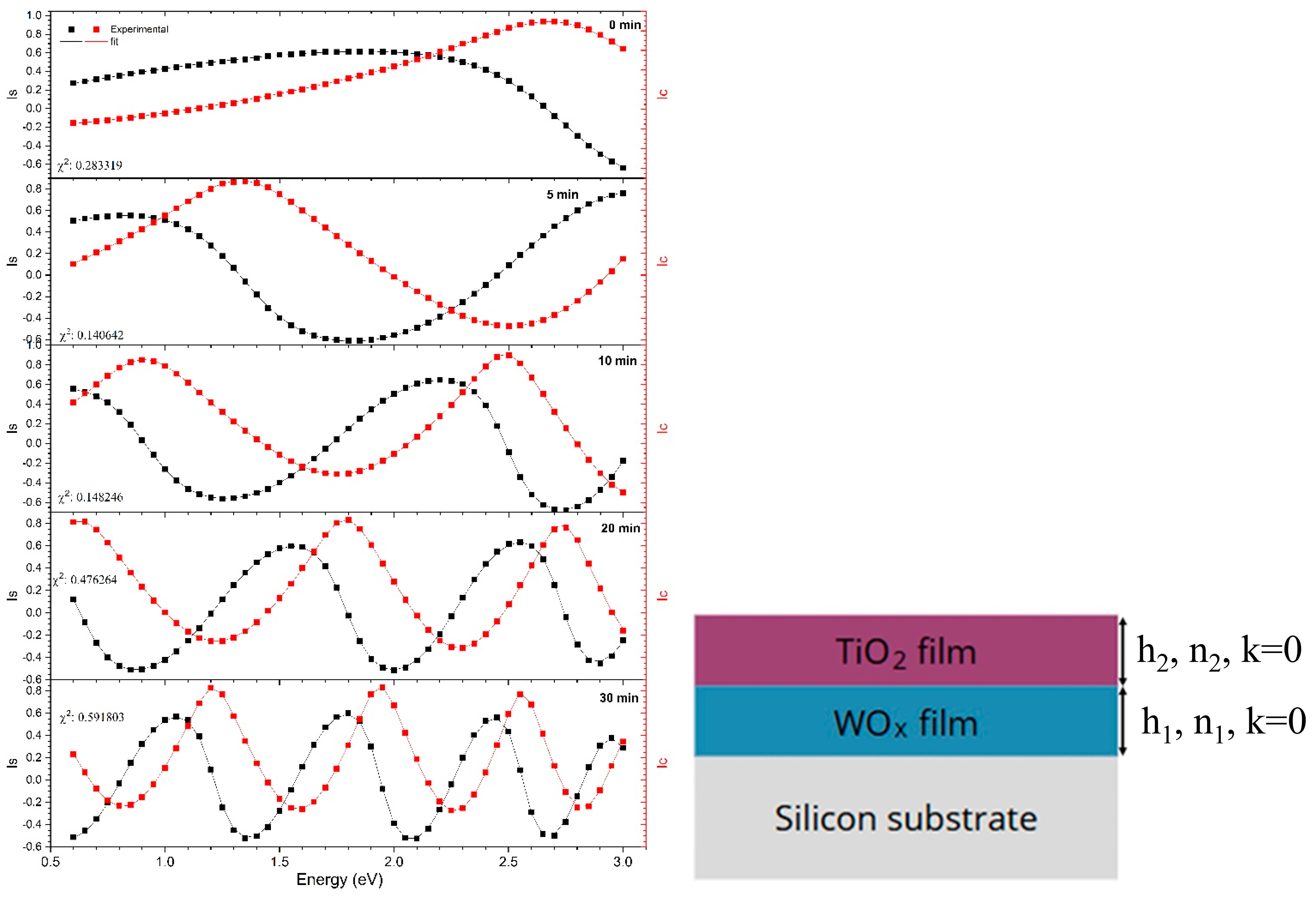
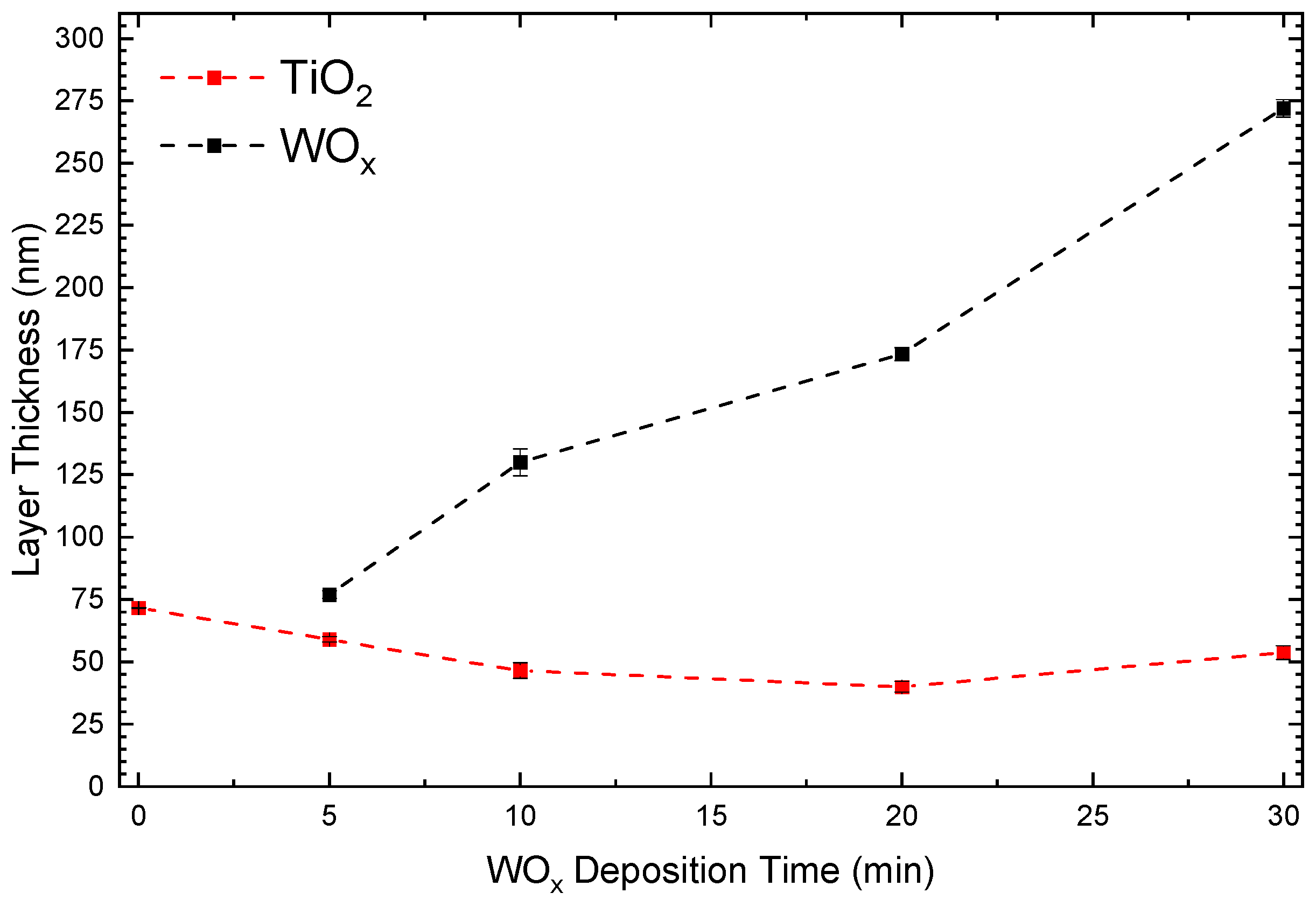
3.1. Optical Modeling and Thickness Evolution by Ellipsometry
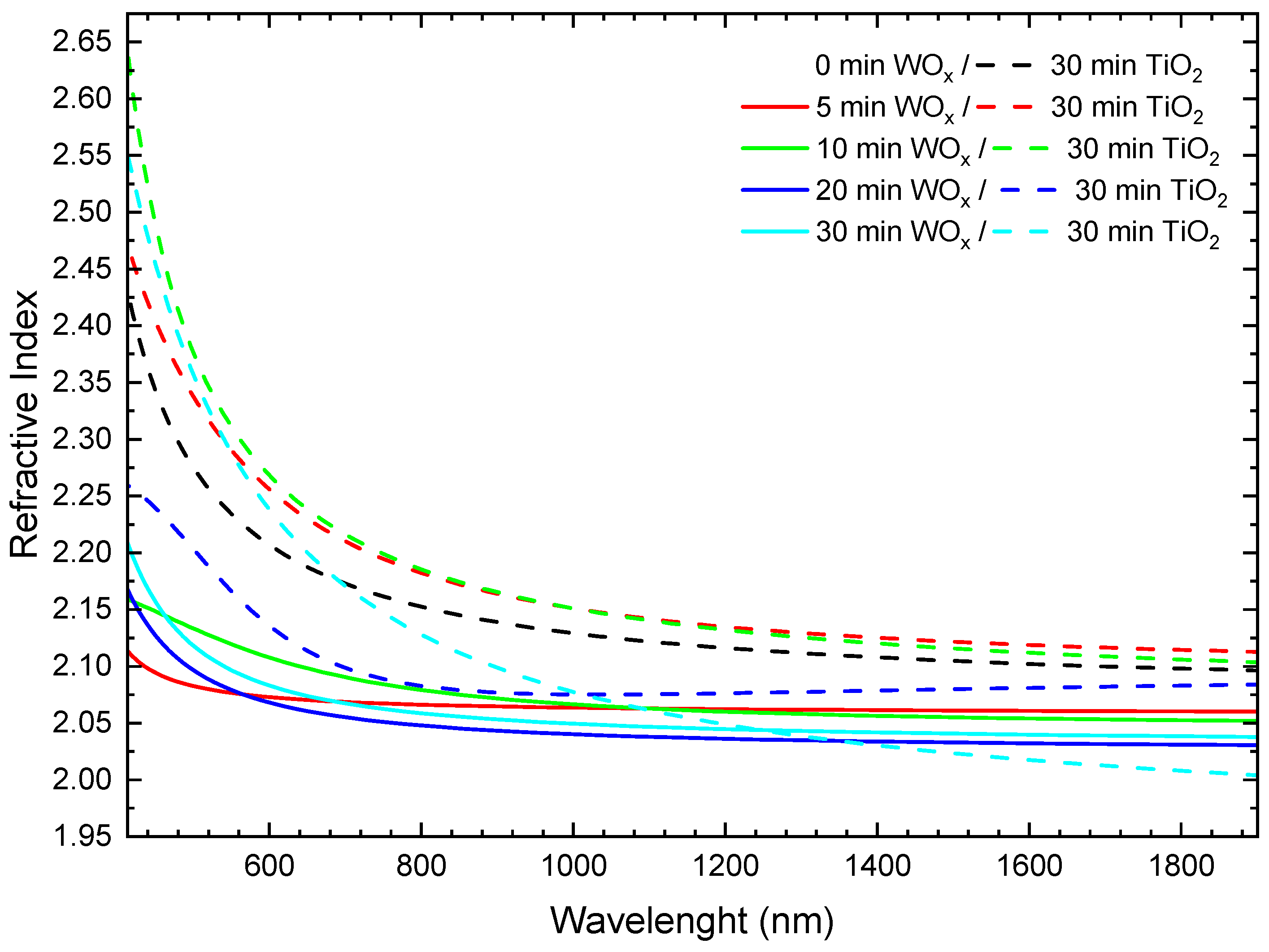
3.2. Refractive Index and Optical Contrast
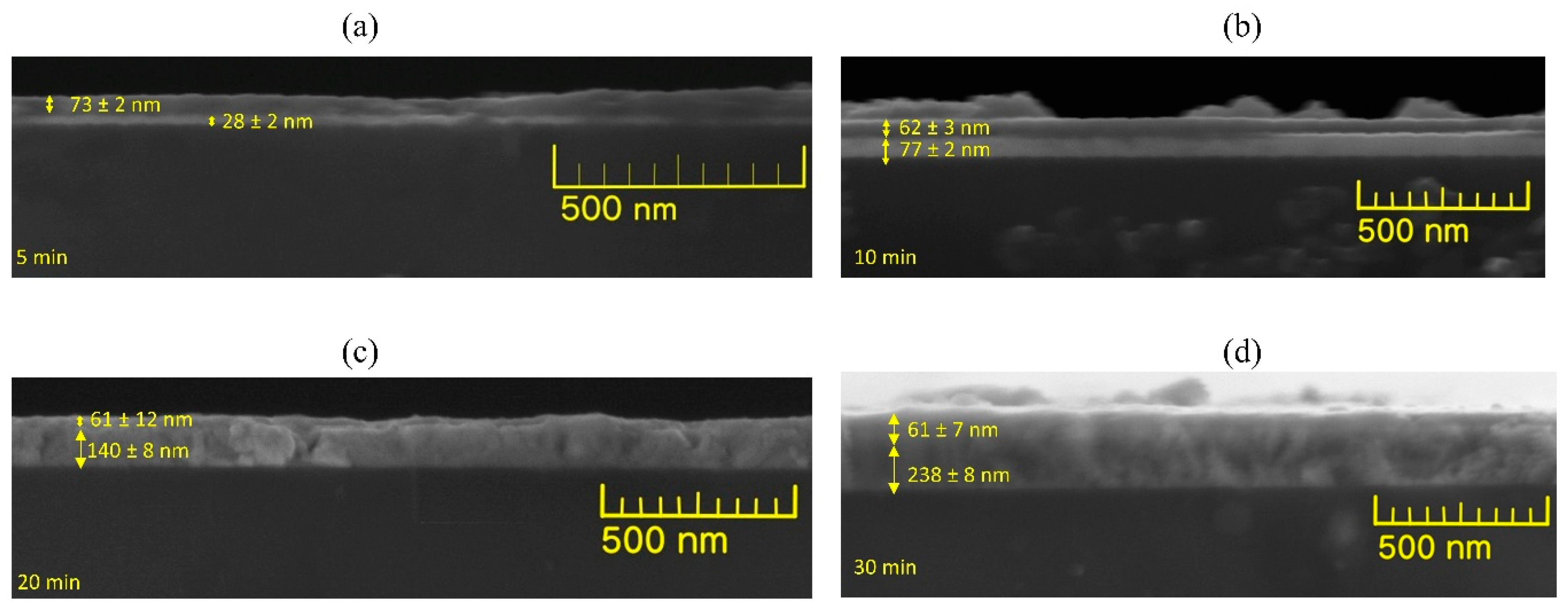
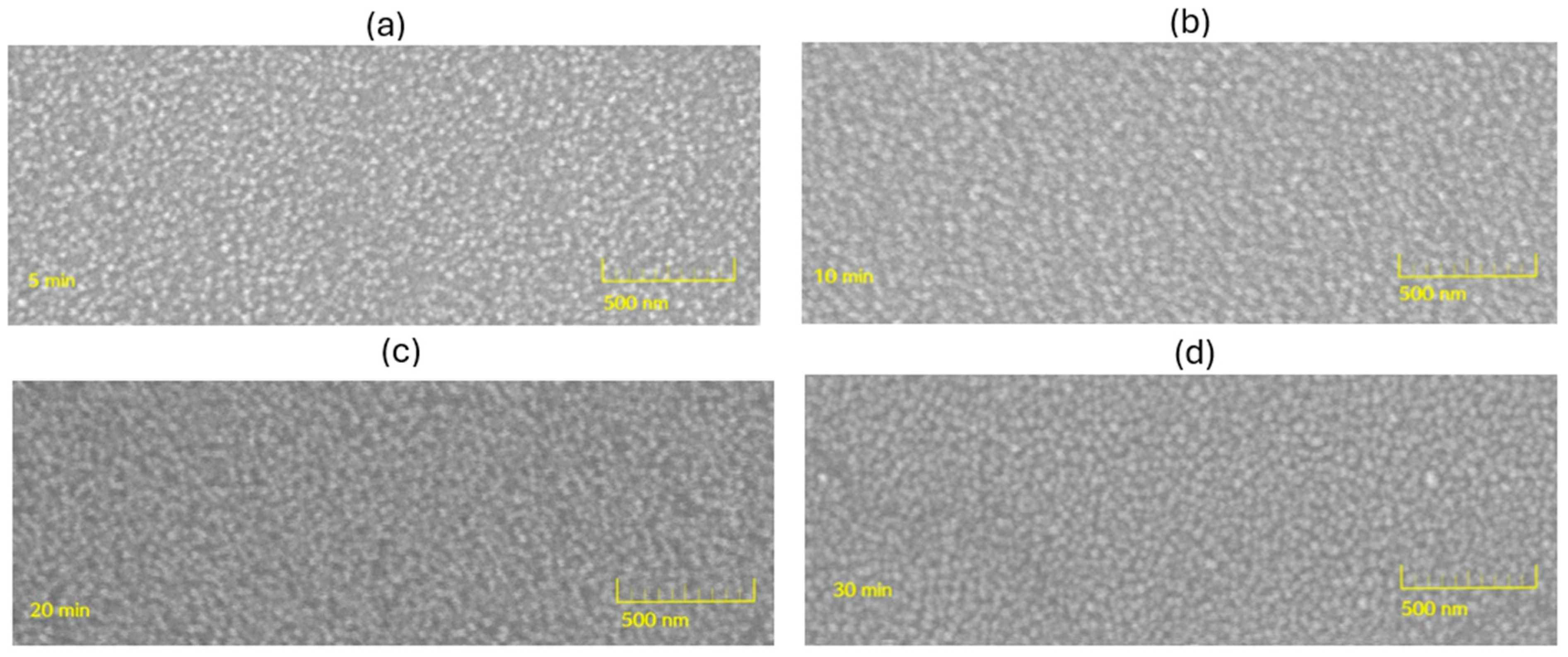
3.3. Morphology and Layer Integrity from Cross-Sectional FE-SEM
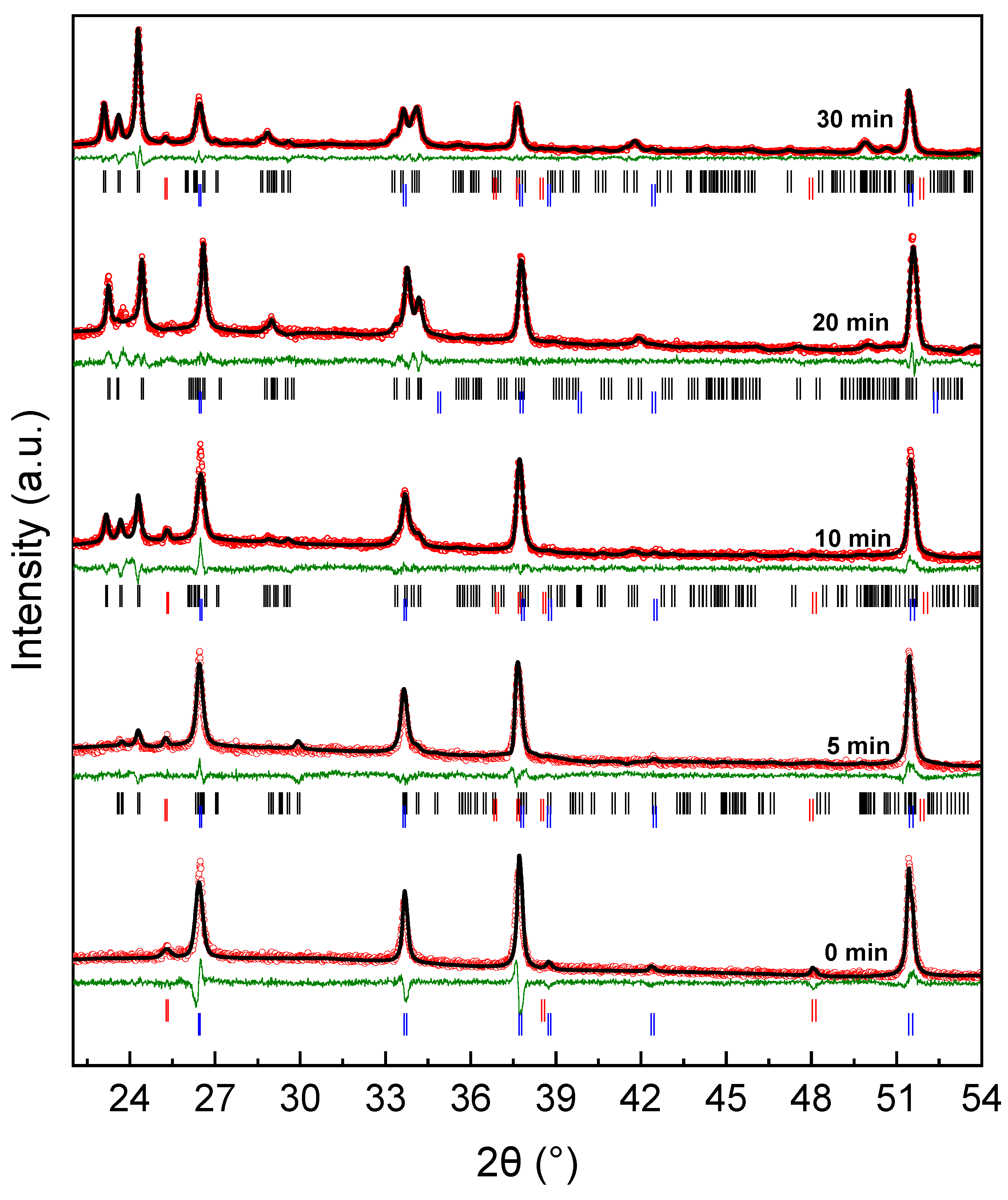
3.4. Crystalline Structure from X-Ray Diffraction
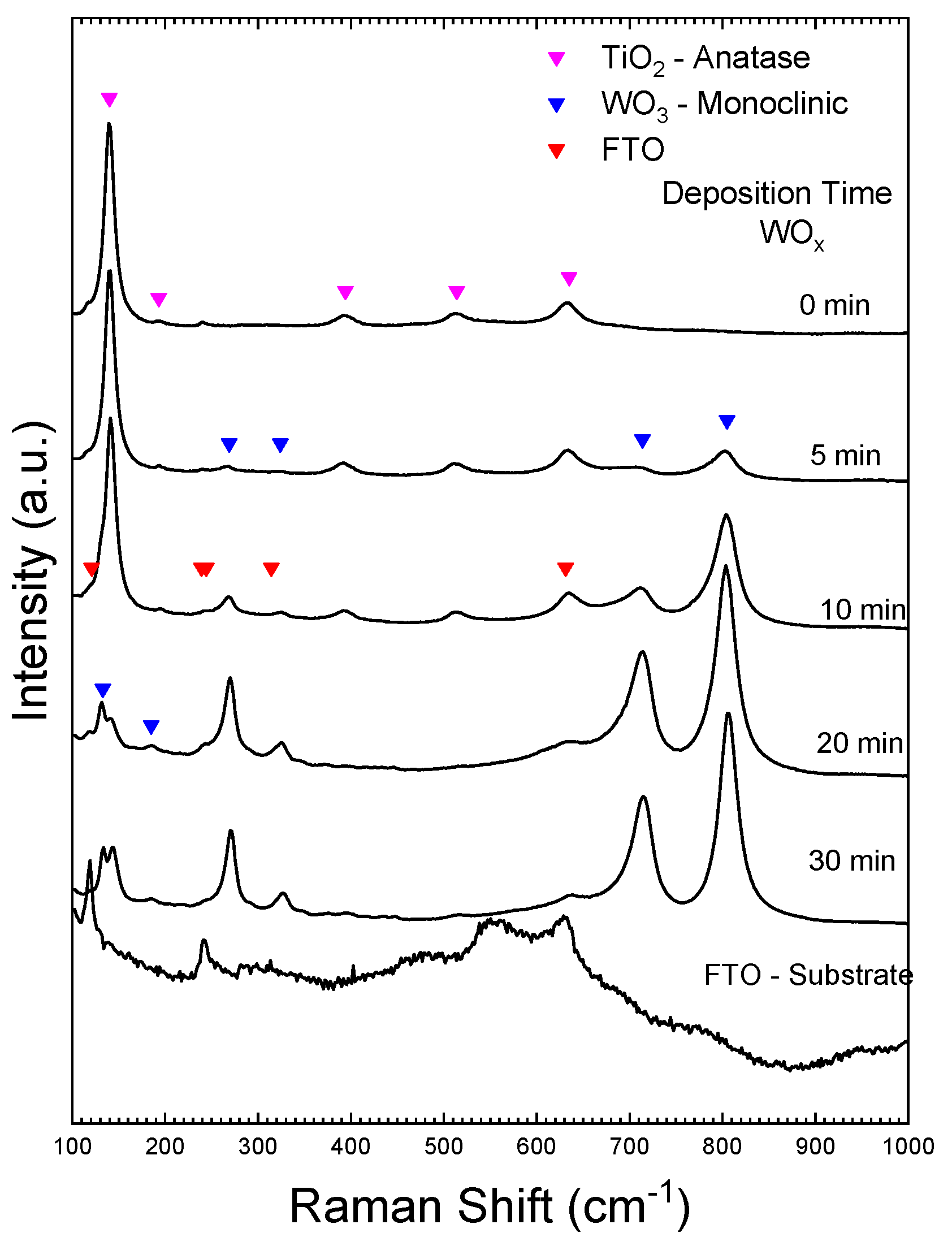
3.5. Vibrational Characterization by Raman Spectroscopy
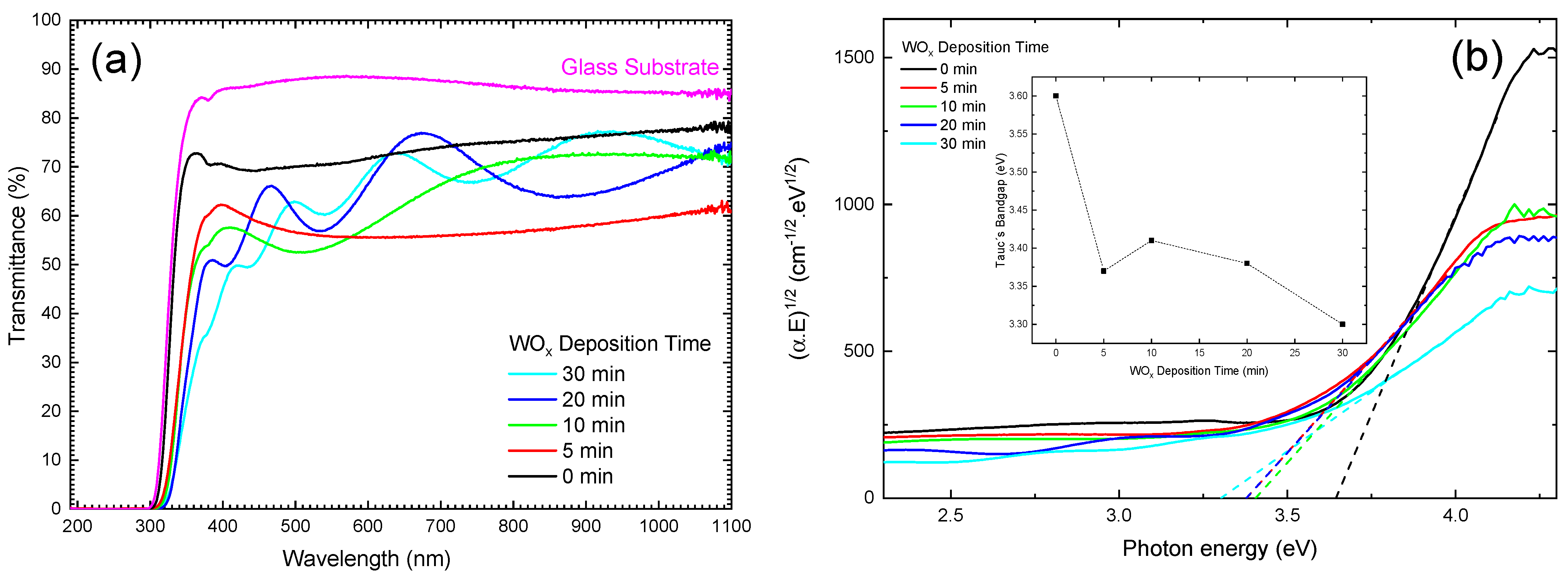
3.6. Optical Transmittance and Band Gap Analysis
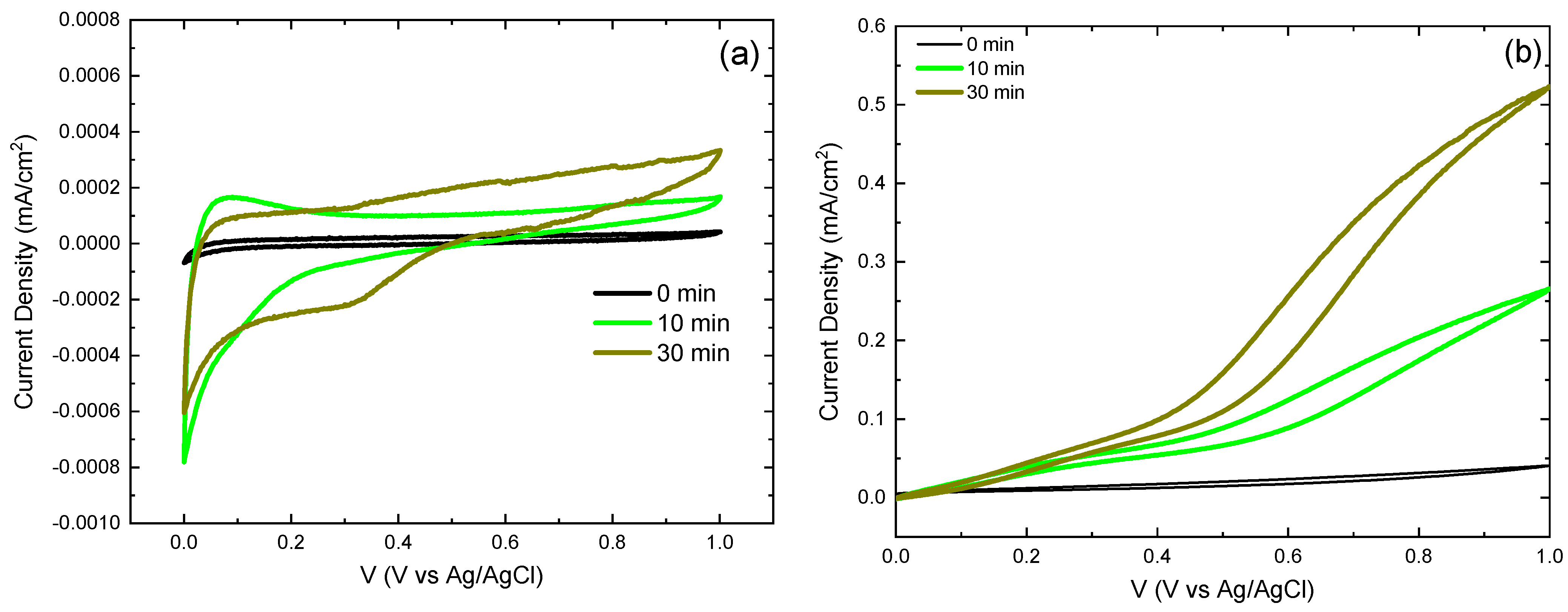
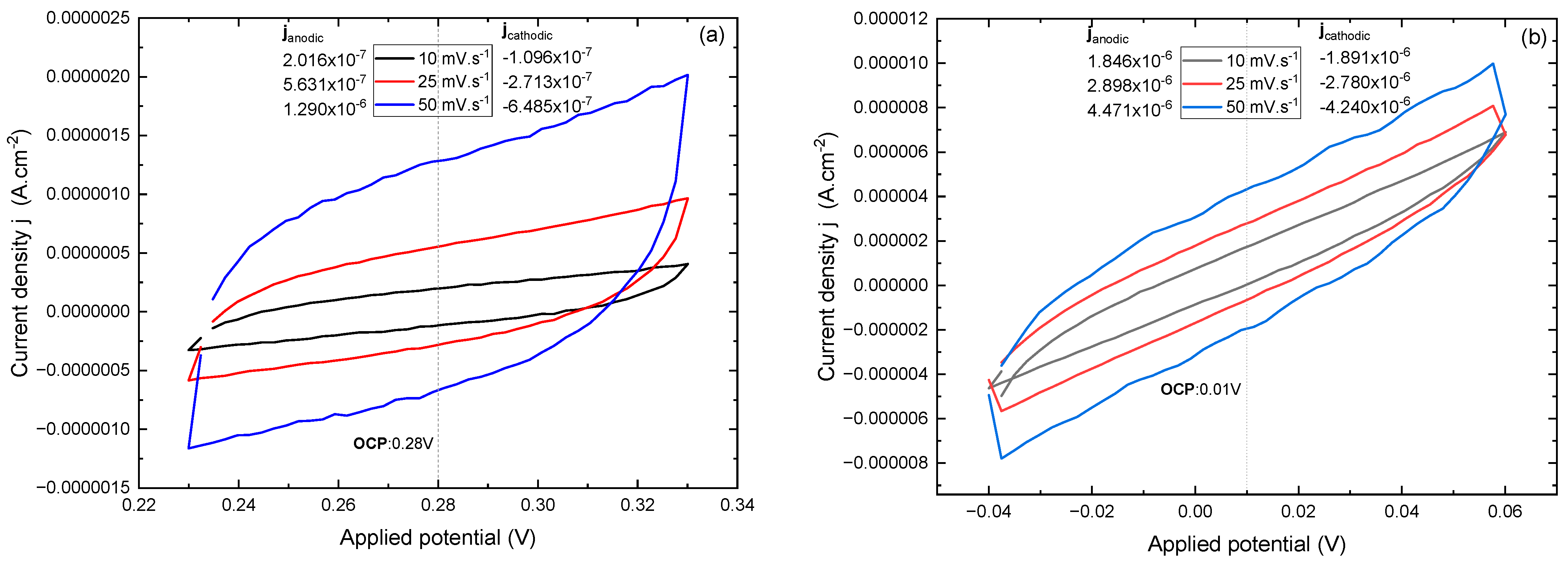
3.7. Electrochemical Behavior by Cyclic Voltammetry
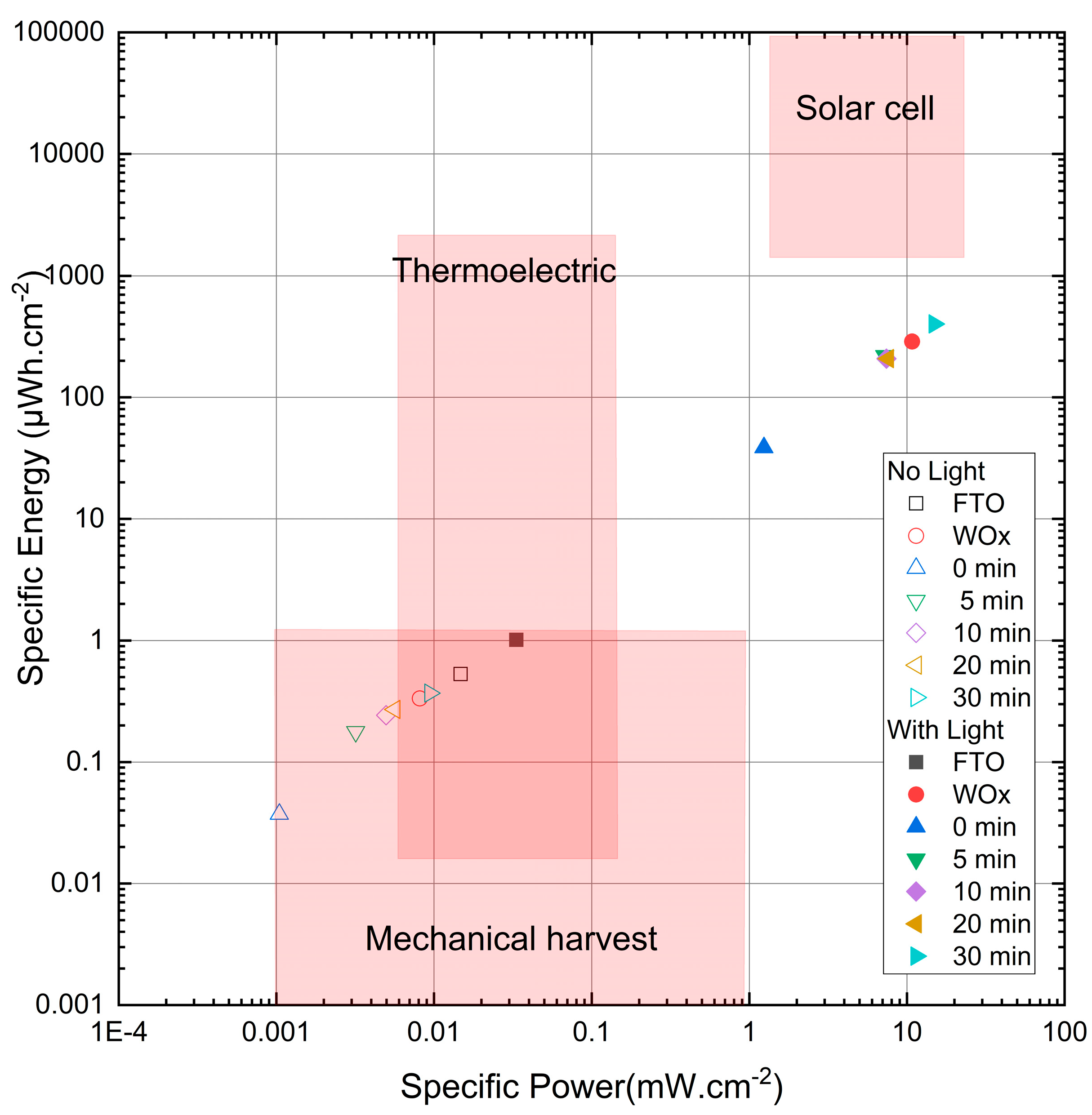
3.8. Energy and Power Density Evaluation
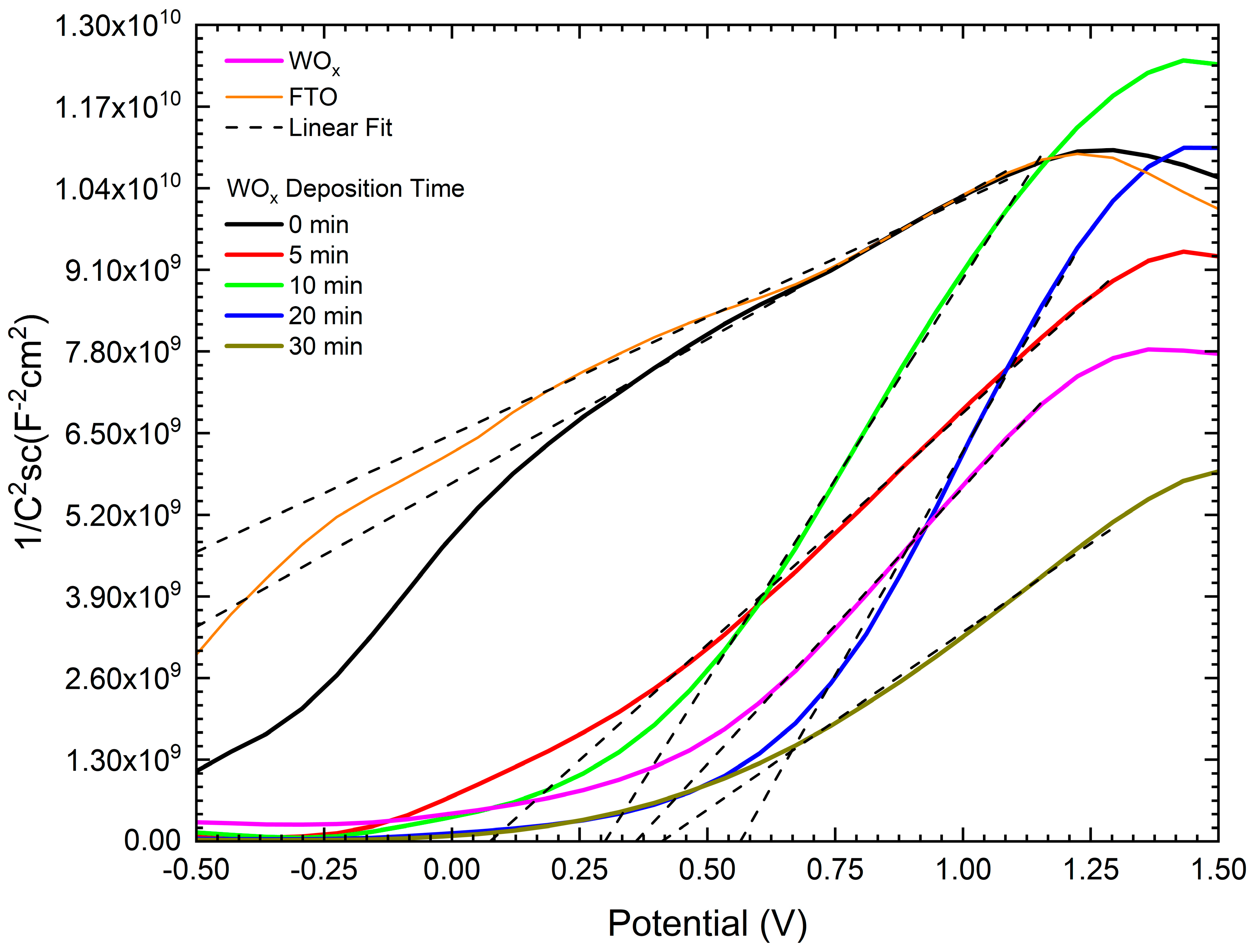
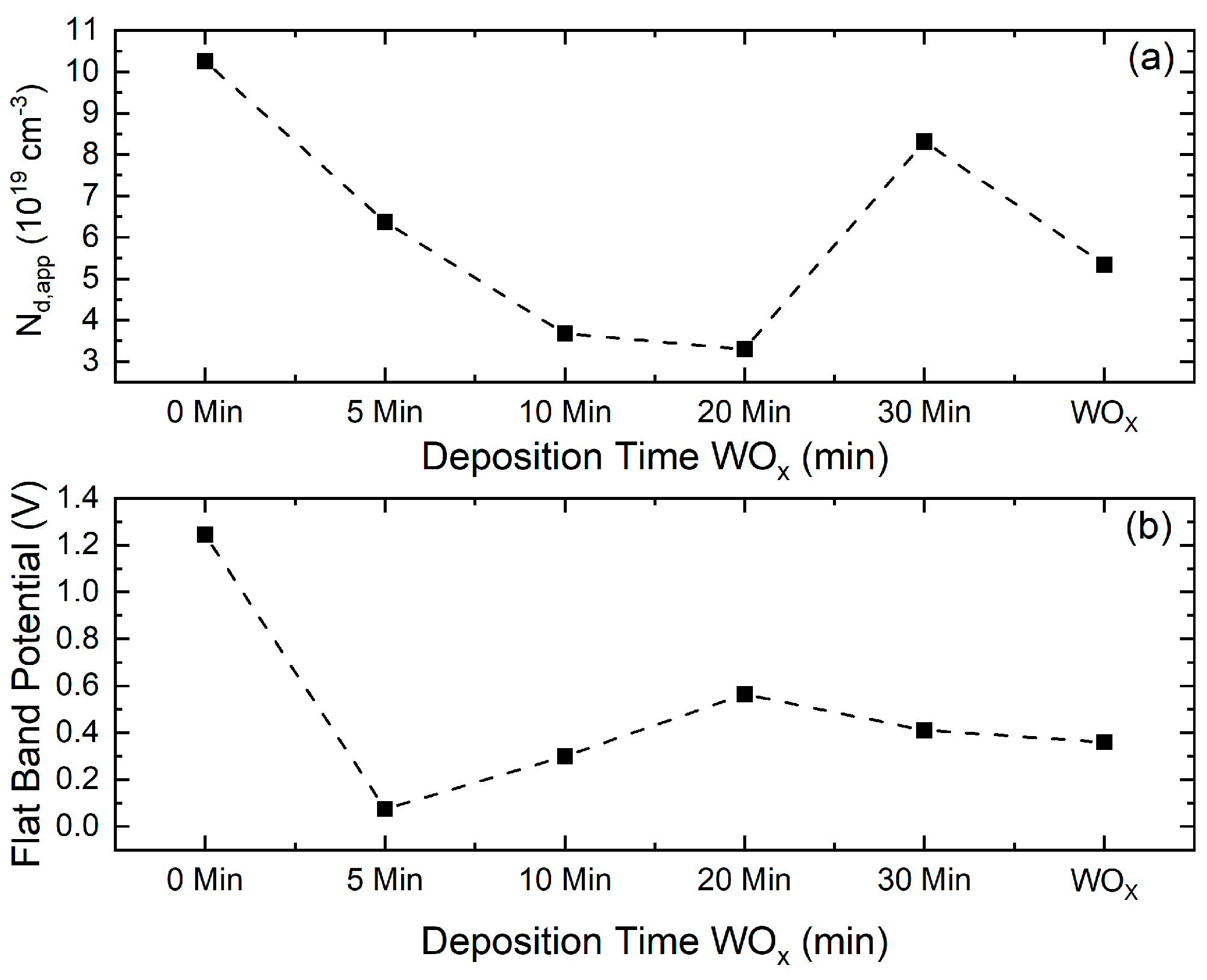
3.9. Semiconductor Properties from Mott–Schottky Analysis
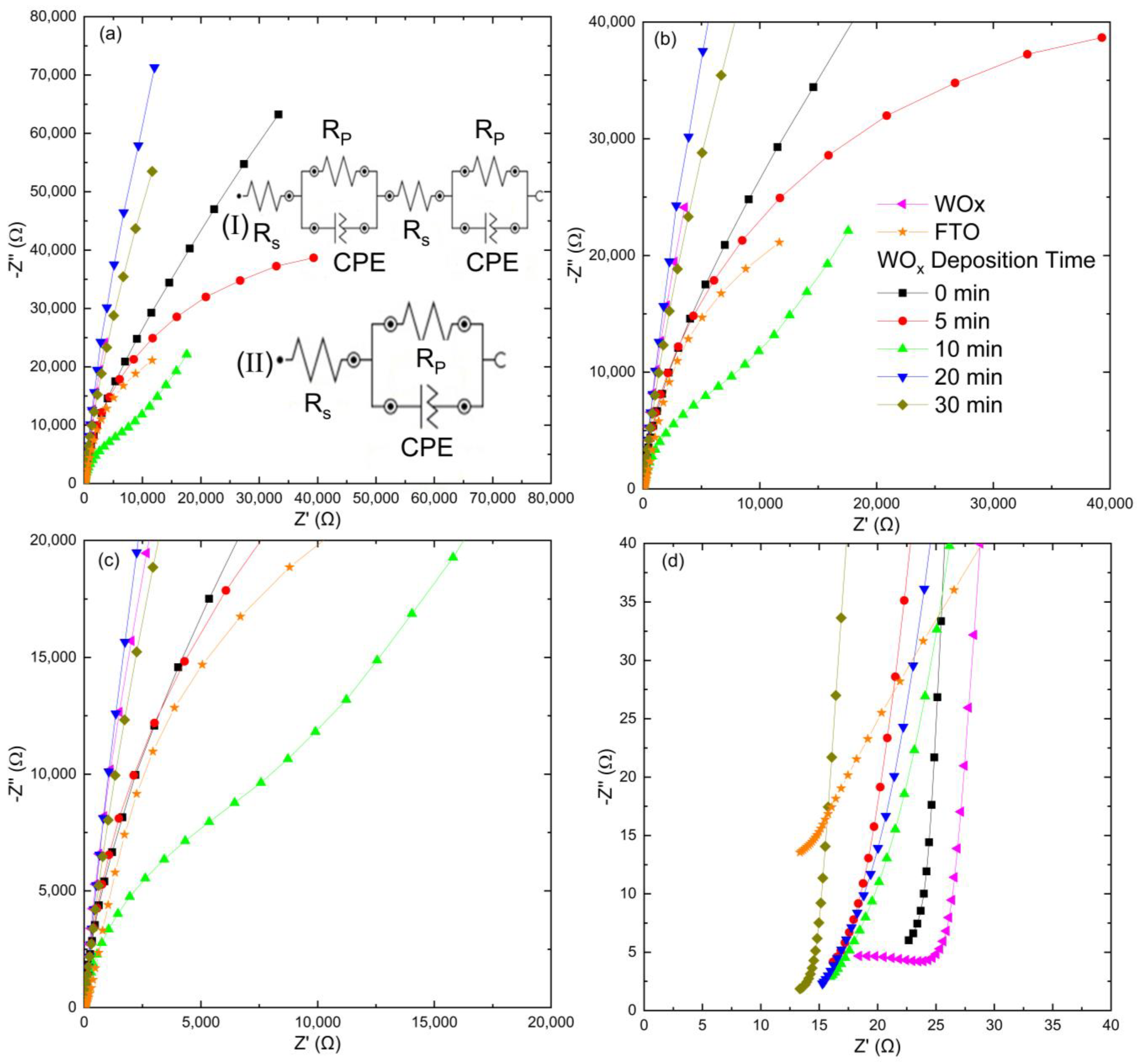
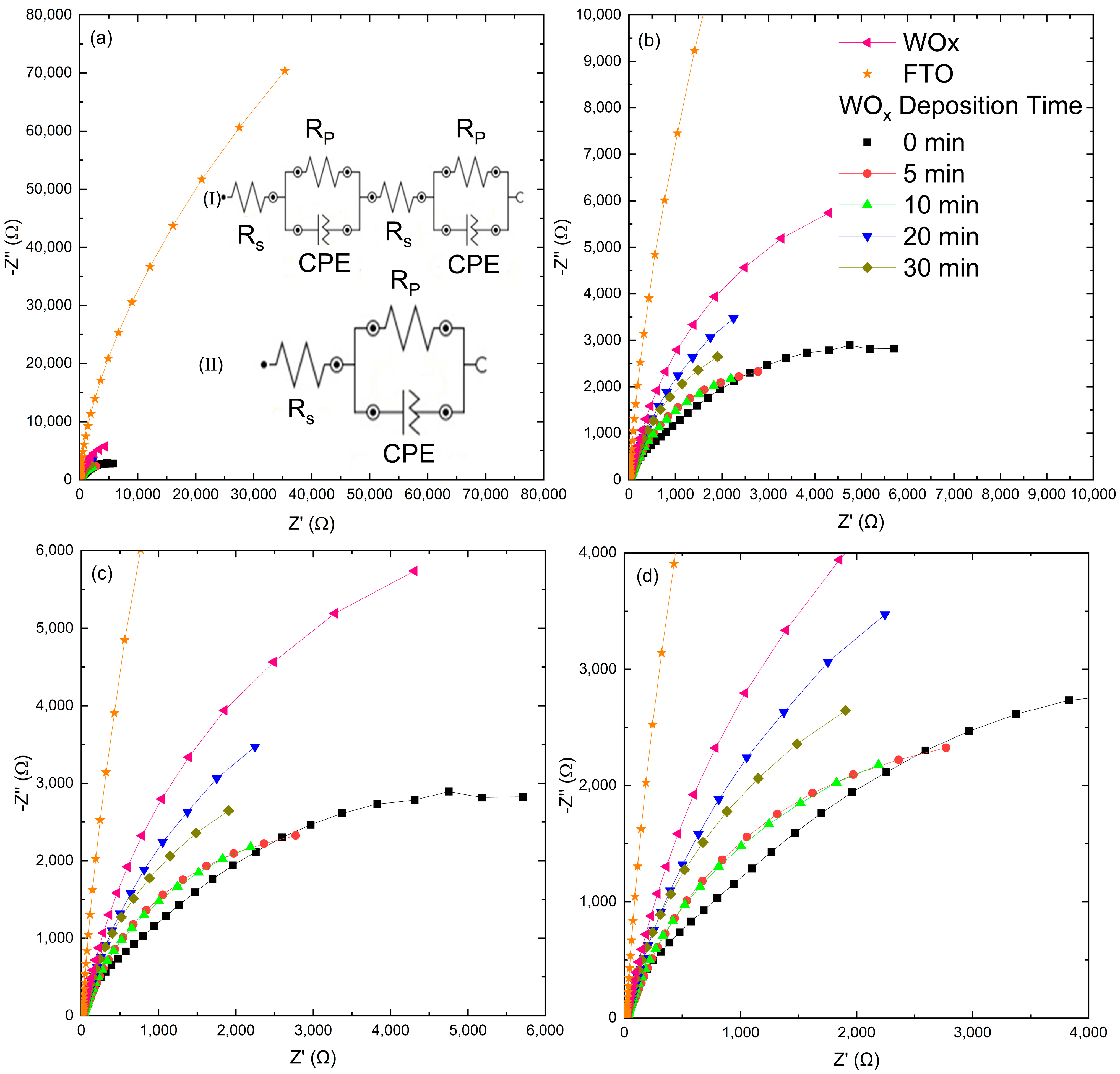
3.10. Semiconductor Properties from Electrochemical Impedance Spectroscopy
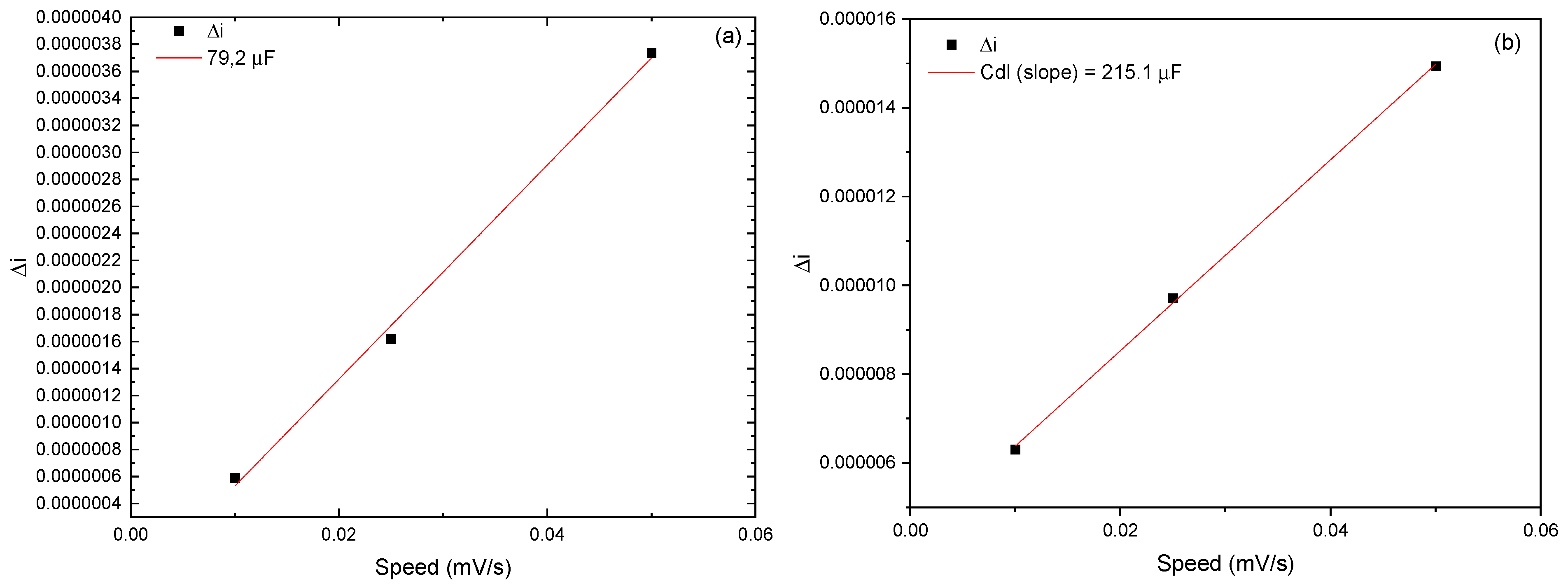
3.11. Quantification of the Interfacial Capacitive Contribution and Photoelectrochemical Implications
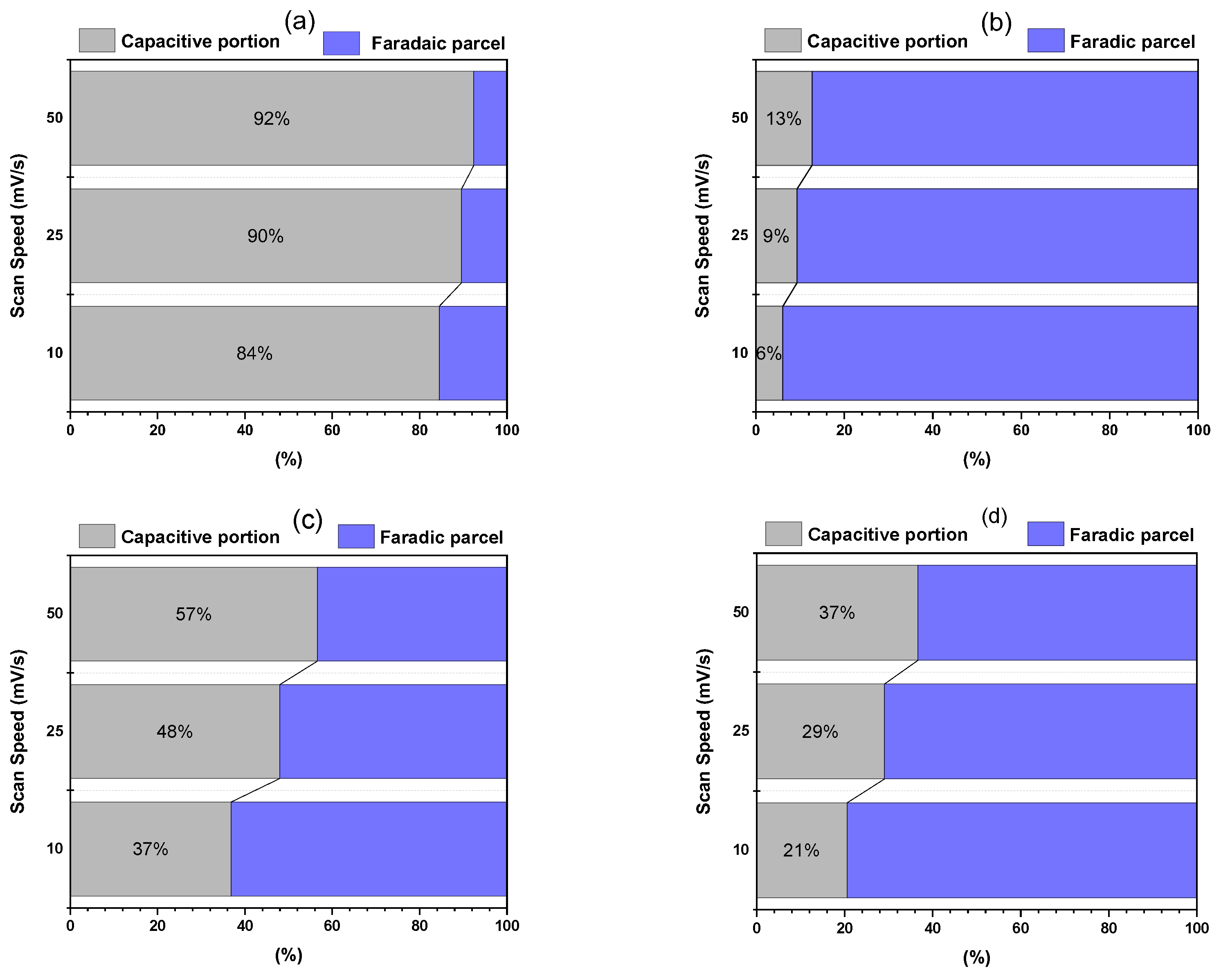
3.12. Capacitive vs. Faradaic Decomposition by the Dunn Method
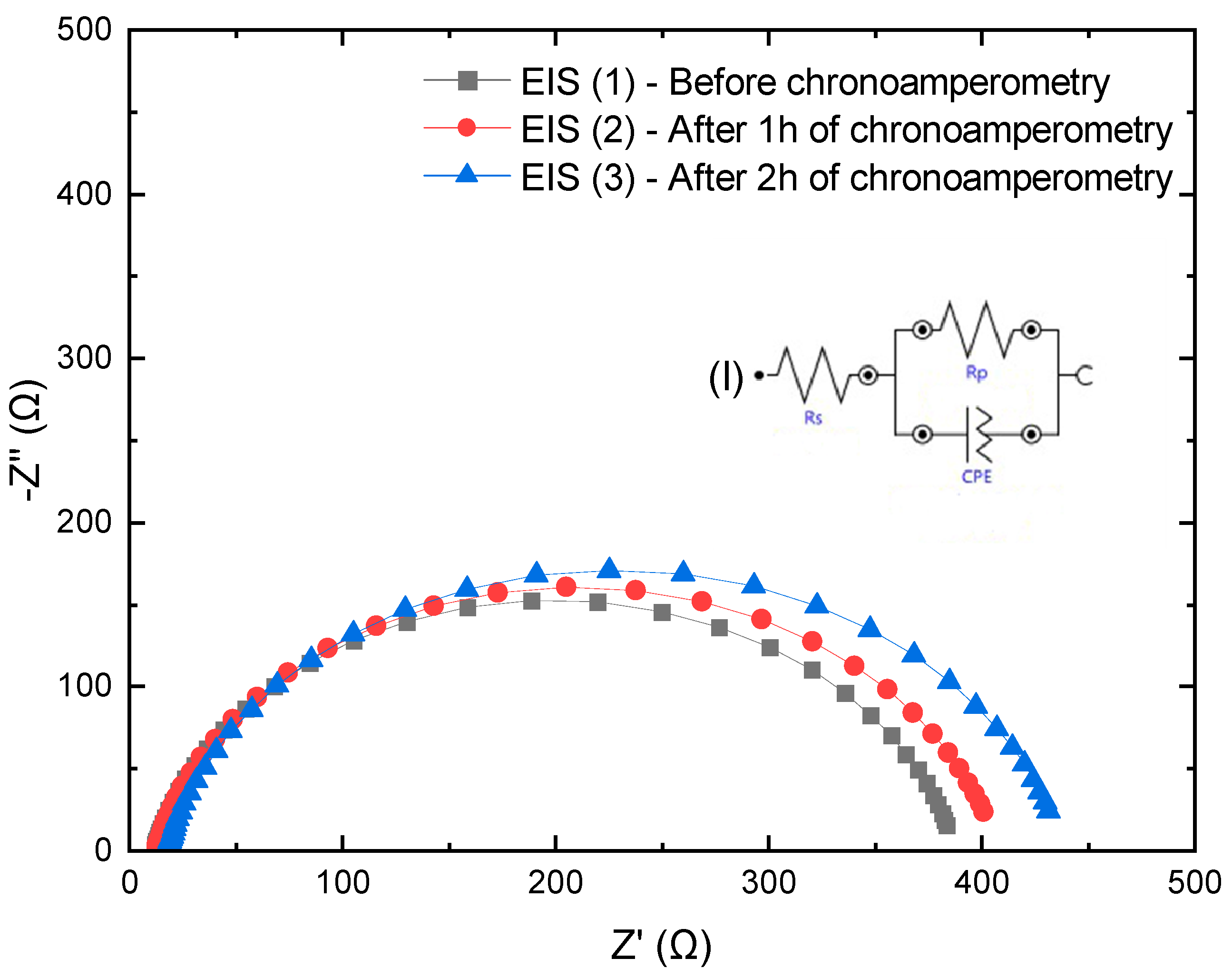
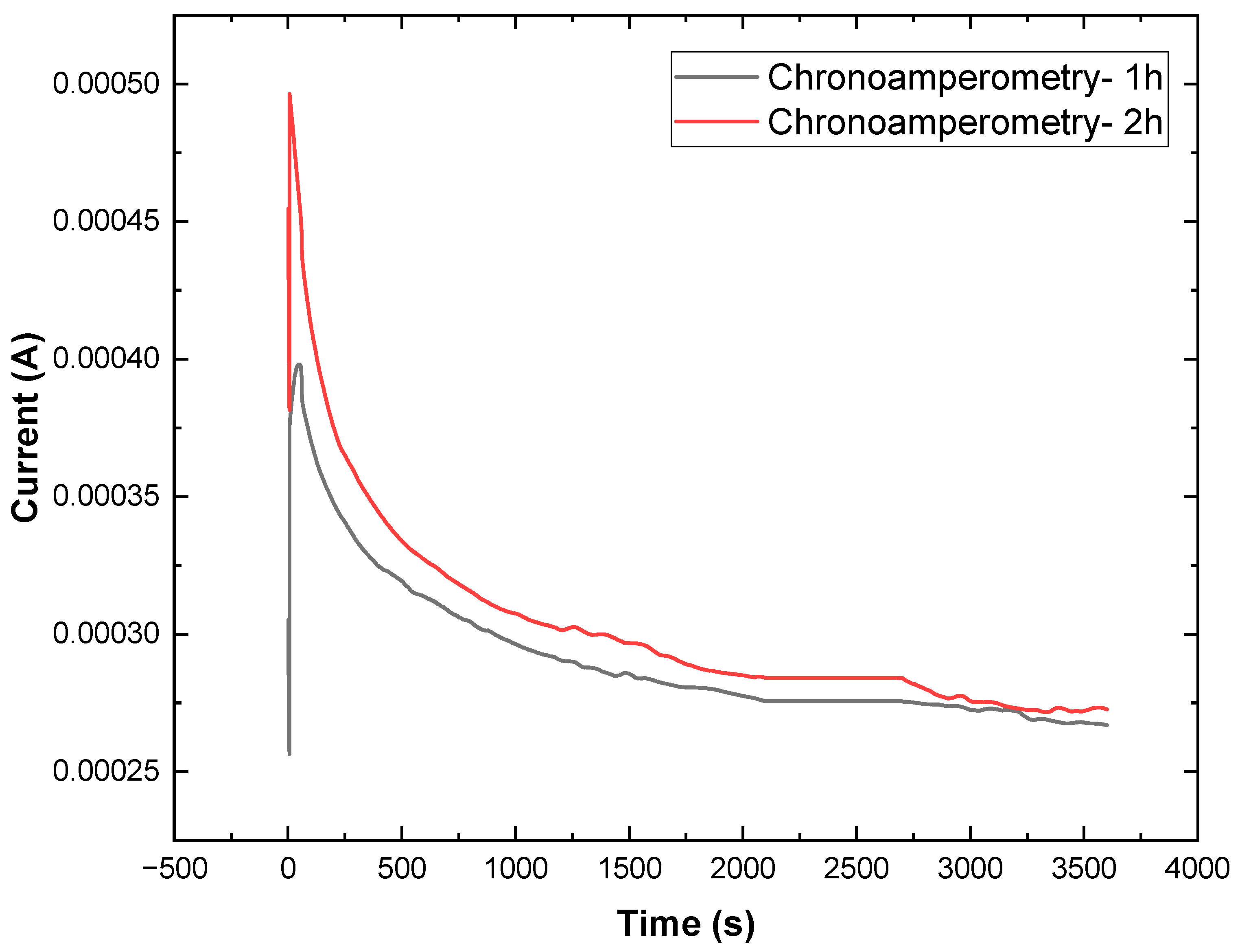
3.13. Stability Monitoring of TiO2/WOx by EIS Before and After Chronoamperometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| WOx | Tungsten oxide |
| TiO2 | Titanium dioxide |
| PEC | Photoelectrochemical |
| DC | Direct current |
| FTO | Fluorine-doped tin oxide |
| FE-SEM | Field-emission scanning electron microscopy |
| XRD | X-ray diffraction |
| OCP | Open-circuit potential |
| EIS | Electrochemical impedance spectroscopy |
| CV | Cyclic voltammetry |
| MS | Mott–Schottky |
| ND | Majority donor density |
| ND,APP | Majority apparent donor density |
| VFB | Flat-band potential |
| Rs | Solution resistance |
| Rp | Charge transfer resistance |
| CPE | Constant phase element |
| χ2 | Goodness of fit (chi-squared) |
| Eg | Optical band gap |
| Nd | Donor density |
| Ps | Specific power |
| Es | Specific energy |
| AM 1.5G | Air mass 1.5 global (solar illumination standard) |
| RHE | Reversible hydrogen electrode |
| OER | Oxygen evolution reaction |
| CA | Chronoamperometry |
| Cdl | Double-layer capacitance |
| ECSA | Electrochemically active surface area |
| Cs | Specific capacitance |
| Rf | Roughness factor |
| fF | Faradaic fraction |
| FRA | Frequency Response Analyzer |
| sccm | Standard cubic centimeters per minute |
References
- Li, S.; Xu, W.; Meng, L.; Tian, W.; Li, L. Recent Progress on Semiconductor Heterojunction-Based Photoanodes for Photoelectrochemical Water Splitting. Small Sci. 2022, 2, 2100112. [Google Scholar] [CrossRef]
- Pessoa, R.S.; Fraga, M.A.; Santos, L.V.; Massi, M.; Maciel, H.S. Nanostructured thin films based on TiO2 and/or SiC for use in photoelectrochemical cells: A review of the material characteristics, synthesis and recent applications. Mater. Sci. Semicond. Process. 2015, 29, 56–68. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, B.; Luo, G.; Yu, J.; Cao, S. S-scheme heterojunction photocatalysts based on 2D materials. Mater. Today 2024, 81, 137–158. [Google Scholar] [CrossRef]
- Shabbir, A.; Sardar, S.; Mumtaz, A. Mechanistic investigations of emerging type-II, Z-scheme and S-scheme heterojunctions for photocatalytic applications–A review. J. Alloys Compd. 2024, 1003, 175683. [Google Scholar] [CrossRef]
