Abstract
Two new species of Glenurus Hagen, 1866 are herein described. Glenurus maya sp. n. is described from Southern Mexico and Guatemala, and G. oswaldi sp. n. from the Antilles. A lectotype for G. heteropteryx Gerstaecker, 1885 is determined, and G. discors Navás, 1920 is revalidated as a valid species. Additionally, the previously described species known to Mexico and Central America are redescribed, and larvae of two species are described for the first time. Digital images and distributional maps are provided for all treated species, and an identification key to the genus is provided.
1. Introduction
Myrmeleontidae is the largest family of the order Neuroptera, with nearly 2120 valid species distributed around the world [1]. These insects are frequently associated with arid and semiarid environments due to the fossorial lifestyle commonly adopted by larvae of the majority of species [2], and although many species simply inhabit areas with an abundance of sandy and dry, loose earth, others are adapted to explore a whole myriad of different habitats [3,4,5]. Glenurini, the only tribe of the Nemoleontinae occurring in the New World, comprises 118 species of Nearctic and Neotropical antlions [6], and its species are particularly known to explore many different habitats in the larval stage. Dimarella Banks, 1913 inhabits loose sand at the base of tree roots [7]; Navasoleon Banks, 1943 lives on bare rock surfaces [8]; Ripalda Navás, 1915 can be found on caves on forest areas [5]; and Eremoleon Banks, 1901 can be found in habitats ranging from loose sand on top of rock crevices, to termite frass, bat guano, animal burrows, and cave entrances [4].
One of the most striking genera of the Glenurini is Glenurus Hagen, 1866 (Figure 1 and Figure 2). The adults are relatively large, with densely marked wings with subapical dark bands and whitish or sometimes pinkish apexes [9,10,11,12,13]. Glenurus larvae (Figure 1e) are known to inhabit particulate organic matter inside tree holes, underneath rock overhangs or exposed tree roots, and animal burrows such as Gopher tortoise burrows [14,15]. The genus currently comprises nine species distributed from the United States to Argentina, although there are still two Asian species classified in this genus (G. atomatus Yang, 1986 and G. posticus Navás, 1913), but they should be transferred to different genus or genera as pointed out by Stange [16], Machado [10], and later demonstrated by Zheng and Liu [17].
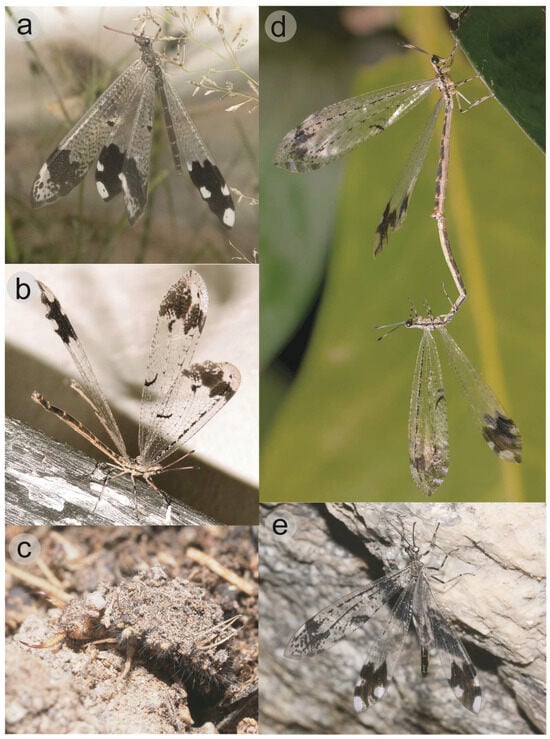
Figure 1.
Live specimens of Glenurus. (a) Glenurus luniger, (b) G. proi by Cheryl Harleston López Espino, (c) G. proi larva by Barry Sullender, (d) G. proi copulating pair by Francisco Alberto Sánchez Almaguer, (e) G. snowii by James Bailey.
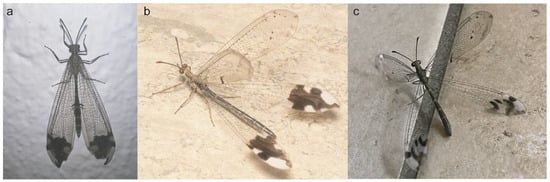
Figure 2.
Live specimens of Glenurus. (a) Glenurus heteropteryx by fpcojeisonfarenas (iNaturalist), (b) G. discors by Don Marsille, (c) G. oswaldi sp. n. by cricri dendrobium (iNaturalist).
Despite being large and charismatic and the increased interest in the literature in the last years [9,10,15,18,19,20], many of the Glenurus species still lack an extensive taxonomic treatment. Images of many species are still rare, the taxonomic keys provided for identification are all regional in nature [10,12,20,21,22,23], and original descriptions are focused and almost fully based on wing markings. Furthermore, for only five out of the nine species, the biology of their larval stage is known [15], but none has been properly described before.
Among the genus extensive distribution, Brazil, Costa Rica, Mexico, and the United States are tied with largest diversity with three species each [1]. However, the analysis of Neotropical specimens from the Colección Nacional de Insectos (CNIN) and Florida State Collection of Arthropods (FSCA) revealed a new species from Mexico and Guatemala, elevating Mexico as the country with the largest Glenurus diversity, with four species. Moreover, the examination of the G. heteropteryx syntypes and specimens revealed that three somewhat cryptic species were under the same name (Figure 2 and Figure 3), leading to the reanalysis of previous synonyms of G. heteropteryx and the description of a new species from the Antilles.
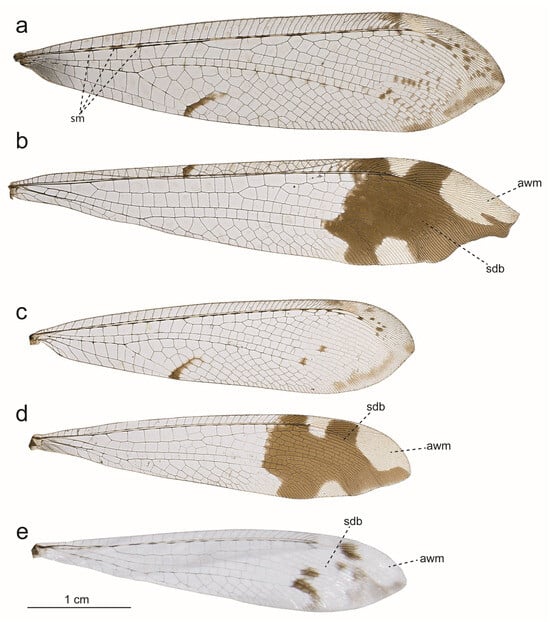
Figure 3.
Fore and hind wings of Glenurus species without a subapical dark band on forewing. (a) Forewing and (b) hind wing of G. heteropteryx; (c) forewing and (d) hind wing of G. discors; (e) hind wing of G. oswaldi sp. n. Abbreviations: awm: apex white mark, sdb: subapical dark band, sm: subcostal markings.
In this sense, we aim to redescribe and investigate the Glenurus species distributed throughout Mexico and Central America, describe the new species, and produce an identification key for all Glenurus species.
2. Materials and Methods
44 specimens were examined, including types and specimens from prior collecting events and deposited at the entomological collections of Colección Nacional de Insectos (Mexico City, Mexico), Colección de Insectos de la Universidad Autónoma de Querétaro, Museum of Comparative Zoology, Museo Entomológico de León, and Florida State Collection of Arthropods. The selection of these specific collections was based on their known holdings of type specimens and historically relevant material for the species treated, as well as logistical accessibility to the authors. Types were analyzed through high-definition photographs sent by their respective institutions. The specimens were identified using the keys provided by Banks [24] and Stange [13], and comparison with types and original descriptions. For the study of genital structures, last four abdominal segments were removed and cleared with a 10% KOH solution, washed with distilled water, 10% acetic acid, and 70% alcohol, following the protocol of Cummings [25]. Cleared terminalias were then stored in microvials with glycerin and kept together with the respective adult specimens. Series of high-resolution photographs were taken using a Discovery V8 stereomicroscope or a Carl Zeiss AxioZoom V16 with an AxioCam 305 camera (Carl Zeiss, Oberkochen, Germany), both stereomicroscopes with a stacking system. Final images and plates were edited using Adobe Photoshop 2021.
General morphology terminology follows Stange [26], Machado [10], and Marquez-Lopez et al. [27]; wing venation follows Breitkreuz et al. [28] and wing spaces are from Machado and Oswald [29]. Distribution maps were made in QGIS software version 3.34.14 with the Neotropical region and their provinces from Morrone [30] and Morrone et al. [31] and for Nearctic regions and their provinces were taken from data published in the CONABIO Geoportal. Maps were constructed with original records from specimens examined from CNIN, IBUNAM (Mexico City), and FSCA. Distribution data published in GBIF [32] and iNaturalist were also downloaded for a more complete treatment of known distribution information of the species. Distributional data from iNaturalist was curated by the authors, where each individual identification was verified and confirmed before downloading. New records are highlighted in bold on each species distribution section, and new records prevenient from iNaturalist observations were marked with a *. The identification key was produced adapting the keys in Banks [24], Stange [13], Petko et al. [20], Machado [10], and original steps.
3. Results
3.1. Taxonomy
3.1.1. Glenurus Hagen
- Glenurus Hagen, 1866: 372 [33] [Type species Formicaleo grata Say, designated by Banks, 1927: 67]. Hagen, 1873: 392 [34] [morphology]. Banks, 1922: 58 [21] [key]. Banks, 1927: 67 [24] [description, type designation, key]. Stange, 1970: 11, 22 [35] [description, distribution, species list, taxonomy]. Penny, 1977: 44 [36] [distribution, species list]. Penny et al., 1997: 77 [37] [distribution, species list, taxonomy]. Stange, 2000 [15] [larvae habitat and biology]. Stange in Penny, 2002: 284 [13] [biology, diagnosis]. Stange, 2004: 177 [16] [distribution, species list, taxonomy]. Ardila-Camacho, 2014 [9] [discussion]. Badano et al., 2018: 936, 937 [5] [phylogeny]. Machado et al., 2019: 8 [6] [phylogeny]. Machado, 2020 [10] [discussion]. Machado and Martins, 2023 [38] [genera list]. Oswald, 2025 [1] [genera list].
- = Ledoscius Navás, 1918: 493 [39] [Type species Ledoscius penningtoni Navás, by original designation and monotypy]. Navás, 1924: 107 [40] [synonymy].