- Wang, H.; Fu, W.; Yang, X.; Huang, Z.; Li, J.; Zhang, H.; Wang, Y. Recent advancements in heterostructured interface engineering for hydrogen evolution reaction electrocatalysis. J. Mater. Chem. 2020, 8, 6926–6956. [Google Scholar] [CrossRef]
- Li, X.Z.; Li, F.B.; Yang, C.L.; Ge, W.K. Photocatalytic activity of WOx-TiO2 under visible light irradiation. J. Photochem. Photobiol. A Chem. 2001, 141, 209–217. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.; Hu, X.; Xu, L.; Chen, G.; Li, X. Hollow spherical WO3/TiO2 heterojunction for enhancing photocatalytic performance in visible-light. J. Water Process Eng. 2021, 40, 101943. [Google Scholar] [CrossRef]
- Rostami, M.; Badiei, A.; Ganjali, M.R.; Rahimi-Nasrabadi, M.; Naddafi, M.; Karimi-Maleh, H. Nano-architectural design of TiO2 for high performance photocatalytic degradation of organic pollutant: A review. Environ. Res. 2022, 212, 113347. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, G.; Hu, X.; Wang, W.; Du, Z.; Wang, Y.; Gong, X.-Z.; Tan, H.; Guo, F.; Tang, J. Hollow flower-like WO3@TiO2 heterojunction microspheres for the photocatalytic degradation of rhodamine B and tetracycline. RSC Adv. 2025, 15, 12629–12644. [Google Scholar] [CrossRef]
- He, X.; Gong, Y.; Niu, L.; Li, C. Development of Defect-Rich WO3-x/TiO2 Heterojunction Toward Dual-Functional Enhancement: Boosting SERS and Photocatalytic Performance. Nanomaterials 2025, 15, 521. [Google Scholar] [CrossRef]
- Castro, I.A.; Byzynski, G.; Dawson, M.; Ribeiro, C. Charge transfer mechanism of WO3/TiO2 heterostructure for photoelectrochemical water splitting. J. Photochem. Photobiol. A Chem. 2017, 339, 95–102. [Google Scholar] [CrossRef]
- Chappanda, K.N.; Smith, Y.R.; Rieth, L.W.; Tathireddy, P.; Misra, M.; Mohanty, S.K. TiO2–WO3 Composite Nanotubes from Co–Sputtered Thin Films on Si Substrate for Enhanced Photoelectrochemical Water Splitting. J. Electrochem. Soc. 2014, 161, H431–H437. [Google Scholar] [CrossRef]
- Sotelo-Vazquez, C.; Quesada-Cabrera, R.; Ling, M.; Scanlon, D.O.; Kafizas, A.; Thakur, P.K.; Lee, T.; Taylor, A.; Watson, G.W.; Palgrave, R.G.; et al. Evidence and Effect of Photogenerated Charge Transfer for Enhanced Photocatalysis in WO3/TiO2 Heterojunction Films: A Computational and Experimental Study. Adv. Funct. Mater. 2017, 27, 1605413. [Google Scholar] [CrossRef]
- Dobromir, M.; Apetrei, R.P.; Rebegea, S.; Manole, A.; Nica, V.; Luca, D. Synthesis and characterization of RF sputtered WO3/TiO2 bilayers. Surf. Coat. Technol. 2016, 285, 197–202. [Google Scholar] [CrossRef]
- Qamar, M.; Drmosh, Q.; Ahmed, M.I.; Qamaruddin, M.; Yamani, Z.H. Enhanced photoelectrochemical and photocatalytic activity of WO3-surface modified TiO2 thin film. Nanoscale Res. Lett. 2015, 10, 54. [Google Scholar] [CrossRef]
- Puddu, V.; Mokaya, R.; Li Puma, G. Novel one step hydrothermal synthesis of TiO2/WO3 nanocomposites with enhanced photocatalytic activity. Chem. Commun. 2007, 4749–4751. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; Wolcott, A.; Fitzmorris, R.C.; Zhang, J.Z.; Zhao, Y. Quasi-core-shell TiO2/WO3 and WO3/TiO2 nanorod arrays fabricated by glancing angle deposition for solar water splitting. J. Mater. Chem. 2011, 21, 10792–10800. [Google Scholar] [CrossRef]
- Silva, L.C.; Barrocas, B.; Melo Jorge, M.E.; Sério, S. Photocatalytic Degradation of Rhodamine 6G Using TiO2/WO3 Bilayered Films Produced by Reactive Sputtering. In Proceedings of the 6th International Conference on Photonics, Optics and Laser Technology, Coruña, Spain, 25–27 January 2018; SciTePress-Science and Technology Publications: Setúbal, Portugal, 2018; pp. 334–340. [Google Scholar]
- Neto, N.F.A.; de Jesus Pereira, A.L.; Leite, D.M.G.; da Silva, J.H.D.; da Silva Pelissari, M.R. Evaluation of ITO/TiO2/Co3O4 as a non-enzymatic heterojunction electrode to glucose electrooxidation. Ionics 2021, 27, 1597–1609. [Google Scholar] [CrossRef]