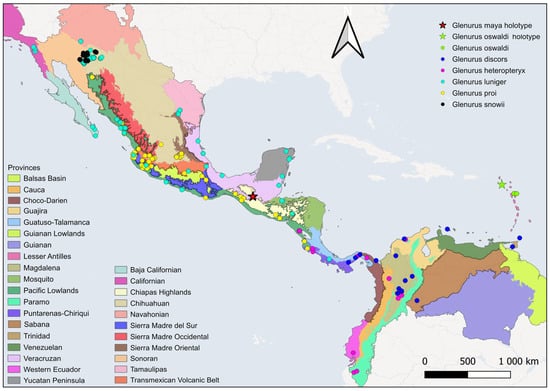 Figure 4. Distributional map with data from iNaturalist for the Glenurus species treated in this work. Map from CONABIO’s GeoPortal (2025), Sistema Nacional de Información sobre Biodiversidad (SNIB): http://www.conabio.gob.mx/informacion/gis/ (accessed on 29 November 2025).
Figure 4. Distributional map with data from iNaturalist for the Glenurus species treated in this work. Map from CONABIO’s GeoPortal (2025), Sistema Nacional de Información sobre Biodiversidad (SNIB): http://www.conabio.gob.mx/informacion/gis/ (accessed on 29 November 2025).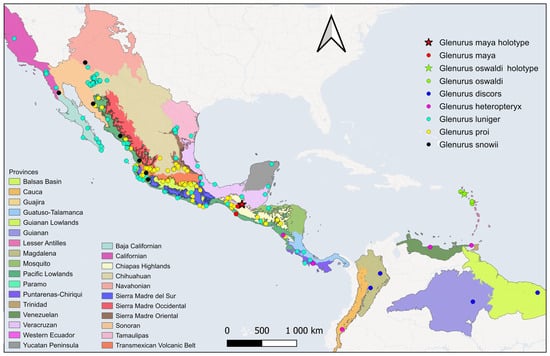 Figure 5. Distributional map with data from collections for the Glenurus species treated in this work. Map from CONABIO’s GeoPortal (2025), Sistema Nacional de Información sobre Biodiversidad (SNIB): http://www.conabio.gob.mx/informacion/gis/ (accessed on 29 November 2025).
Figure 5. Distributional map with data from collections for the Glenurus species treated in this work. Map from CONABIO’s GeoPortal (2025), Sistema Nacional de Información sobre Biodiversidad (SNIB): http://www.conabio.gob.mx/informacion/gis/ (accessed on 29 November 2025).- Included species. G. croesus Banks, 1922; G. discors Navás, 1920; G. gratus (Say, 1839); G. heteropteryx Gerstaecker, 1885; G. incalis Banks, 1922; G. luniger Gerstaecker, 1893; G. maya sp. n.; G. oswaldi sp. n.; G. peculiaris (Walker, 1860); G. penningtoni (Navás, 1918); G. proi Navás, 1930; G. snowii Banks, 1907.
- Larvae known. G. gratus, G. luniger, G. proi.
- Diagnosis. Antennae long and slender, nearly as long as the combined length of the head and thorax; pronotum longer than wide; legs slender and elongated, fore femur nearly as long as the combined length of the head and prothorax; hind leg slightly longer than other legs; tibial spurs long and well-developed, reaching at least tarsomere II, barely curved, mostly curving at tip; midtibiae slightly bent; fore and hind wings with a subapical dark brown band from pterostigma to wing posterior margin (absent in the forewing of G. discors, G. heteropteryx, and G. oswaldi sp. n.); forewing RP origin distal to CuA fork; cubital area with a large dark brown stripe or large spot between anterior and posterior fork branches, and near end of posterior CuA fork branch; poststigmal area in both wings whitish; forewing anterior banksian line present or absent; hind wing posterior wing margin below rhegma with a whitish area (absent in G. croesus); female posterior gonapophysis very short, with posterior margin roundish; female lateral gonapophysis and ectoproct with some stout acicular setae; larvae mandible with two teeth; larvae palpi with three segments; rastra absent.
- Remarks. The adult of Glenurus is easily recognizable from other Glenurini genera by the presence of subapical dark bands on their hind wings (Figure 3). In G. oswaldi sp. n. the hind wing subapical bands might be broken [18] or barely visible, but the whitish poststigmal area and the short and weakly produced posterior gonapophysis reinforce the genus identification. Some Ripalda species also bear a whitish poststigmal area [5], but the posterior gonapophysis is very different, as well as the wing marking patterns and larvae morphology. The larvae of Glenurus can be readily distinguished from other genera of Glenurini by possessing only two mandibular teeth instead of three, and the labial palpi has three segments.
This genus was historically regarded as morphologically uniform [13,35], and this was reflected in recent phylogenies which recovered Glenurus as monophyletic, both with morphological [5] and molecular data [6]. Despite the apparent morphological uniformity of Glenurus species, some characters appear to be useful for distinguishing species, such as fore and hind wing pigmentation (mainly on subcostal, mediocubital areas, and wing apex), apex white markings shape, and frons and leg coloration.
- Biology. Larvae biology has been commented before in the literature [3,11,13,14,15]. All known larvae are associated with particulate decomposed matter, sawdust and dry wood debris, either inside tree holes, animal burrows and/or underneath rock overhangs, fallen logs or tree stumps. Glenurus proi larvae is known to inhabit large ant mounds as well (Figure 1e). Glenurus croesus, G. discors, G. heteropteryx, G. incalis, G. maya sp. n., G. oswaldi sp. n., and G. peculiaris larvae are still undiscovered. The feeding habits of G. gratus has been mentioned by [14], suggesting that larvae can be specific when choosing prey. Adult biology, on the other hand, has not been described in detail before. Oviposition habits are unknown [11], although adults have been described to fly often after midnight [13].
3.1.2. Glenurus discors Navás
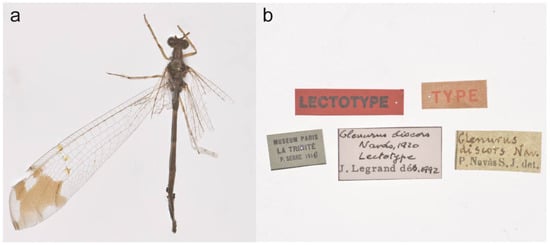
Figure 6.
Glenurus discors holotype. (a) Female holotype, (b) type labels. J. Legrand label reads as “Glenurus discors Navás, 1920 Lectotype J. Legrand det. 1992”.
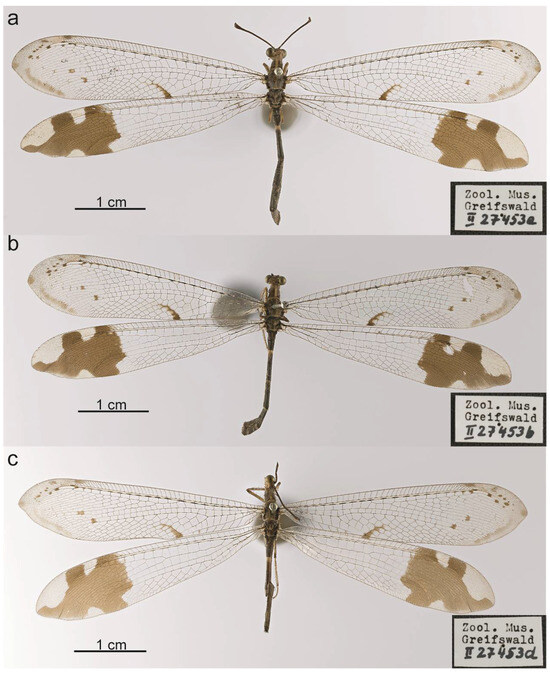
Figure 7.
Glenurus heteropteryx paralectotypes, in agreement with G. discors diagnosis. (a) Specimen II27453a, (b) specimen II27453b, (c) specimen II27453d.
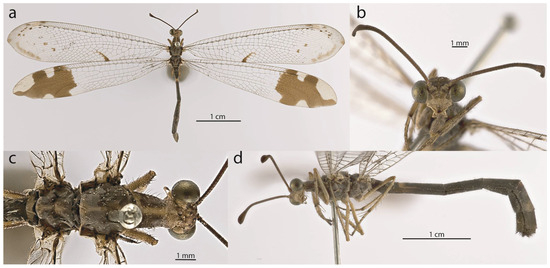
Figure 8.
Glenurus discors. (a) G. heteropteryx paralectotype habitus, (b) frons, (c) thorax, dorsal view, (d) thorax, legs, and abdomen; lateral view.
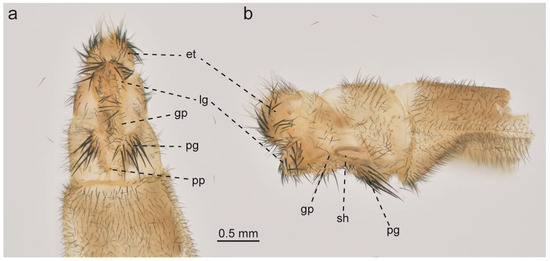
Figure 9.
Glenurus discors, female genitalia. (a) ventral view, (b) lateral view. Abbreviations: et: ectoproct, gp: gonapophyseal plate, lg: lateral gonapophysis, pg: posterior gonapophysis, pp: pregenital plate, sh: spermatheca.
- Glenurus discors Navás, 1920: 202 [41] [Holotype: female, La Trinité (MNHN)]. Banks, 1922: 58 [21] [taxonomy]. Navás 1923: 187 [42] [taxonomy]. Stange 1970: 22 [35] [synonym]. Penny 1977: 44 [36] [distribution]. Stange 2004: 178 [16] [distribution, taxonomy, type].
- Distribution. Panama (Gerstaecker 1885), France: Martinique (Navás 1920), Colombia (Ardilla-Camacho 2014), Brazil (Machado 2020), French Guyana, Curaçao *, Trinidad *, Saint Lucia *.
- Diagnosis. Pronotum dark brown with a medial longitudinal pale stripe, and two weak sublateral pale brown bands; forewing subcostal area hyaline; forewing subapical dark band absent, forewing apex with a dark brown spot at rhegma and another dark brown spot on the area above, and with a few small, smaller spots throughout apex membrane, which has a whitish hue; hind wing subapical dark band complete, with apex white mark oblong or falcate.