- Escaliante, L.C.; Azevedo Neto, N.F.; Mendoza, H.E.; Xiao, C.; Kandel, R.; da Silva, J.H.D.; Osterloh, F.E. Sputter-Coated TiO2 Films as Passivation and Hole Transfer Layers for Improved Energy Conversion with Solar Fuel WO3/CuWO4 Photoanodes. ACS Appl. Mater. Interfaces 2024, 16, 69229–69238. [Google Scholar] [CrossRef]
- Mahjabin, S.; Haque, M.; Sobayel, K.; Selvanathan, V.; Jamal, M.S.; Bashar, M.S.; Sultana, M.; Hossain, M.I.; Shahiduzzaman; Algethami, M.; et al. Investigation of Morphological, Optical, and Dielectric Properties of RF Sputtered WOx Thin Films for Optoelectronic Applications. Nanomaterials 2022, 12, 3467. [Google Scholar] [CrossRef]
- Mahjabin, S.; Mahfuzul Haque, M.; Khan, S.; Selvanathan, V.; Jamal, M.; Bashar, M.; Alkhammash, H.I.; Hossain, M.I.; Shahiduzzaman, M.; Amin, N.; et al. Effects of oxygen concentration variation on the structural and optical properties of reactive sputtered WOx thin film. Sol. Energy 2021, 222, 202–211. [Google Scholar] [CrossRef]
- Khare, C.; Sliozberg, K.; Meyer, R.; Savan, A.; Schuhmann, W.; Ludwig, A. Layered WO3/TiO2 nanostructures with enhanced photocurrent densities. Int. J. Hydrogen Energy 2013, 38, 15954–15964. [Google Scholar] [CrossRef]
- Lv, Z.; Yang, D.; Mo, J.; Jin, Z.; Chang, S. Construction of TiO2/WO3/TiO2 double heterojunction films for excellent electrochromic performance. Sci. Rep. 2024, 14, 11443. [Google Scholar] [CrossRef] [PubMed]
- Chuenkruit, A.; Thongjoon, W.; Aiempanakit, M.; Aiempanakit, C.; Aiempanakit, K. Preparation of WO3 on TiO2 Nanotubes for Electrochromic-Enhanced Photocatalytic Activity. Curr. Appl. Sci. Technol. 2025, 25, e0263966. [Google Scholar] [CrossRef]
- Mak, C.L.; Lai, B.; Wong, K.H.; Choy, C.L.; Mo, D.; Zhang, Y.L. Spectroellipsometric study of sol–gel derived potassium sodium strontium barium niobate films. J. Appl. Phys. 2001, 89, 4491–4496. [Google Scholar] [CrossRef]
- Forouhi, A.R.; Bloomer, I. Optical dispersion relations for amorphous semiconductors and amorphous dielectrics. Phys. Rev. B 1986, 34, 7018–7026. [Google Scholar] [CrossRef]
- Forouhi, A.R.; Bloomer, I. Optical properties of crystalline semiconductors and dielectrics. Phys. Rev. B 1988, 38, 1865–1874. [Google Scholar] [CrossRef]
- Eiamchai, P.; Chindaudom, P.; Pokaipisit, A.; Limsuwan, P. A spectroscopic ellipsometry study of TiO2 thin films prepared by ion-assisted electron-beam evaporation. Curr. Appl. Phys. 2009, 9, 707–712. [Google Scholar] [CrossRef]
- Madhavi, V.; Kondaiah, P.; Hussain, O.M.; Uthanna, S. Structural, Optical, and Luminescence Properties of Reactive Magnetron Sputtered Tungsten Oxide Thin Films. ISRN 2012, 2012, 801468. [Google Scholar] [CrossRef]
- Berg, S.; Nyberg, T. Fundamental understanding and modeling of reactive sputtering processes. Thin Solid Films 2005, 476, 215–230. [Google Scholar] [CrossRef]
- Thornton, J.A. Influence of apparatus geometry and deposition conditions on the structure and topography of thick sputtered coatings. J. Vac. Sci. Technol. 1974, 11, 666–670. [Google Scholar] [CrossRef]
- Kusano, E. Structure-Zone Modeling of Sputter-Deposited Thin Films: A Brief Review. Appl. Sci. Converg. Technol. 2019, 28, 179–185. [Google Scholar] [CrossRef]
- Yu, G.; Xie, X.; Pan, L.; Bao, Z.; Cui, Y. Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2013, 2, 213–234. [Google Scholar] [CrossRef]
- Boukamp, B.A. Nonlinear Least Squares Fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Lethy, K.J.; Beena, D.; Vinod Kumar, R.; Pillai, V.M.; Ganesan, V.; Sathe, V. Structural, optical and morphological studies on laser ablated nanostructured WO3 thin films. Appl. Surf. Sci. 2008, 254, 2369–2376. [Google Scholar] [CrossRef]
- Boulova, M.; Lucazeau, G. Crystallite Nanosize Effect on the Structural Transitions of WO3 Studied by Raman Spectroscopy. J. Solid State Chem. 2002, 167, 425–434. [Google Scholar] [CrossRef]
- Christou, K.; Louloudakis, D.; Vernardou, D.; Katsarakis, N.; Koudoumas, E. One-pot synthesis of WO3 structures at 95 °C using HCl. J. Solgel Sci. Technol. 2015, 73, 520–526. [Google Scholar] [CrossRef]
- Chen, W.-F.; Koshy, P.; Sorrell, C.C. Effects of film topology and contamination as a function of thickness on the photo-induced hydrophilicity of transparent TiO2 thin films deposited on glass substrates by spin coating. J. Mater. Sci. 2016, 51, 2465–2480. [Google Scholar] [CrossRef]
- Guillén C Band Gap Energy and Lattice Distortion in Anatase TiO2 Thin Films Prepared by Reactive Sputtering with Different Thicknesses. Materials 2025, 18, 2346. [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Sachs, M.; Park, J.-S.; Pastor, E.; Kafizas, A.; Wilson, A.A.; Francàs, L.; Gul, S.; Ling, M.; Blackman, C.; Yano, J.; et al. Effect of oxygen deficiency on the excited state kinetics of WO3 and implications for photocatalysis. Chem. Sci. 2019, 10, 5667–5677. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Takahashi, Y.; Akimoto, Y. Characterization of photocatalytic hybrid TiO2–WOX thin films deposited via co-sputtering. Thin Solid Films 2024, 789, 140195. [Google Scholar] [CrossRef]
- Alruwaili, M.; Roy, A.; Alhabradi, M.; Yang, X.; Chang, H.; Tahir, A.A. Heterostructured WO3–TiVO4 thin-film photocatalyst for efficient photoelectrochemical water splitting. Heliyon 2024, 10, e25446. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.-X.; Ding, S.-S.; Wang, Y.; Wang, X.-R.; Huang, W.-Q.; Li, K.; Huang, G.-F. Type-II/type-II band alignment to boost spatial charge separation: A case study of g-C3N4 quantum dots/a-TiO2/r-TiO2 for highly efficient photocatalytic hydrogen and oxygen evolution. Nanoscale 2020, 12, 6037–6046. [Google Scholar] [CrossRef]
- Anandhu, T.P.R.; Mohan, R.; Cherusseri, J.; Varma, S.J. High areal capacitance and enhanced cycling stability of binder-free, pristine polyaniline supercapacitor using hydroquinone as a redox additive. Electrochim. Acta 2022, 425, 140740. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, N.; Zhao, X.; Fang, Z.; Zhang, X.; Liu, Y.; Bai, Z.; Dou, S.; Yu, G. Hierarchical nanoarchitectured hybrid electrodes based on ultrathin MoSe2 nanosheets on 3D ordered macroporous carbon frameworks for high-performance sodium-ion batteries. J. Mater. Chem. A 2020, 8, 2843–2850. [Google Scholar] [CrossRef]
- Allen, J.B.; Larry, R. Faulkner Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Iqbal, A.; Kafizas, A.; Sotelo-Vazquez, C.; Wilson, R.; Ling, M.; Taylor, A.; Blackman, C.; Bevan, K.; Parkin, I.; Quesada-Cabrera, R. Charge Transport Phenomena in Heterojunction Photocatalysts: The WO3/TiO2 System as an Archetypical Model. ACS Appl. Mater. Interfaces 2021, 13, 9781–9793. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.-X.; Liang, Y.; Liu, X.-X.; Diamond, D.; Lau, K.-T. Electrodeposition and pseudocapacitive properties of tungsten oxide/polyaniline composite. J. Power Sources 2011, 196, 4842–4848. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S.W. Flat-Band Potential of a Semiconductor: Using the Mott–Schottky Equation. J. Chem. Educ. 2007, 84, 685. [Google Scholar] [CrossRef]
- Sivula, K. Mott–Schottky Analysis of Photoelectrodes: Sanity Checks Are Needed. ACS Energy Lett. 2021, 6, 2549–2551. [Google Scholar] [CrossRef]
- Mohd Raub, A.A.; Bahru, R.; Mohd Nashruddin, S.N.A.; Yunas, J. Advances of nanostructured metal oxide as photoanode in photoelectrochemical (PEC) water splitting application. Heliyon 2024, 10, e39079. [Google Scholar] [CrossRef]