- Description. Head: Antennae clubbed with 38–42 flagellomeres, dark brown. Flagellomeres dark brown, distally lighter. Vertex slightly raised, brown, anterior row with two sublateral dark brown marks and two dark brown marks medially that connect with each other and with middle row. Middle row with four dark brown marks, and posterior row with two sublateral and two medial oblong dark brown patches. Vertex with setae short dark decumbent setae, a few scattered short white setae near epicranial mark, which is dark brown. Interantennal area brown. Frons brown near antennae base, fading to light brown near clypeus. Frons with few scattered delicate white setae. Interocular distance as broad as scape diameter. Clypeus brown with rows of long dark setae at frontoclypeal margin. Labrum brown with a row of setae at distal margin. Mandible well developed, dark, slightly less dark at exterior margins. Palpi brown, and dark brown near joints, with distal palpomere dark brown and fusiform. Palpimacula round. Thorax: Thorax mostly dark brown. Pronotum longer than wide, dark brown, with a narrow pale longitudinal sublateral line at each lateral margin, a pale longitudinal line at middle, and sometimes two subapical pale spots near furrow. Pronotum with some short dark setae, and long, anteriorly curved setae at lateral margins. Prescutum dark, with two pale spots on lateroposterior margins. Prescutum with short dark setae and a few erect, delicate setae. Mesoscutum dark, and some scattered short dark setae. Mesoscutellum dark, slightly paler on posterior margin, and a narrow pale longitudinal line starting subapically and broadening until posterior margin. Mesoscutellum with a few decumbent white setae near posterolateral margin. Metaprescutum dark with a pale narrow longitudinal line at middle, broadening near posterior margins. Metascutum dark, with velvety patches at middle. Metascutellum dark, with a pale narrow longitudinal line at middle, broadening near posterior margins, with white setae pointed posteriorly. Thorax laterally dark, with meso and metepisternum darker dorsally and paler ventrally, with many scattered white setae. Wings: Wings broad, lanceolate. Forewing slightly shorter than hind wing, in repose the apex of the hind wing extends after forewing. Forewing: veins dark brown dashed with pale brown. Membrane hyaline before apex, except around a few scattered crossveins that are surrounded by a brown tint. Costal area simple. Subcostal area hyaline. Presectoral area with eight crossveins. Cubital area with a large brown oblique streak at the end of the second branch of CuA fork. Pterostigma whitish to pinkish. Apex with veins and membrane with a whitish hue, with no subapical dark band, a dark brown spot at rhegma and on the area above it, and a few scattered dark brown spots. Apex posterior margin sometimes with a weak, dark concave streak. Hind wing: slightly narrower than forewing. Veins brown, slightly dashed with pale brown, except for Sc and RA veins, which are evidently dashed. Membrane hyaline before subapical dark band. Apex white on poststigmal area, with white area oblong or falcate, and subapical dark band solid, encompassing a white mark on pterostigma, and another white mark at posterior wing margin, roundish to oblong. Presectoral area wider than posterior area. Posterior area narrow, with cells longer than wide or as wide as long. Legs: Coxae dark, sometimes ventrally pale brown. Coxae with many delicate, long, white setae. Femora pale with an apical dark band, and dark spots at setal bases which sometimes combine forming a darker suffusion on femora external face. Femora with many short decumbent setae, mostly dark, shorter than femoral width, and a few setae at most subequal to femora width. Forefemoral sense hair much shorter than femur, at most subequal in length to femoral width, and mid femoral sense hair similar in length to forefemoral sense hair. Tibia pale, with an apical and a subbasal dark band, and small dark spots at setal bases. Tibia with many short, decumbent dark setae slightly shorter than tibial width, and a few long dark bristles. Hind tibia longer than femur. Tibial spurs well developed, fore and midtibial spurs reaching tarsomere III and metatibial spurs reaching tarsomere II. Tarsomeres pale, with distal margin dark. Distal tarsomere longer than basitarsus, which is longer than remaining tarsomeres. Basitarsus long, a bit longer than twice the length of tarsomere II. Pretarsal claws well developed, roughly two thirds the length of distal tarsomere. Abdomen: Abdomen dark, with median transversal pale to yellowish bands on tergites III-V, and narrowly pale on tergites and sternites posterior margins. Abdominal sclerites covered with short dark and white setae, which are longer nearing terminalia. Female terminalia: tergite IX dark beset with many short dark setae. Ectoproct with many delicate setae dorsally and posteriorly, and many digging setae on ectoproct sub ventral and ventral regions. Pregenital plate very small, sclerotized, and subtriangular. Anterior gonapophysis inconspicuous. Posterior gonapophysis very short, posterior margin rounded, with many long digging setae. Lateral gonapophysis shorter than ectoproct in lateral view, with many thick digging setae, longer than those in ectoproct and shorter than those on posterior gonapophysis. Gonapophyseal plates elongate and parallel.
- Larva. Unknown.
- Remarks. This species was described by Navás [41] based on the holotype from La Trinité (Martinique: France). The holotype was erroneously mentioned as a male by Navás [41], since it is a female as presented here (Figure 6). The type locality was also erroneously placed in Trinidad by Stange [35], which was followed by subsequent authors [13,16,36]. In the original description Navás [41] mentioned that G. discors was very similar to G. heteropteryx but they could be separated based on the hindwing length (same length as the forewing in G. discors, but longer in G. heteropteryx), the rhegmal area of the forewing with less spots in G. discors, and some differences on the white mark of the hindwing (Figure 3). After that, Banks [21] stated that G. discors agreed with G. heteropteryx, and that the characters mentioned by Navás [41] to separate them are variable, which was later contested by Navás [42], mentioning that he had compared G. discors with the traditional G. heteropteryx. After that the species was only mentioned again by Stange [35], who agreed with Banks [21] and synonymized G. discors under G. heteropteryx, which was posteriorly followed by all subsequent authors [13,16,36].
Herein, after examining the four female syntypes of G. heteropteryx, it is clear that the series is composed by two species, one specimen that is herein designated as the lectotype of G. heteropteryx (ZIMG II27453c) (Figure 10a) and the three paralectotypes (ZIMG II27453a, II27453b, II27453d) that perfectly agree with the holotype of G. discors (Figure 7). In this sense, we are proposing that G. discors is a valid species. The examination of a larger series of specimens allowed us to clearly distinguish them, reinforcing that the characters mentioned by Navás [41] to separate both species are in fact useful. Furthermore, we also add two other important characters to distinguish them: the presence of dark marks on the subcostal area of the forewing of G. heteropteryx, and the dark brown frons, scape and pedicel of G. heteropteryx.
Figure 10.
Glenurus heteropteryx lectotype. (a) habitus, dorsal view, (b) frons, (c) thorax, dorsal view, (d) thorax, legs and abdomen, lateral view.
With the revalidation of G. discors some of the records attributed to G. heteropteryx have to be reinterpreted now. The type series of G. heteropteryx are all from Chiriqui (Panama), but as discussed above, all three paralectotypes actually belong G. discors. Stange [13] mentioned that G. heteropteryx are very likely to be found in Costa Rica, but the figure of the wings presented by the author clearly belongs to G. discors. Furthermore, the records of G. heteropteryx from Colombia [9] and Brazil [10] also belong to G. discors.
- Examined material. PANAMA: Chiriqui (3♀, EMAU [paralectotypes]).
3.1.3. Glenurus heteropteryx Gerstaecker
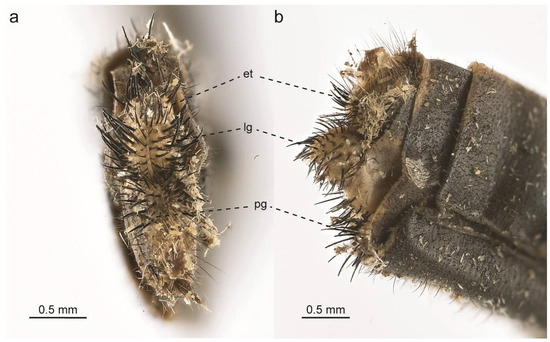
Figure 11.
Glenurus heteropteryx lectotype female terminalia. (a) Posterior view, (b) lateral view. Abbreviations: et: ectoproct, lg: lateral gonapophysis, pg: posterior gonapophysis.
- Glenurus heteropteryx Gerstaecker, 1885: 17 [43] [Lectotype: female: Panama: Chiriqui (ZIMG—II27453c)]. Navás 1920: 203 [41] [taxonomy]. Banks, 1922: 58 [21] [taxonomy, key]. Navás 1923: 187 [41] [taxonomy]. Navás 1935: 362 [44] [distribution]. Stange 1970: 22 [35] [taxonomy, distribution]. Penny 1977: 44 [36] [distribution]. Whittington 2002: 382 [45] [distribution]. Stange 2002: 285 [13] [redescription, distribution, taxonomy]. Stange 2004: 178 [16] [distribution, taxonomy, type]. Ardilla-Camacho et al., 2014: 692 [9] [distribution]. Giacomino 2015: 154 [18] [distribution]. Machado 2020: 137 [10] [distribution, key]. Machado and Martins 2023 [38] [distribution].
- Distribution. Panama (Gerstaecker 1885), Ecuador (Navás 1935), Venezuela (Stange 1970), Trinidad (Whittington 2002), Nicaragua, Costa Rica *.
- Diagnosis. Pronotum dark brown with a medial longitudinal pale stripe, and two weak sublateral pale brown bands; forewing subcostal area with dark brown marks; forewing subapical dark band absent, forewing apex with a dark brown spot at rhegma and another dark brown spot on the area above, and with a speckled apex membrane, which has a whitish hue; hind wing subapical dark band complete, with apex white mark strongly falcate with a deep indentation.
- Redescription. Head: Antennae clubbed with 38–42 flagellomeres, dark brown. Flagellomeres dark brown, distally lighter. Vertex slightly raised, brown, anterior row with two sublateral dark brown marks and two dark brown marks medially that connect with each other and with middle row. Middle row with four dark brown marks, and posterior row with two sublateral and two medial oblong dark brown patches. Vertex with setae short dark decumbent setae, a few scattered short white setae near epicranial mark, which is dark brown. Interantennal area dark brown, merging with frons transversal dark brown band. Frons dark brown near antennae base and brown near clypeus. Frons with few scattered delicate white setae. Interocular distance as broad as scape diameter. Clypeus brown with rows of long dark setae at frontoclypeal margin. Labrum brown with a row of setae at distal margin. Mandible well developed, dark, slightly less dark at exterior margins. Palpi brown, and dark brown near joints, with distal palpomere dark brown and fusiform. Palpimacula round. Thorax: Thorax mostly dark brown. Pronotum longer than wide, dark brown, with a narrow pale longitudinal sublateral line at each lateral margin, a pale longitudinal line at middle, and sometimes two subapical pale spots near furrow. Pronotum with some short dark setae, and long, anteriorly curved setae at lateral margins. Prescutum dark, with two pale spots on lateroposterior margins. Prescutum with short dark setae and a few erect, delicate setae. Mesoscutum dark, and some scattered short dark setae. Mesoscutellum dark, slightly paler on posterior margin, and a narrow pale longitudinal line starting subapically and broadening until posterior margin. Mesoscutellum with a few decumbent white setae near posterolateral margin. Metaprescutum dark with a pale narrow longitudinal line at middle, broadening near posterior margins. Metascutum dark, with velvety patches at middle. Metascutellum dark, with a pale narrow longitudinal line at middle, broadening near posterior margins, with white setae pointed posteriorly. Thorax laterally dark, with meso and metepisternum darker dorsally and paler ventrally, with many scattered white setae. Wings: Wings broad, lanceolate. Forewing slightly shorter than hind wing, in repose the apex of the hind wing extends after forewing. Forewing: veins dark brown dashed with pale brown. Membrane hyaline before apex, except around a few scattered crossveins that are surrounded by a brown tint. Costal area simple. Subcostal area with dark brown infuscations. Presectoral area with eight crossveins. Cubital area with a large brown oblique streak at the end of the second branch of CuA fork. Pterostigma whitish to pinkish. Apex with veins and membrane with a whitish hue, with no subapical dark band, a dark brown spot at rhegma and on the area above it, and many scattered dark brown spots throughout wing apex. Apex posterior margin sometimes with a weak, dark concave streak. Hind wing: slightly narrower than forewing. Veins brown, slightly dashed with pale brown, except for Sc and RA veins which are evidently dashed. Membrane hyaline before subapical dark band. Apex white on poststigmal area, with white area strongly falcate, deeply indented on posterodistal margin, and subapical dark band solid, encompassing a white mark on pterostigma, and another white mark at posterior wing margin, roundish to oblong. Presectoral area wider than posterior area. Posterior area narrow, with cells longer than wide. Legs: Coxae dark, sometimes ventrally pale brown. Coxae with many delicate, long, white setae. Femora pale with an apical dark band, and dark spots at setal bases which sometimes combine forming a darker suffusion on femora external face. Femora with many short decumbent setae, mostly dark, shorter than femoral width, and a few setae at most subequal to femora width. Forefemoral sense hair much shorter than femur, at most subequal in length to femoral width, and mid femoral sense hair similar in length to forefemoral sense hair. Tibia pale, with an apical and a subbasal dark band, and small dark spots at setal bases. Tibia with many short, decumbent dark setae slightly shorter than tibial width, and a few long dark bristles. Hind tibia longer than femur. Tibial spurs well developed, fore and midtibial spurs reaching tarsomere III and metatibial spurs reaching tarsomere II. Tarsomeres pale, with distal margin dark. Distal tarsomere longer than basitarsus, which is longer than remaining tarsomeres. Basitarsus long, a bit longer than twice the length of tarsomere II. Pretarsal claws well developed, roughly two thirds the length of distal tarsomere. Abdomen: Abdomen dark, with median transversal pale to yellowish bands on tergites III–V, and narrowly pale on tergites and sternites posterior margins. Abdominal sclerites covered with short dark and white setae, which are longer nearing terminalia. Female terminalia: tergite IX dark beset with many short dark setae. Ectoproct with many delicate setae dorsally and posteriorly, and many digging setae on ectoproct sub ventral and ventral regions. Pregenital plate very small, sclerotized, and subtriangular. Anterior gonapophysis inconspicuous. Posterior gonapophysis very short, posterior margin rounded, with many long digging setae. Lateral gonapophysis shorter than ectoproct in lateral view, with many thick digging setae, longer than those in ectoproct and shorter than those on posterior gonapophysis. Gonapophyseal plates elongate and parallel.
- Larva. Unknown.
- Remarks. During the development of this work, we discovered that the type series of G. heteropteryx deposited at ZIMG is actually composed by four female syntypes, and not a female holotype as mentioned by Stange [16] (Figure 7 and Figure 10a). This agrees with the original description of the species, since Gerstaecker [43] did not clearly designate a holotype, but mentioned the word female, and presented body and wing lengths with variation, suggesting the description was based in more the one female. However, when studying the four syntypes it was clear to us that the series is composed of two distinctive species. The first species, represented by one of the syntypes (ZIMG II27453c), is characterized by the frons, scape and pedicel dark brown, the forewing with dark marks in the subcostal area, and the apex speckled with many dark spots, and the hindwing slightly longer than the forewing (Figure 10). While the second species, represented by the remaining three syntypes (ZIMG II27453a, II27453b, II27453d) has the frons, scape, and pedicel brown, the forewing subcostal area without dark marks, and the apex with only two dark spots near the rhegma, and the hind and forewing with the same length (Figure 7). In fact, most of the characters used here to distinguish both species were actually presented before by Navás [41,42] to separate G. discors and G. heteropteryx, which were considered as synonyms since Stange [34]. Furthermore, the species represented by three of the syntypes, is actually in accordance with the description and the female holotype of G. discors. In this sense, in order to avoid the creation of a new name, we are herein designating the sole syntype (ZIMG II27453c) as the lectotype of G. heteropteryx. Consequently, the three paralectotypes (ZIMG II27453a, II27453b, II27453d) now represent specimens of G. discors.
As mentioned in the remarks section above, with the revalidation of G. discors the distribution records associated with G. heteropteryx needed to be reviewed. The records from Colombia and Brazil presented respectively by Ardila-Camacho et al. [9] and Machado [10] are now interpreted as belonging to G. discors. The record from Guadeloupe presented by Stange [16] very likely represents G. oswaldi sp. n. The records from Ecuador, Venezuela, and Trinidad previously presented in the literature [35,44,45] are impossible to check, since no figures or details about the specimens mentioned in those papers were provided. However, images of Glenurus specimens from Ecuador at the iNaturalist platform show individuals that, despite bearing wings that are more intensely speckled, in general are in accordance with G. heteropteryx and not G. discors. In this sense, we are provisionally keeping the records from these three countries associated to G. heteropteryx. In fact, the only not doubtful record for G. heteropteryx is the one represented by the lectotype from Panama. Other specimens that agree with G. heteropteryx can be seeing in photos on the iNaturalist from Panama.
- Examined material. NICARAGUA: Managua: Ticuantepe: Montibelli, alt. 370 m, 12.021346, −86.232194, V-2003, col. D. Roiz (1♀, MEL). PANAMA: Chiriqui (1♀, EMAU [lectotype]).
3.1.4. Glenurus luniger Gerstaecker
Figure 12.
Glenurus luniger, holotype. (a) Habitus, dorsal view, (b) frons, (c) thorax, dorsal view, (d) thorax and legs, lateral view.

Figure 13.
Glenurus luniger, regional variations. (a) Holotype, from Panama, (b) Mexican specimen, from Baja California.
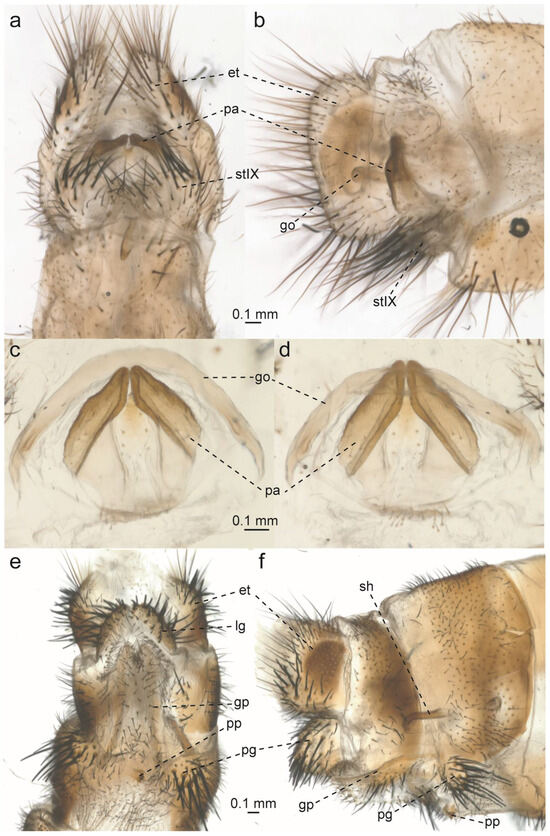
Figure 14.
Glenurus luniger, male and female terminalia and genitalia. (a) Male terminalia, ventral view, and (b) lateral view. (c) Male genitalia, ventral view, and (d) dorsal view. (e) Female genitalia, ventral view, and (f) lateral view. Abbreviations: et: ectoproct, go: gonarcus, gp: gonapophyseal plate, lg: lateral gonapophysis, pa: parameres, pg: posterior gonapophysis, pp: pregenital plate, sh: spermatheca, stIX: ninth sternite.

Figure 15.
Glenurus luniger, third instar larva. (a) Dorsal view, (b) ventral view.
- Glenurus luniger Gerstaecker, 1893: 125 [46] [Holotype female, Chiriqui (ZIMG)]. Banks, 1938: 420 [22] [distribution, redescription]. Stange, 1970: 22 [35] [taxonomy, types]. Penny, 1977: 44 [36] [distribution]. Henry et al., 1992: 434 [47] [fore and hind wing illustration]. Penny et al., 1997: 77 [37] [distribution, taxonomy]. Stange, 2000: 228 [15] [biology, distribution]. Oswald et al., 2002: 581 [48] [distribution]. Stange in Penny, 2002: 285 [13] [distribution, redescription, taxonomy]. Stange, 2004:178 [16] [distribution, taxonomy, type]. Badano et al., 2018: 936 [5] [habitus photograph, phylogeny]. Machado et al., 2019: 425 [6] [habitus photograph, phylogeny]. Oswald, 2025 [1] [species list].
- Distribution. Belize *, Costa Rica (Stange, 2002), El Salvador *, Guatemala *, Honduras *, Mexico (BCS, CHIS, JAL, SIN, SON, VER (Oswald et al., 2002), BC, OAX, TA, COL *, NL *, SLP *, NAY *, GRO *, MOR *, YUC *, ROO *), Nicaragua, Panama (Gerstaecker, 1893), USA (AZ, CA).
- Diagnosis. Forefemora pale brown, sometimes with a brownish tint on external face; pronotum dark brown with a medial longitudinal pale stripe, sometimes with two sublateral pale brown bands; hind wing subcostal area before subapical dark band hyaline.