- Bilal, S.; Akbar, A.; Shah, A.-H.A. Highly Selective and Reproducible Electrochemical Sensing of Ascorbic Acid Through a Conductive Polymer Coated Electrode. Polymers 2019, 11, 1346. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Espinosa-Angeles, J.C.; Goubard-Bretesché, N.; Quarez, E.; Payen, C.; Sougrati, M.-T.; Crosnier, O.; Brousse, T. Investigating the Cycling Stability of Fe2WO6 Pseudocapacitive Electrode Materials. Nanomaterials 2021, 11, 1405. [Google Scholar] [CrossRef]
- Aydın, H.; Kurtan, U.; Demir, M.; Karakuş, S. Synthesis and Application of a Self-Standing Zirconia-Based Carbon Nanofiber in a Supercapacitor. Energy Fuels 2022, 36, 2212–2219. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.-W.; Zhang, J.-F.; Wu, D.; Tian, Z.-Q.; Tian, Z.-W.; Shi, K. New Strategy for Electrochemical Micropatterning of Nafion Film in Sulfuric Acid Solution. Electrochim. Acta 2014, 146, 125–133. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Zahedi, F. Electrochemical reduced graphene oxide-polyaniline as effective nanocomposite film for high-performance supercapacitor applications. Electrochim. Acta 2017, 245, 575–586. [Google Scholar] [CrossRef]
- Shen, C.; Xu, S.; Xie, Y.; Sanghadasa, M.; Wang, X.; Lin, L. A Review of On-Chip Micro Supercapacitors for Integrated Self-Powering Systems. J. Microelectromech. Syst. 2017, 26, 949–965. [Google Scholar] [CrossRef]
- Yang, W.; Prabhakar, R.R.; Tan, J.; Tilley, S.D.; Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 2019, 48, 4979–5015. [Google Scholar] [CrossRef]
- Chiu, M.; Tseng, W.; Tang, H.; Chang, Y.; Chen, C.; Hsu, W.; Chang, W.; Wu, C.; Li, L. Band Alignment of 2D Transition Metal Dichalcogenide Heterojunctions. Adv. Funct. Mater. 2017, 27, 1603756. [Google Scholar] [CrossRef]
- O’Hayre, R.; Nanu, M.; Schoonman, J.; Goossens, A. Mott−Schottky and Charge-Transport Analysis of Nanoporous Titanium Dioxide Films in Air. J. Phys. Chem. C 2007, 111, 4809–4814. [Google Scholar] [CrossRef]
- Oliva, F.Y.; Avalle, L.B.; Santos, E.; Cámara, O.R. Photoelectrochemical characterization of nanocrystalline TiO2 films on titanium substrates. J. Photochem. Photobiol. A Chem. 2002, 146, 175–188. [Google Scholar] [CrossRef]
- Lee, K.K.; Chin, W.S.; Sow, C.H. Cobalt-based compounds and composites as electrode materials for high-performance electrochemical capacitors. J. Mater. Chem. A 2014, 2, 17212–17248. [Google Scholar] [CrossRef]
- Kavan, L.; Tétreault, N.; Moehl, T.; Grätzel, M. Electrochemical Characterization of TiO2 Blocking Layers for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 16408–16418. [Google Scholar] [CrossRef]
- Bolts, J.M.; Wrighton, M.S. Correlation of photocurrent-voltage curves with flat-band potential for stable photoelectrodes for the photoelectrolysis of water. J. Phys. Chem. 1976, 80, 2641–2645. [Google Scholar] [CrossRef]
- Gillet, M.; Lemire, C.; Gillet, E.; Aguir, K. The role of surface oxygen vacancies upon WO3 conductivity. Surf. Sci. 2003, 532–535, 519–525. [Google Scholar] [CrossRef]
- Semikina, T.V. Optical properties of dielectric layers with CeO2. Semicond. Phys. Quantum Electron. Optoelectron. 2004, 7, 291–296. [Google Scholar] [CrossRef]
- Mayadas, A.F.; Shatzkes, M. Electrical-Resistivity Model for Polycrystalline Films: The Case of Arbitrary Reflection at External Surfaces. Phys. Rev. B 1970, 1, 1382–1389. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium dioxide (TiO2)-based photocatalyst materials activity enhancement for contaminants of emerging concern (CECs) degradation: In the light of modification strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Kasap, S.O.; Kabir, M.Z.; Ramaswami, K.O.; Johanson, R.E.; Curry, R.J. Charge collection efficiency in the presence of non-uniform carrier drift mobilities and lifetimes in photoconductive detectors. J Appl Phys 2020, 128, 124501. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.A. Real surface area measurements in electrochemistry. Pure Appl. Chem. 1991, 63, 711–734. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef]