- Redescription. Antennae clubbed with 50 flagellomeres, dark brown, slightly darker at base and at the club. Flagellomeres brown. Vertex slightly raised, pale brown, anterior row with three dark brown patches, the middle one larger and sometimes merged with a dark brown patch connecting to middle row patches; middle row as a dark transversal band sometimes divided into four large patches, posterior row with two sublateral oblong dark patches and two submedial dark brown spots. Vertex with a few dark decumbent setae, and a few scattered short setae near epicranial mark, which is dark brown. Interantennal area pale brown. Frons dark brown near antennae base with a pale brown patch near clypeus. Frons with few scattered white setae. Interocular distance as broad as or subequal to scape diameter. Clypeus dark brown, pale brown at lateral margins, with rows of long dark setae at frontoclypeal margin. Labrum dark brown, pale at lateral margins, with a row of setae at distal margin. Mandible well developed, dark, slightly less dark at exterior margins. Palpi dark brown, and pale brown near joints, with distal palpomere brown and fusiform. Palpimacula round. Thorax: Thorax dark with pale areas, or medially pale brown and laterally dark brown. Pronotum longer than wide, dark brown, with a narrow pale longitudinal sublateral line at each lateral margin, a narrow to large pale longitudinal line at middle. Pronotum with some short dark setae, and long, anteriorly curved setae at lateral margins. Mesoprescutum dark, with a pale medial line medially, or pale brown with lateral margins dark brown. Prescutum with a few erect, short dark setae. Mesoscutum dark with pale patches at middle, sometimes connecting to mesoprescutum pale brown areas, with some scattered short dark setae. Mesoscutellum dark, pale on posterior margin, with two pale sublateral spots, and a narrow pale longitudinal line starting subapically and broadening until posterior margin, or almost entirely pale brown with a few greyish brown lines medially. Mesoscutellum with a few decumbent setae near posterolateral margin. Metaprescutum pale brown with two submedial dark brown patches, or almost entirely pale brown, with lateral margins dark brown. Metascutum dark, with pale patches at middle. Metascutellum dark, pale at posterior margin, with white setae pointed posteriorly. Thorax laterally dark, with many scattered white setae. Wings: Wings broad, lanceolate. Forewing slightly or evidently shorter than hind wing, in repose the apex of the hind wing extends after forewing. Forewing: Sc and CuA veins dark brown dashed with pale brown, and remaining veins dark, or dashed similarly to Sc and CuA. Membrane hyaline before subapical dark band, except around a few scattered crossveins that are surrounded by a brown tint, especially on presectoral, radial, mediocubital, cubital and posterior areas. Subcostal area membrane suffused with brown at Radial area crossveins. Cubital area with a large brown stripe at the end of the second branch of CuA fork and scattered small brown spots near posterior margin extending until forewing apex. Pterostigma whitish to pinkish. Forewing apex with veinlets and membrane white. Subapical dark band complete to partially broken into small dark brown spots, frequently with a “smudge-like” brown mark near posterior wing margin. Anterior banksian line present, sometimes weakly evident, posterior banksian line absent. Hind wing: slightly narrower than forewing. Veins brown, slightly dashed with pale brown, except for Sc, RA, and MP veins, which are evidently dashed. Membrane hyaline before subapical dark band, except sometimes on posterior and prefork areas. Apex white on poststigmal area, and subapical dark band solid, encompassing a white mark on pterostigma, and other white mark near posterior wing margin, roundish to oblong. Legs: Coxae dark, sometimes ventrally pale brown. Coxae with many delicate, long, white setae. Femora pale with an apical dark band, and dark spots at setal bases which sometimes combine forming a darker suffusion on femora external face. Femora with many decumbent setae, mostly dark, shorter than femoral width, and a few setae at most subequal to femora width. Forefemoral sense hair much shorter than femur, at most subequal in length to femoral width, and mid femoral sense hair similar in length to forefemoral sense hair. Tibia pale, with an apical and a subbasal dark band, and small dark spots at setal bases. Tibia with many short, decumbent dark setae slightly shorter than tibial width, and a few long dark bristles. Metathoracic tibia longer than femur. Tibial spurs well developed, fore and midtibial spurs reaching tarsomere III and metatibial spurs reaching tarsomere II. Tarsomeres pale, with distal margin dark. Distal tarsomere longer than basitarsus, which is longer than remaining tarsomeres. Basitarsus long, a bit longer than twice the length of tarsomere II. Pretarsal claws well developed, roughly two thirds the length of distal tarsomere. Abdomen: Abdomen dark, narrowly pale on tergites and sternites posterior margins. Abdominal sclerites covered with short dark and white setae, which are longer nearing terminalia. Male terminalia: Male tergite IX rounded, with many long dark setae on tergite margins. Male sternite IX split medially, appearing ventrally as two rounded sclerites beset with short dark setae basally and many long dark posteriorly oriented setae on posterior margins. Male ectoproct dark, covered with long dark posteriorly oriented setae. Gonarcus arching dorsal to parameres, slender, with simple apodemes. Parameres subquadrangular, almost three times longer than high, sclerotized, basally bent inwards. Pelta membranous, with many short setae. Female terminalia: tergite IX dark beset with many short dark setae. Ectoproct with many delicate setae dorsally and posteriorly, and many digging setae on ectoproct sub ventral and ventral regions. Pregenital plate very small, sclerotized, and subtriangular. Anterior gonapophysis inconspicuous. Posterior gonapophysis very short, posterior margin rounded, with many long digging setae. Lateral gonapophysis shorter than ectoproct in lateral view, with many thick digging setae, longer than those in ectoproct and shorter than those on posterior gonapophysis. Gonapophyseal plates elongate and parallel. Spermatheca with the distal half broader and darker than proximal half.
- Larva. Head: Mandibles short, pale brown, darker at tip and at teeth, and shorter than head capsule. Mandibles with a few scattered short dark setae dorsally, and many long setae at mandible external margins, which are subequal in length to mandible width at base, and shorter than mandible width at mandible mid length. Mandible internal margins with rows of star shaped dolichasters until distal teeth. Head capsule greyish brown, darker posteriorly, and as wide as long, with lateral margins rounded. Head capsule dorsally covered with star-shaped dolichasters, covered with conical dolichasters ventrally. Clypeo-labrum greyish brown, narrower than head capsule on dorsal view, with a triple sinuated posterior margin. Clypeo-labrum posterior margin with a row of long, thick dark star shaped dolichasters. Ocular tubercle raised, with setae between stemmata. Distal palpomere fusiform. Thorax: Thoracic tergites covered with short dark setae and thick dark dolichasters, and a few long, dark setae on anterior and posterior tergite margins. Sternum pale brown, with a large dark brown patch sublaterally, near coxae insertions, with many short dark setae and a few long dark setae medially. Sternum covered with conical dolichasters. Pronotum pentagonal, dark brown with anterior margin pale brown. Meso and metathorax dark brown with a pale brown tint laterally and medially. Mesonotum anterior subsegment narrow. Mesothoracic spiracle borne on a short tubercle, which is dark brown and as wide as long. Mesothoracic and metathoracic setiferous processes short, scoli-like, covered with long dark bristles. Legs: Coxae pale brown with brown patches and beset with dark setae. Femora pale brown beset with short dark setae and rows of long dark setae on external face. Tibiae pale brown, with three opposing rows of short dark setae. Tarsi pale brown. Pretarsal claws dark, fore and mid pretarsal claws almost 1/3 of tarsi length, while hind pretarsal claws at least half of hind tarsus. Abdomen: Abdominal tergites dark brown, and lateral margins pale brown. Tergites and sternites covered with short, dark setae, and tergites with transversal rows of thicker and longer setae medially. Abdominal setiferous processes pale brown, well developed, covered with long dark setae. Sternite VIII short, dark brown, covered with short dark setae and a pair of thick and long dark setae submedially on posterior margin. Segment IX pale brown, dark brown basally, longer than wide, with well-developed digging setae.
- Remarks. This species has a widespread distribution across the Southern Nearctic and the Central America Neotropics, similarly to G. proi (Figure 4 and Figure 5). It is being registered from the Mexican states of Baja California, Oaxaca, and Tamaulipas for the first time. Additionally, it is the most morphologically variable species treated in this work, with a clinal variation along North to Central America (Figure 13). The Neotropical specimens, including the holotype (Figure 13a), tend to bear a narrower and longer hind wing, with a narrow posterior area, and the posterior branch of the MP2 fork reaching the second bifurcation of the anterior MP2 branch at most, with the distalmost white spot on the wing apex more falcate, and the white mark on posterior wing margin usually merge with the wing margin. On the other hand, Nearctic specimens (Figure 13b) tend to bear a broader and shorter hind wing, with a higher posterior area, and the posterior branch of MP2 fork reaching the third bifurcation of the anterior MP2 branch. Also, the distalmost white mark on the wing apex is usually rounder, and the white mark on the posterior wing margin usually “float” and does not touch the wing margins. Additionally, Nearctic specimens are darker, with a dark thorax and a very narrow medial pale longitudinal band dorsally. Neotropical specimens are usually less dark than Nearctic specimens, with a broader pale medial longitudinal band dorsally on thorax, with the mesoscutellum almost all pale brown colored. Specimens from central Mexico tend to bear intermediate characteristics. Moreover, as in other Glenurus species, there are slight variations in the apical field patterns: the forewing apical dark band can be completely solid from the anterior to posterior margins (Figure 13a), or nearly solid but broken into clustered dark spots (Figure 13b).
Nearctic specimens are more similar in coloration and structure to G. snowii, as they tend to be darker, its subapical dark band tend to be broken and its hind wing broader, while the Neotropical specimens are more similar to G. incalis. Glenurus luniger can be readily diagnosed from G. snowii by the forewing subapical dark band, which is generally more complete, and by the hind wing subcostal area which is clear of suffusion. Glenurus luniger and G. incalis can be diagnosed mostly by their geographic distribution. The latter species is known only from central Peru and northwestern Brazil, while G. luniger ranges from western USA to Panama. Additionally, in G. incalis, the forewing Costal area just before the pterostigma is much higher and dense with bifurcated crossveins. In G. luniger, the Costal area just before the hypostigmatic cell is shorter, and the dark infuscation of the subapical dark band covers a larger portion of the Costal area.
The larva was previously known by Stange [15], but it is being described for the first time here. It inhabits leaf litter under rock overhangs, and dry wood debris in tree stumps [15].
- Examined material. MEXICO: Baja California: Ensenada: Rancho Mike’s Sky, palapas cerca de cascada, 03.viii.2021, 31°05.976′ N, 115°37.302′ W, 1227 m, Trampa de Luz, Contreras, Cancino, Luna, Martins, Marquez (2♀, CNIN); Baja California Sur: Los Cabos: Sierra de la Laguna, Rancho Ecológico Sol de Mayo, Cabañas: 13.viii.2021, 238 m, Trampa de Luz, Contreras, Cancino, Luna, Martins, Marquez (1♀1♂, CNIN); 14.viii.2021, 282 m, Trampa de Luz, Contreras, Cancino, Luna, Martins, Marquez (1♀, CNIN); Golfo de California: Isla Espiritu Santo, 03.xi.1986, L. Cervantes (2♀, CNIN); Mulegé: San Bruno, Hotel Costa Serena, terreno aledaño, 11.viii.2021, 27°09.832′ N, 112°09.895′ W, 6 m, trampa de luz, Luna, Contreras, Barba, Ramírez (2♀, CNIN); Loreto: Misión San Fco. Javier de Viggé-Biaundó, Arroyo San Javier, 11.viii.2021, 25°51.347′ N, 111°33.005′ W, 393 m, trampa de luz, Luna, Contreras, Barba, Ramírez (1♀, CNIN); Jalisco: Est. de Biología, 28.vi.1980, A. Pescador (1?, CNIN); Oaxaca: Huatulco: Parque Nal. Huatulco, Estación el Sabanal, 30.vii.2005, 15°48′10.7″ N, 96°11′39.4″ O, 109 m, TL1, S. Zaragoza (1♀, CNIN); Sonora: Álamos, 15–20.vii.1958, R. L. Westcott (1♀, CNIN); Rio Cuchajachi 7 mi. S. Álamos, 22.III.1985, M–30, reared Rock overhang, R. Miller, L. Stange (1 larva, FSCA); Veracruz: Motzorongo, i.1934 (?, CNIN); Santiago Tuxtla, C del Vigia, 02.v.1964, H Perez R (1♂, CNIN). NICARAGUA: Managua, Ticuantepe: Montibelli, 370 m, 12.021346, −86.232194, V-2003, col. David Roiz (1♀, MEL).