- Nanda, J.; Augustyn, V. Transition Metal Oxides for Electrochemical Energy Storage; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Wang, S.; Wan, K.; Feng, J.; Yang, Y.; Wang, S. BiVO4 photoanodes with enhanced photoelectrochemical performance: Preparation, modification and emerging applications. J. Mater. Sci. Technol. 2025, 217, 182–220. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel–Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Huang, S.; Bao, R.; Wang, J.; Yi, J.; Zhang, Z.; Liu, L.; Han, Y.; Li, Z.; Min, D.; Zhang, W.; et al. Synergistic effect of oxygen vacancy defects and TiO2/WO3 heterostructures in photocatalytic hydrogen production and dye degradation. J. Alloys Compd. 2023, 961, 170945. [Google Scholar] [CrossRef]
- Zhang, X.; Chi, Z.; Xu, B.; Jiang, L.; Zhou, X.; Zhang, Y.; Liu, S.; Xu, J. Multifunctional organic fluorescent materials derived from 9,10-distyrylanthracene with alkoxyl endgroups of various lengths. Chem. Commun. 2012, 48, 10895–10897. [Google Scholar] [CrossRef]
- Shao, H.; Lin, Z.; Xu, K.; Taberna, P.-L.; Simon, P. Electrochemical study of pseudocapacitive behavior of Ti3C2Tx MXene material in aqueous electrolytes. Energy Storage Mater. 2019, 18, 456–461. [Google Scholar] [CrossRef]
- Speldrich, S.; Wark, M.; Wittstock, G. Metal Oxide Protection Layers for Enhanced Stability and Activity of WO3 Photoanodes in Alkaline Media. ACS Appl. Energy Mater. 2023, 6, 9602–9614. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, M.; Rodríguez-Gutiérrez, I.; Vega-Poot, A.; García-Rodríguez, R.; Rodríguez-Gattorno, G.; Oskam, G. Charge transfer and recombination kinetics at WO3 for photoelectrochemical water oxidation. Electrochim. Acta 2017, 258, 900–908. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ho, H.H.; Zahran, Z.N.; Chandra, D.; Katsuki, T.; Tsubonouchi, Y.; Hoshino, N.; Shiroishi, H.; Ishizaki, M.; Kurihara, M.; Chu, H.M.; et al. Highly stable and efficient photoelectrochemical water oxidation at an anisotropically crystallized monoclinic WO3 film with predominant growth of the (202) plane. J. Mater. Chem. A 2025, 13, 17898–17909. [Google Scholar] [CrossRef]
- Jiang, H.; Hong, J.J.; Wu, X.; Surta, T.W.; Qi, Y.; Dong, S.; Li, Z.; Leonard, D.P.; Holoubek, J.J.; Wong, J.C.; et al. Insights on the Proton Insertion Mechanism in the Electrode of Hexagonal Tungsten Oxide Hydrate. J. Am. Chem. Soc. 2018, 140, 11556–11559. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications. In Modern Aspects of Electrochemistry; Kluwer Academic Publishers: Boston, MA, USA, 2002; pp. 143–248. [Google Scholar]
- Knöppel, J.; Kormányos, A.; Mayerhöfer, B.; Hofer, A.; Bierling, M.; Bachmann, J.; Thiele, S.; Cherevko, S. Photocorrosion of WO3 Photoanodes in Different Electrolytes. ACS Phys. Chem. Au 2021, 1, 6–13. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, W.; Huang, Y.; Zhi, C. Proton-Insertion-Enhanced Pseudocapacitance Based on the Assembly Structure of Tungsten Oxide. ACS Appl. Mater. Interfaces 2014, 6, 18901–18910. [Google Scholar] [CrossRef]
- Logar, A.; Kozlica, D.K.; Vodeb, O.; Gaberšček, M.; Hodnik, N.; Strmčnik, D. Bubble Trouble: Quantifying the Effects of Bubbles on the Electrochemical Interface. ACS Catal. 2025, 15, 6380–6385. [Google Scholar] [CrossRef]
- Grigioni, I.; Di Liberto, G.; Dozzi, M.V.; Tosoni, S.; Pacchioni, G.; Selli, E. WO3/BiVO4 Photoanodes: Facets Matching at the Heterojunction and BiVO4 Layer Thickness Effects. ACS Appl. Energy Mater. 2021, 4, 8421–8431. [Google Scholar] [CrossRef]
- Resasco, J.; Zhang, H.; Kornienko, N.; Becknell, N.; Lee, H.; Guo, J.; Briseno, A.L.; Yang, P. TiO2/BiVO4 Nanowire Heterostructure Photoanodes Based on Type II Band Alignment. ACS Cent. Sci. 2016, 2, 80–88. [Google Scholar] [CrossRef]
- Al-Aisaee, N.; Alhabradi, M.; Yang, X.; Alruwaili, M.; Rasul, S.; Tahir, A.A. Fabrication of WO3 heterostructure photoanode by PVD for photoelectrochemical applications. Sol. Energy Mater. Sol. Cells 2023, 263, 112561. [Google Scholar] [CrossRef]
- Salimi, R.; Sabbagh Alvani, A.A.; Mei, B.T.; Naseri, N.; Du, S.F.; Mul, G. Ag-Functionalized CuWO4/WO3 nanocomposites for solar water splitting. New J. Chem. 2019, 43, 2196–2203. [Google Scholar] [CrossRef]
- Ahmed, K.; Wang, Y.; Bai, Y.; Sekar, K.; Li, W. A carbon nanowire-promoted Cu2O/TiO2 nanocomposite for enhanced photoelectrochemical performance. New J. Chem. 2022, 46, 15495–15503. [Google Scholar] [CrossRef]
- Kim, K.; Moon, J.H. Three-Dimensional Bicontinuous BiVO4/ZnO Photoanodes for High Solar Water-Splitting Performance at Low Bias Potential. ACS Appl. Mater. Interfaces 2018, 10, 34238–34244. [Google Scholar] [CrossRef]
- Noh, E.; Noh, K.-J.; Yun, K.-S.; Kim, B.-R.; Jeong, H.-J.; Oh, H.-J.; Jung, S.-C.; Kang, W.-S.; Kim, S.-J. Enhanced Water Splitting by Fe2 O3-TiO2-FTO Photoanode with Modified Energy Band Structure. Sci. World J. 2013, 2013, 723201. [Google Scholar] [CrossRef]
| Deposition Parameter | Value |
|---|---|
| Residual Pressure (Torr) | 1.0 × 10−5 |
| Working Pressure (Torr) | 3.0 × 10−3 |
| WOx Layer | |
| DC Power (W) | 150 |
| ΦAr (sccm) | 10 |
| ΦO2 (sccm) | 10 |
| Deposition Time (min) | 0, 5, 10, 20, 30 |
| TiO2 Layer | |
| DC Power (W) | 300 |
| ΦAr (sccm) | 10 |
| ΦO2 (sccm) | 2 |
| Deposition Time (min) | 30 |
| Samples | Medium Length (nm) |
|---|---|
| 5 min | 29.5 ± 8 |
| 10 min | 47.9 ± 7 |
| 20 min | 38.5 ± 5 |
| 30 min | 46.5 ± 3 |
| WO3 Deposition Time (min) | Structure | a (Å) | b (Å) | c (Å) | β (°) | V (Å3) | ρ (g.cm−3) | D (nm) | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| 0 | TiO2 | 3.7849 ± 5 | - | 9.5744 ± 29 | - | 137.16 ± 42 | 3.87 ± 1 | - | 4.74 |
| 5 | WO3 | 7.3286 ± 8 | 7.5465 ± 8 | 7.5076 ± 18 | 91.83 ± 2 | 415.00 ± 12 | 7.42 ± 2 | - | 2.01 |
| TiO2 | 3.7923 ± 9 | - | 9.5538 ± 2 | - | 137.40 ± 4 | 3.86 ± 1 | |||
| 10 | WO3 | 7.3266 ± 15 | 7.5159 ± 12 | 7.6781 ± 16 | 90.39 ± 1 | 422.79 ± 14 | 7.29 ± 2 | 36 ± 3 | 2.11 |
| TiO2 | 3.7840 ± 10 | - | 9.5421 ± 11 | - | 136.63 ± 5 | 3.88 ± 5 | - | ||
| 20 | WO3 | 7.2873 ± 2 | 7.5497 ± 3 | 7.6517 ± 4 | 90.65 ± 1 | 420.95 ± 3 | 7.32 ± 1 | 44 ± 5 | 1.88 |
| TiO2 | 3.8175 ± 1 | - | 9.5034 ± 6 | - | 138.50 ± 1 | 3.84 ± 1 | - | ||
| 30 | WO3 | 7.3274 ± 1 | 7.5366 ± 2 | 7.7022 ± 2 | 90.67 ± 1 | 425.32 ± 2 | 7.24 ± 3 | 41 ± 1 | 1.97 |
| TiO2 | 3.7930 ± 6 | - | 9.5576 ± 7 | - | 137.50 ± 3 | 3.86 ± 3 | - |
| Samples | VFB (V) | Nd,app (×1019 cm−3) |
|---|---|---|
| 0 min | 1.24 ± 2 | 10.26 ± 7 |
| 5 min | 0.074 ± 9 | 6.37 ± 3 |
| 10 min | 0.29 ± 1 | 3.68 ± 3 |
| 20 min | 0.56 ± 2 | 3.30 ± 4 |
| 30 min | 0.41 ± 2 | 8.32 ± 9 |
| WOx | 0.36 ± 1 | 5.34 ± 3 |
| Samples | CPE1 | CPE2 | Χ2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Y0 (µS·sn) | n | Y0 (µS·sn) | n | ||||||
| 0 min | 22.3 | 144 | - | - | 15.2 | 0.933 | - | - | 0.300 |
| 5 min | 17.3 | 83.2 | - | - | 17.80 | 0.940 | - | - | 0.135 |
| 10 min | 17.70 | 13.2 | - | - | 25.00 | 0.918 | - | - | 0.233 |
| 20 min | 16.70 | 1.51 × 103 | - | - | 22.40 | 0.933 | - | - | 0.231 |
| 30 min | 13.90 | 355 | - | - | 27.30 | 0.946 | - | - | 0.052 |
| WOx | 1.49 | 17.4 | 7.93 | 232 | 46.7 | 0.604 | 62.70 | 0.965 | 0.022 |