3.1.5. Glenurus maya sp. n. Tavares
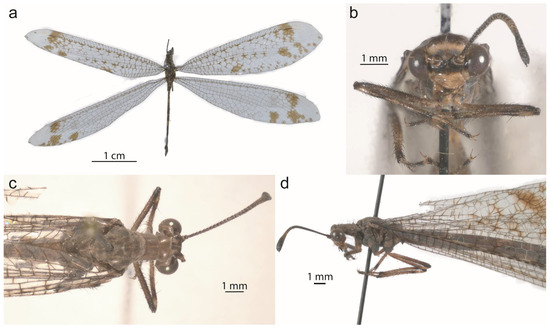
Figure 16.
Glenurus maya sp. n. (a) Paratype habitus, dorsal view, (b) frons, (c) holotype thorax, dorsal view, (d) holotype thorax and legs, lateral view.
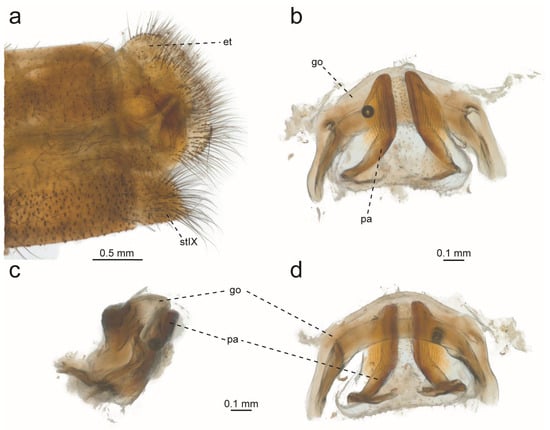
Figure 17.
Glenurus maya sp. n. male terminalia and genitalia. (a) Male terminalia, lateral view, (b) male genitalia, ventral view, (c) lateral view, and (d) dorsal view. Abbreviations: et: ectoproct, go: gonarcus, pa: parameres, stIX: ninth sternite.
- Diagnosis. Pronotum dark brown with a medial longitudinal pale stripe, and two sublateral pale brown bands; forewing subapical dark band reduced into three dark brown patches; fore and hind wing apex and poststigmal areas mostly whitish; hind wing subcostal area partially suffused with dark brown; forefemoral sense hair length roughly two times the femoral width.
- Description. Head: Antennae clubbed with 36 flagellomeres, dark brown, slightly lighter before the club. Flagellomeres dark brown, distally pale brown. Vertex slightly raised, pale or almost yellowish brown, anterior and middle row fused as a dark transversal band sometimes divided into four large patches, posterior row with two sublateral and two medial oblong dark patches. Vertex without setae, a few scattered short setae near epicranial mark, which is dark brown. Interantennal area dark brown, merging with frons transversal dark brown band, which extends to genal area between frons and eye margins. Frons dark brown near antennae base and pale or yellowish brown near clypeus. Frons with few scattered delicate white setae. Interocular distance as broad as scape diameter. Clypeus pale with rows of long dark setae at frontoclypeal margin. Labrum pale with a row of setae at distal margin. Mandible well developed, dark, slightly less dark at exterior margins. Palpi brown, and dark brown near joints, with distal palpomere dark brown and fusiform. Palpimacula round. Thorax: Thorax dark with pale areas. Pronotum longer than wide, dark brown, with a narrow pale longitudinal sublateral line at each lateral margin, a narrow pale longitudinal line at middle, and two subapical pale spots near furrow. Pronotum with some short dark setae, and long, anteriorly curved setae at lateral margins. Prescutum dark, with two pale spots on lateroposterior margins. Prescutum with a few erect, delicate setae. Mesoscutum dark with pale patches at middle, and some scattered short dark setae. Mesoscutellum dark, pale on posterior margin, with two pale sublateral spots, and a narrow pale longitudinal line starting subapically and broadening until posterior margin. Mesoscutellum with a few decumbent white setae near posterolateral margin. Metaprescutum dark with a pale narrow longitudinal line at middle. Metascutum dark, with pale patches at middle. Metascutellum dark, pale at posterior margin, with white setae pointed posteriorly. Thorax laterally dark, with meso and metepisternum darker dorsally and paler ventrally, with many scattered white setae. Wings: Wings broad, lanceolate. Forewing slightly shorter than hind wing, in repose the apex of the hind wing extends after forewing. Forewing: veins dark brown dashed with pale brown. Membrane hyaline before subapical dark band, except around a few scattered crossveins that are surrounded by a brown tint. Costal area with a few bifurcated crossveins. Subcostal area membrane suffused with brown at Costal and Radial area crossveins. Presectoral area with eight crossveins, and presectoral area and prefork area with some bifurcated crossveins. Mediocubital area with a series of brown spots from the origin of CuA fork until rhegma. Cubital area with a large brown spot at the end of the second branch of CuA fork and scattered small brown spots near posterior margin extending until forewing apex. Pterostigma whitish to pinkish. Apex with veins and membrane white, with a narrow and broken subapical dark band, which is brown to light brown. Hind wing: slightly narrower than forewing. Veins brown, slightly dashed with pale brown, except for Sc and RA veins which are evidently dashed. Membrane hyaline before subapical dark band. Subcostal area suffused with a dark brown tint. Apex white, and subapical dark band reduced to three brown spots at pterostigma, at posterior wing margin opposite to pterostigma and at rhegma. Presectoral area wider than posterior area. Posterior area narrow, with cells longer than wide. Legs: Coxae dark, and prothoracic coxae with a transversal pale band at middle. Coxae with many delicate, long, white setae. Femora pale with an apical dark band, and dark spots at setal bases which sometimes combine forming a darker suffusion. Femora with many decumbent setae, mostly dark, shorter than femoral width. Forefemoral sense hair short, much shorter than femur, and midfemoral sense hair shorter than forefemoral sense hair. Tibia pale, with an apical and a subbasal dark band, and small dark spots at setal bases. Tibia with many short, decumbent dark setae slightly shorter than tibial width, and a few long dark bristles. Metathoracic tibia longer than femur. Tibial spurs well developed, pro and midtibial spurs reaching tarsomere III and metatibial spurs reaching tarsomere II. Tarsomeres pale, with distal margin dark. Distal tarsomere longer than basitarsus, which is longer than remaining tarsomeres. Basitarsus short, roughly twice the length of tarsomere II. Pretarsal claws well developed, roughly two thirds the length of distal tarsomere. Abdomen: Abdomen dark, with median transversal pale bands on tergites III-V, and narrowly pale on tergites and sternites posterior margins. Abdominal sclerites covered with short dark and white setae, which are longer nearing terminalia. Male tergite IX rounded, with many long dark setae on tergite margins. Male sternite IX not split medially, with a rounded posterior margin, and many long dark posteriorly oriented setae. Male ectoproct dark, covered with long dark posteriorly oriented setae. Gonarcus arching dorsal to parameres, broad, with bifid apodemes. Parameres subquadrangular, slightly longer than high, sclerotized, basally bent inwards. Pelta membranous, with many short setae.
- Type specimens. Holotype: Guatemala: Huehuete: Yalambojoch. Ixcansan, 1600 m, 26.vii.2000, Col. J. Monzon, 00060785 (♂—FSCA). Paratypes: Mexico: Chiapas: Rancho Grande, 1.viii.1976, 0060784 (♂—FSCA); Santa Rosa, v.1971 (?—CNIN).
- Etymology. This species is named after the Mayans, the Mesoamerican indigenous ethnic group of people native to southern Mexico and northern Central America, which is also the known distribution of the new species.
- LSID: urn:lsid:zoobank.org:act:D43C297A-28DB-444F-AAA2-359985D9CE89
- Remarks. This species is unique among its congeners as its subapical dark band on both wings is reduced to dark brown patches, while the whitish areas are much more prominent (Figure 16a). The forefemoral sense hair is also longer, although is shorter in length than the forefemur. The male specimens also have the sternite IX not split medially (Figure 17), differing from G. proi and G. luniger. The gonarcus arms bifid distal ends is another unique characteristic to the known Glenurus male genitalia. Nothing is known of this species biology, female morphology, or its larvae.
3.1.6. Glenurus oswaldi Tavares, Marquez, Zheng, Machado, Contreras sp. n.
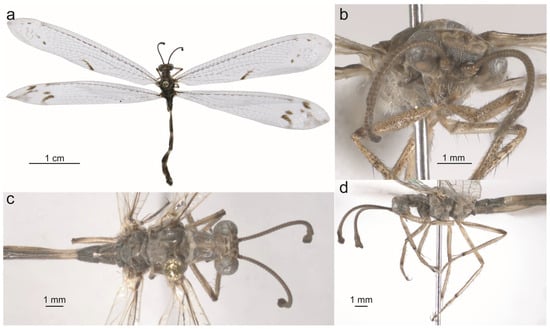
Figure 18.
Glenurus oswaldi sp. n. holotype. (a) Habitus, dorsal view, (b) frons, (c) thorax, dorsal view, (d) thorax and legs, lateral view.
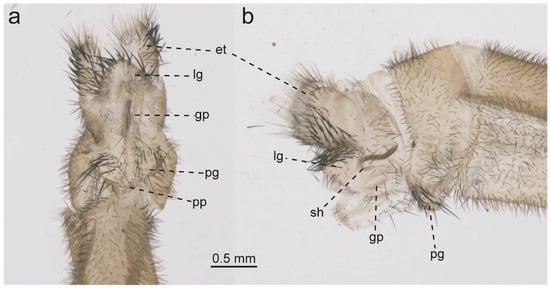
Figure 19.
Glenurus oswaldi sp. n. female genitalia. (a) Ventral view, (b) lateral view. Abbreviations: et: ectoproct, gp: gonapophyseal plate, lg: lateral gonapophysis, pg: posterior gonapophysis, pp: pregenital plate, sh: spermatheca.
- Diagnosis. Pronotum dark brown with a medial longitudinal pale stripe, and two weak sublateral pale brown bands; forewing subcostal area hyaline; forewing subapical dark band absent, forewing apex with a dark brown spot at rhegma and another dark brown spot on the area above, and with a few small, smaller spots throughout apex membrane, which has a whitish hue; hind wing subapical dark band divided into three to four large dark patches.
- Description. Head: Antennae clubbed with 38–40 flagellomeres, dark brown. Flagellomeres dark brown, distally lighter. Vertex slightly raised, brown, anterior row with two sublateral dark brown marks and two dark brown marks medially that connect with each other and with middle row. Middle row with four dark brown marks, and posterior row with two sublateral and two medial oblong dark brown patches. Vertex without setae, a few scattered short setae near epicranial mark, which is dark brown. Interantennal area dark brown, merging with frons transversal dark brown band, which extends to genal area between frons and eye margins. Frons dark brown near antennae base and brown near clypeus. Frons with few scattered delicate white setae. Interocular distance as broad as scape diameter. Clypeus brown with rows of long dark setae at frontoclypeal margin. Labrum brown with a row of setae at distal margin. Mandible well developed, dark, slightly less dark at exterior margins. Palpi brown, and dark brown near joints, with distal palpomere dark brown and fusiform. Palpimacula round. Thorax: Thorax mostly dark brown. Pronotum longer than wide, dark brown, with a narrow pale longitudinal sublateral line at each lateral margin, a narrow pale longitudinal line at middle, and two subapical pale spots near furrow. Pronotum with some short dark setae, and long, anteriorly curved setae at lateral margins. Prescutum dark, with two pale spots on lateroposterior margins. Prescutum with a few erect, delicate setae. Mesoscutum dark, and some scattered short dark setae. Mesoscutellum dark, slightly paler on posterior margin, and a narrow pale longitudinal line starting subapically and broadening until posterior margin. Mesoscutellum with a few decumbent white setae near posterolateral margin. Metaprescutum dark with a pale narrow longitudinal line at middle. Metascutum dark, with velvety patches at middle. Metascutellum dark, pale at posterior margin, with white setae pointed posteriorly. Thorax laterally dark, with meso and metepisternum darker dorsally and paler ventrally, with many scattered white setae. Wings: Wings broad, lanceolate. Forewing slightly shorter than hind wing, in repose the apex of the hind wing extends after forewing. Forewing: veins dark brown dashed with pale brown. Membrane hyaline before apex, except around a few scattered crossveins that are surrounded by a brown tint. Costal area simple. Presectoral area with eight crossveins. Cubital area with a large brown oblique streak at the end of the second branch of CuA fork. Pterostigma whitish to pinkish. Apex with veins and membrane with a whitish hue, with no subapical dark band, a dark brown spot at rhegma and on the area above it, and a few scattered dark brown spots. Apex posterior margin sometimes with a weak, dark concave streak. Hind wing: slightly narrower than forewing. Veins brown, slightly dashed with pale brown, except for Sc and RA veins which are evidently dashed. Membrane hyaline before subapical dark band. Apex white, and subapical dark band broken into three large dark patches: just after pterostigma, at wing apex, and at rhegma which sometimes breaks down into two separate patches. Presectoral area wider than posterior area. Posterior area narrow, with cells longer than wide. Legs: Coxae dark, sometimes ventrally pale brown. Coxae with many delicate, long, white setae. Femora pale with an apical dark band, and dark spots at setal bases, which sometimes combine forming a darker suffusion on femora external face. Femora with many decumbent setae, mostly dark, shorter than femoral width, and a few setae at most subequal to femora width. Forefemoral sense hair much shorter than femur, at most subequal in length to femoral width, and mid femoral sense hair similar in length to forefemoral sense hair. Tibia pale, with an apical and a subbasal dark band, and small dark spots at setal bases. Tibia with many short, decumbent dark setae slightly shorter than tibial width, and a few long dark bristles. Hind tibia longer than femur. Tibial spurs well developed, fore and midtibial spurs reaching tarsomere III and metatibial spurs reaching tarsomere II. Tarsomeres pale, with distal margin dark. Distal tarsomere longer than basitarsus, which is longer than remaining tarsomeres. Basitarsus long, a bit longer than twice the length of tarsomere II. Pretarsal claws well developed, roughly two thirds the length of distal tarsomere. Abdomen: Abdomen dark, with median transversal pale to yellowish bands on tergites III–V, and narrowly pale on tergites and sternites posterior margins. Abdominal sclerites covered with short dark and white setae, which are longer nearing terminalia. Female terminalia: tergite IX dark beset with many short dark setae. Ectoproct with many delicate setae dorsally and posteriorly, and many digging setae on ectoproct sub ventral and ventral regions. Pregenital plate very small, sclerotized, and subtriangular. Anterior gonapophysis inconspicuous. Posterior gonapophysis very short, posterior margin rounded, with many long digging setae. Lateral gonapophysis shorter than ectoproct in lateral view, with many thick digging setae, longer than those in ectoproct and shorter than those on posterior gonapophysis. Gonapophyseal plates elongate and parallel.
- Types. Holotype: British West Indies: Northern Antilles: Saba: Booby Hill, 4.ix.1993, BLT, T. Van Oosteren, FSCA 00060786 (♀—FSCA).
- Etymology. This species is named after Dr. John D. Oswald from Texas A & M University, who has contributed immensely to neuropterology, particularly for creating and maintaining the Lacewing Digital Library.
- LSID: urn:lsid:zoobank.org:act:9B00EF0F-6FCC-4766-981A-E6B172A6CB45
- Remarks. The new species, together with G. discors and G. heteropteryx, belongs to the morphotype group with no subapical dark band on the forewings (Figure 3), but it can be easily separated from the other two species based on its broken subapical band in the hindwings. Glenurus oswaldi sp. n. was actually imaged and discussed before by Giacomino [18] as a new record of G. heteropteryx from Guadeloupe. This species appears to be restricted to the Antilles, with the type registered from the island of Saba (Netherlands) and the specimen registered by Giacomino [18]. Stange [16] in his catalogue mentioned G. heteropteryx occurring in Guadeloupe, but this record might also belong to the new species. Nothing is known of this species biology, male morphology, or its larvae.
3.1.7. Glenurus proi Navás
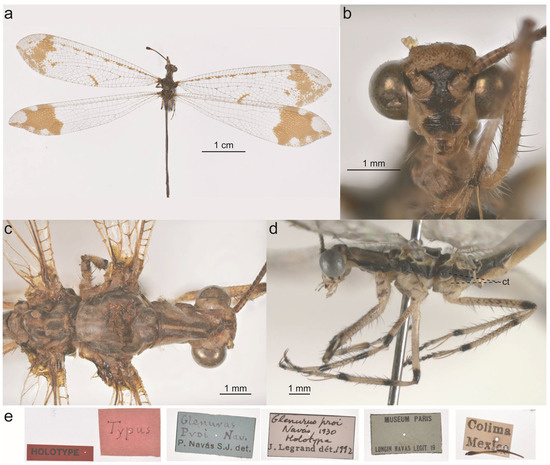
Figure 20.
Glenurus proi. (a) Holotype habitus, dorsal view, with type labels, (b) frons, and (c) thorax, dorsal view. (d) Thorax and legs, lateral view, from another specimen. (e) Type labels. Abbreviations: ct: contrasting thorax.
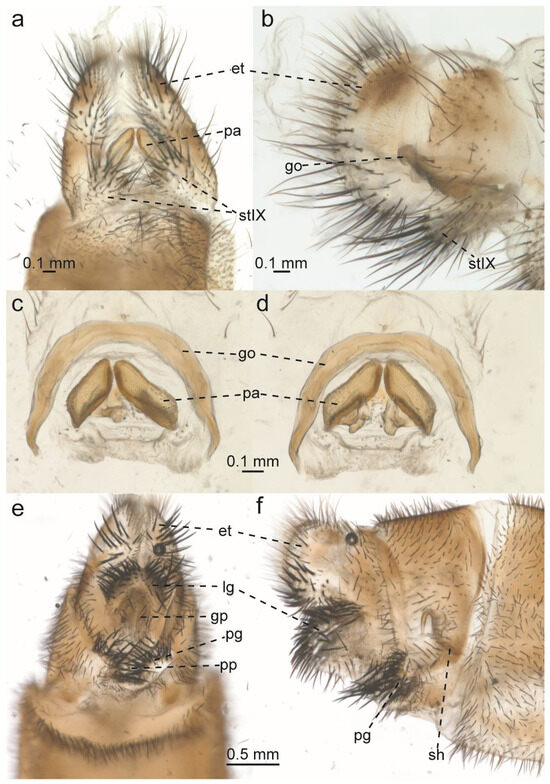
Figure 21.
Glenurus proi male and female terminalia and genitalia. (a) Male terminalia, ventral view, (b) lateral view. (c) Male genitalia, ventral view, (d) dorsal view. (e) Female genitalia, ventral view, (f) lateral view. Abbreviations: et: ectoproct, go: gonarcus, gp: gonapophyseal plate, lg: lateral gonapophysis, pa: parameres, pg: posterior gonapophysis, pp: pregenital plate, sh: spermatheca, stIX: ninth sternite.

Figure 22.
Glenurus proi third instar larva. (a) Dorsal view, (b) ventral view.
- Glenurus proi Navás, 1929: 17 [49] [Holotype female, Colima, México (MNHN)]. Stange, 1970: 23 [35] [taxonomy, type]. Penny, 1977: 44 [36] [distribution]. Stange, 2000: 228 [15] [biology, distribution]. Oswald et al., 2002: 581 [48] [distribution]. Penny, 2002 [13] [habitus illustration]. Stange in Penny, 2002: 282 [13] [distribution, redescription, taxonomy]. Stange, 2004: 179 [16] [distribution, taxonomy, type]. Badano et al., 2018: 936 [5] [phylogeny]. Oswald, 2025 [1] [species list] Marquez-López et al., 2024: 13, 16 [27] [genitalia].
- Distribution. Costa Rica (Stange in Penny, 2002), El Salvador *, Guatemala *, Honduras (Stange, 2004), Mexico (COL (Navás, 1929; Stange, 2000), CHIS, SON (Oswald et al., 2002), MOR, QRO, SI, NL *, AGU *, NAY *, JAL *, MICH *, GRO *, PUE *, OAX *, HGO *, GUA*), Nicaragua.
- Diagnosis. Pronotum pale brown with two dark brown submedial longitudinal stripes; Wings’ costal veins dashed with pale and dark brown; subapical dark band breaking into irregular spots; thorax pale brown contrasting with dark brown pterothoracic pleura; hindleg basitarsus longer than distal tarsomere; larvae with head capsule wider than long, and shorter than mandibles.