| Samples | Rs1 ( | CPE1 | CPE2 | Χ2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Y0 (µS·sn) | n | Y0 (µS·sn) | n | ||||||
| 0 min | 20.5 | 0.658 | 1.49 | 7.51 × 10−3 | 46.1 | 0.964 | 111.0 | 0.807 | 0.060 |
| 5 min | 16.40 | 9.15 | - | - | 313.0 | 0.741 | - | - | 0.436 |
| 10 min | 3.66 | 7.66 × 10−3 | 9.54 | 6.20 | 59.0 | 0.703 | 340 | 0.807 | 0.005 |
| 20 min | 16.40 | 14.2 | - | - | 323.0 | 0.832 | - | - | 0.019 |
| 30 min | 13.7 | 8.05 | - | - | 386 | 0.866 | - | - | 0.009 |
| WOx | 6.76 | 15.8 | 3.04 | 15.7 | 36.5 | 0.629 | 172.0 | 0.895 | 0.005 |
| Samples (Without Lighting) | Cdl (µF) | ECSA (Cs = 25) cm2 | ECSA (Cs = 40) cm2 | ECSA (Cs = 45) cm2 |
|---|---|---|---|---|
| 0 min | 4.17 × 10−2 | 1.67 × 10−3 | 1.04 × 10−3 | 9.26 × 10−3 |
| 30 min | 79.2 | 3.17 | 1.98 | 1.76 |
| Samples (With Lighting) | Cdl (µF) | ECSA (Cs = 25) cm2 | ECSA (Cs = 40) cm2 | ECSA (Cs = 45) cm2 |
| 0 min | 70.5 | 2.82 | 1.76 | 1.57 |
| 30 min | 215.1 | 8.6 | 5.37 | 4.78 |
| Samples | CPE | Χ2 | |||
|---|---|---|---|---|---|
| Y0 (µS·sn) | n | ||||
| 30 min-EIS (1) | 11.1 | 377 | 95.7 | 0.867 | 0.003 |
| 30 min-EIS (2) | 12.5 | 395 | 130 | 0.868 | 0.001 |
| 30 min-EIS (3) | 18.0 | 420 | 120 | 0.869 | 0.009 |
| System | Electrolyte | J (1.23 VRHE (mA·cm-2)) | VFB (VRHE) | ND/NA (cm-3) | Reference |
|---|---|---|---|---|---|
| TiO2/WOx | 0.5 M Na2SO4 | ~0.19 | 0.41 | 8.32 × 1019 | This work |
| WO3/BiVO4 | 0.5 M Na2SO4 | ~1.0 | — | — | [97] |
| TiO2/BiVO4 (Ta:TiO2 NWs) | 0.5 M Na2SO4 | 2.1 | TiO2 ≈ 0.19; BiVO4 ≈ 0.08 | Ta:TiO2 ~1 × 1020 | [98] |
| CuWO4/WO3/Ag | 0.5 M phosphate | 0.205 | -0.045 | ↑ vs. single phases | [100] |
| WO3/Fe2O3 (nanorods, PVD/RF) | 0.1 M phosphate | 0.588 | 0.49 | ~9.57 × 1020 | [99] |
| Cu2O/TiO2 (p–n) | 1 M NaOH | 0.139 | TiO2: 0.76; Cu2O: 0.54; Cu2/TiO2 –0.72; | Cu2O: 5.36 × 1018; TiO2: 1.06 × 1019; TiO2+C-NW: 1.30 × 1019 | [101] |
| BiVO4/ZnO (3D bicontinuous) | 0.5 M Na2SO4 | 4.1 | — | — | [102] |
| Fe2O3/TiO2 | 1 M NaOH | ~5.8 | — | — | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo, L.D.; Sartori, B.; Torres, M.D.M.; Graves, D.A.; Botan-Neto, B.D.; Murase, M.S.W.; Neto, N.F.A.; Leite, D.M.G.; Pessoa, R.S.; da Silva Sobrinho, A.S.; et al. Tuning Optical and Photoelectrochemical Properties of TiO2/WOx Heterostructures by Reactive Sputtering: Thickness-Dependent Insights. Nanomanufacturing 2025, 5, 15. https://doi.org/10.3390/nanomanufacturing5040015
Araujo LD, Sartori B, Torres MDM, Graves DA, Botan-Neto BD, Murase MSW, Neto NFA, Leite DMG, Pessoa RS, da Silva Sobrinho AS, et al. Tuning Optical and Photoelectrochemical Properties of TiO2/WOx Heterostructures by Reactive Sputtering: Thickness-Dependent Insights. Nanomanufacturing. 2025; 5(4):15. https://doi.org/10.3390/nanomanufacturing5040015
Chicago/Turabian StyleAraujo, Lucas Diniz, Bianca Sartori, Matheus Damião Machado Torres, David Alexandro Graves, Benedito Donizeti Botan-Neto, Mariane Satomi Weber Murase, Nilton Francelosi Azevedo Neto, Douglas Marcel Gonçalves Leite, Rodrigo Sávio Pessoa, Argemiro Soares da Silva Sobrinho, and et al. 2025. "Tuning Optical and Photoelectrochemical Properties of TiO2/WOx Heterostructures by Reactive Sputtering: Thickness-Dependent Insights" Nanomanufacturing 5, no. 4: 15. https://doi.org/10.3390/nanomanufacturing5040015
APA StyleAraujo, L. D., Sartori, B., Torres, M. D. M., Graves, D. A., Botan-Neto, B. D., Murase, M. S. W., Neto, N. F. A., Leite, D. M. G., Pessoa, R. S., da Silva Sobrinho, A. S., & Pereira, A. L. J. (2025). Tuning Optical and Photoelectrochemical Properties of TiO2/WOx Heterostructures by Reactive Sputtering: Thickness-Dependent Insights. Nanomanufacturing, 5(4), 15. https://doi.org/10.3390/nanomanufacturing5040015