- Redescription. Head: Antennae clubbed with 37–42 flagellomeres, pale brown, dark brown at the club. Flagellomeres pale brown, with short dark setae. Vertex slightly raised or raised, pale or almost yellowish brown; anterior and middle row as two inconspicuous bands of small brown spots, and posterior row with two medial oblong dark brown patches. Vertex with short brown, anteriorly oriented decumbent setae, a few scattered short setae near epicranial mark, which is dark brown. Interantennal area dark brown, merging with frons dark brown patches and epicranial mark, forming a diamond shape dark brown area with a small triangular pale brown in the middle. Frons pale brown near genae, which are pale brown with dark brown areas near antennae. Frons with few scattered delicate white setae. Interocular distance as broad as pedicel diameter. Clypeus dark brown, pale brown near margins, with rows of long white setae at frontoclypeal margin. Labrum pale, with a subtriangular dark brown patch medially, and with a row of setae at distal margin. Mandible well developed, pale brown basally and dark brown distally. Palpi pale brown, dark brown near joints, with distal palpomere fusiform. Palpimacula round. Thorax: Thorax pale with dark areas. Pronotum longer than wide, pale brown, with two longitudinal submedial dark brown, and with grayish markings near lateral margins. Pronotum with some short dark setae on anterior margin, anteriorly curved white and dark setae at lateral margins, and erect dark setae on posterior margin. Prescutum dark brown, with two submedial pale brown comma shaped patches. Prescutum with erect brown and white setae. Mesoscutum pale brown, dark brown on lateral margins and on two triangular shaped patches near prescutum margins, with some scattered short white setae. Mesoscutellum pale brown, with two submedial dark brown bands, evident but sometimes inconspicuous. Mesoscutellum with a few decumbent white setae near posterolateral margin. Postmesoscutellum dark brown. Metaprescutum pale brown, dark brown on anterior margins and on two sub medial roundish spots. Metascutum dark brown, with pale patches submedially. Metascutellum dark brown, pale brown medially, with white setae posteriorly oriented. Thorax laterally pale brown, contrasting with pterothoracic pleura, which is dark brown with many scattered white setae. Wings: Wings broad, forewing lanceolate and hind wing weakly falcate. Forewing shorter than hind wing, in repose the apex of the hind wing extends after forewing. Fore and hind wing veins dark brown dashed with pale brown, beset with short black setae. Costal vein dark brown dashed with pale brown. Forewing: membrane hyaline before subapical dark band, except around a few scattered crossveins that are surrounded by a brown tint, mostly on mediocubital area. Subcostal area membrane almost fully suffused with brown, sometimes broken into dark spots on a few areas. Anterior banksian line present, posterior banksian line absent. Seven to eight presectoral crossveins, simple. Mediocubital area membrane sometimes weakly suffused with dark brown around crossveins. Crossveins at prefork area simple. Cubital area with a large brown oblique stripe at the end of the second branch of CuA fork. Pterostigma whitish. Apex veinlets white, brown, or dashed, and membrane white or hyaline. Subapical dark band complete, incomplete, or weakly evident, which breaks into small irregular spots and patches on gradates at apex and posterior margin. Hind wing: slightly narrower than forewing. Membrane hyaline before subapical dark band. Apex white with few scattered small dark brown spots at poststigmal area. Hind wing subapical dark band complete but not fully solid, sometimes breaking down into small irregular spots and patches near apex margins. Hind wing subapical dark band with two white spots on apex posterior margin, with the distalmost spot sometimes weakly evident and partially filled with small dark brown irregular spots. Hind wing presectoral area wider than posterior area. Hind wing posterior area narrow, with cells longer than high. Legs: Coxae pale brown, prothoracic coxa with two longitudinal dark brown stripes submedially, remaining coxae with one. Coxae with many delicate, long, white setae. Femora pale brown with an apical dark band, and dark spots at setal bases which sometimes combine forming a slightly dark suffusion. Femora with many decumbent setae, mostly dark, shorter than femoral width, and a few erect dark setae on femora closing face, which are around to or subequal to femoral width. Forefemoral sense hair short, much shorter than femur, and midfemoral sense hair similar in size to forefemoral sense hair. Tibia pale, with an apical and a subbasal dark band, and small dark spots at setal bases. Tibia with many short, decumbent dark setae slightly shorter than tibial width, and a few long dark bristles. Fore and mid tibiae roughly same length as femora. Hind tibia longer than femur. Tibial spurs well developed, fore and midtibial spurs reaching tarsomere III and hind tibial spurs reaching tarsomere II. Tarsomeres pale, with distal margin dark. Basal hind tarsomere longer than distal tarsomere, which is longer than remaining tarsomeres. Pretarsal claws well developed, roughly two thirds the length of distal tarsomere. Abdomen: Abdominal tergites dark brown with basal and distal margins pale brown, tergites IV and V sometimes with a weak transversal subapical pale brown band, and tergite VIII mostly pale brown. Abdominal sternites pale brown, somewhat darker from sternite V–IV. Abdominal sclerites covered with short dark and white setae, which are longer nearing terminalia. Male terminalia: Male tergite IX rounded, with many long dark setae on tergite margins. Male sternite IX split medially, appearing ventrally as two rounded sclerites beset with short dark setae basally and many long dark posteriorly oriented setae on posterior margins. Male ectoproct dark, covered with long dark posteriorly oriented setae. Gonarcus arching dorsal to parameres, slender, with simple apodemes. Parameres subquadrangular, almost two times longer than high, sclerotized, basally bent inwards. Pelta membranous, with many short setae. Female terminalia: tergite IX dark beset with many short dark setae. Ectoproct with many delicate setae dorsally and posteriorly, and many long digging setae on ectoproct sub ventral and ventral regions. Pregenital plate very small, sclerotized, and subtriangular. Anterior gonapophysis inconspicuous. Posterior gonapophysis very short, posterior margin rounded, with many long digging setae. Lateral gonapophysis shorter than ectoproct in lateral view, with many thick digging setae, shorter than those in ectoproct and posterior gonapophysis. Gonapophyseal plates elongate and parallel. Spermatheca posteriorly hook-like.
- Larva: Head: Mandibles pale brown, darker at tip and at tip of the teeth, and longer than head capsule. Mandibles covered with dark setae, which are shorter than mandible width, except at mandible base external face, which are at most the same length as mandible width. Head capsule pale brown, wider than long, dorsally covered with conical dolichasters, and covered with simple dolichasters ventrally. Clypeo-labrum very broad, almost same size as rest of the head capsule on dorsal view, with a triple deeply sinuated posterior margin. Clypeo-labrum posterior margin with a row of long, thick dark dolichasters. Ocular tubercle well raised, with setae between stemmata. Distal palpomere fusiform. Thorax: Thoracic tergites covered with short dark setae, and a few long, dark setae on anterior and posterior tergite margins. Sternum pale brown, with a large dark brown patch sublaterally, near coxae insertion, with many short dark setae and a few long dark setae medially. Pronotum dark brown, with a longitudinal pale brown stripe medially. Pronotum with a small pale brown, dorsally projected process on both anterolateral margins, which are covered with long dark setae. Meso and metathorax pale brown with a brownish tint, with some brown patches and small brown spots on long setal bases at anterior and posterior margins. Mesonotum anterior subsegment narrow. Mesothoracic spiracle borne on tubercle, which is dark brown and longer than wide. Metathorax pale brown. Mesothoracic and metathoracic setiferous processes long, scoli-like, covered with long dark bristles. Legs: Coxae pale brown, with brown patches on external face, and beset with short dark setae. Femora pale brown with an incomplete brown band medially, beset with short dark setae and rows of long dark setae on external face. Tibiae pale brown, with three opposing rows of short dark setae. Tarsi pale brown. Pretarsal claws dark, fore and mid pretarsal claws almost ¼ of tarsi length, while hind pretarsal claws at least half of hind tarsus. Abdomen: Abdominal tergites brown, with two submedial dark brown patches, and lateral margins pale brown. Tergites and sternites covered with short, dark setae, and tergites with a transversal row of thicker setae medially. Abdominal setiferous processes pale brown, scoli-like, covered with long dark setae. Sternite VIII short, pale brown, with a sublateral dark brown semicircular patch on each side, covered with short dark setae and a pair of thick dark setae submedially on posterior margin. Segment IX longer than wide, with poorly developed digging setae.
- Remarks. The holotype was previously stated to be a male [16], but in fact, it is a female (Figure 20a). The species has a wide distribution, occurring from northern Mexico to Costa Rica. It is now registered from Nicaragua and the Mexican states of Morelos, Queretaro, and Sinaloa for the first time. Albeit it shows a bit of morphological variation, the overall pattern seems constant. The head vertex might be raised or short, and the degree of suffusion on forewing subcostal area varies, as does the intensity of the subapical dark band. Glenurus proi can be readily differentiated from the remaining Mexican species by the dashed Costal vein on both wings, the mostly pale brown pronotum, and the contrasting thorax on lateral view.
Glenurus proi larva was previously known by Stange [15], but it is being firstly described here. The larva also shows a very characteristic morphology, as the head capsule is very wide, and the clypeo-labrum posterior margin is conspicuously sinuated (Figure 22). These larvae can be found under large root overhangs and cover themselves with debris similarly to Ascalaphinae larvae, and are very quick moving, in contrast to other Glenurus larvae [15]. It also can inhabit ant mounds (Figure 1e) (Dr. Barry Sullender, personal communication).
- Examined material. MEXICO: Hidalgo, Mpio. Metztitlán, 3 km W San Cristóbal, 1538 m., 20°38′09″ N 98°50′44″ W, 15.VII.2017, Atta mexicana debris Berlese, nest #1, P. E. Skelley, coll. Matorral (1 larva, FSCA); Morelos: Tlaquiltenango, Quilamota, Estación, 05.iv.2013, 18.51157′ N, 99.0367′ W, 1058 m, M. V. Rosas (2♀, CNIN); El Limón, Cuauchichinola, 06.iv.2013, M. V. Rosas (1♀, CNIN); Tepalcingo, Estación El Limón, 03.xi.2012, M. V. Rosas (♀, CNIN); Puebla: Zapotitlán Salinas, Res. Biósf. Tehuacán-Cuicatlán, Jardín Botánico Helia Bravo Hollis, 18°19.956′ N, 97°27.501′ W, 1489 m, 26.viii.2025, Contreras, Lozano, Luna, Marquez, Castrejón, Shannon trap (2♂, CNIN); Querétaro: Jalpan, Saldiveña, 01.ix.2003, S Zambrano (1♀, CNIN); Toliman, km 69 carr Bernal-Peña Blanca, 27.vii.1999, 20°56′ N, 99°50′ W, R Jones (1♀, CNIN); Sinaloa: Panititlan, 21.x.1982, M García (1♀, CNIN); Sonora: Los Álamos, Parque de la Colorada, Sendero Arroyo Alto (Chalatón), 17.ix.2019, 27°00.551′ N, 108°57.206′ W, 510 m, Luz negra y blanca, Contreras, Barba, Cancino, Ardila, Marquez (1♂2♀, CNIN); NICARAGUA: Granada, Volcán Mombacho, Finca San Joaquín, 650 m, 11.825672, −85.988872, trampa Malaise en café bajo cultivo orgánico, 31-III-1998, Col. Jean-Michel Maes (1♀, MEL).
3.1.8. Glenurus snowii Banks
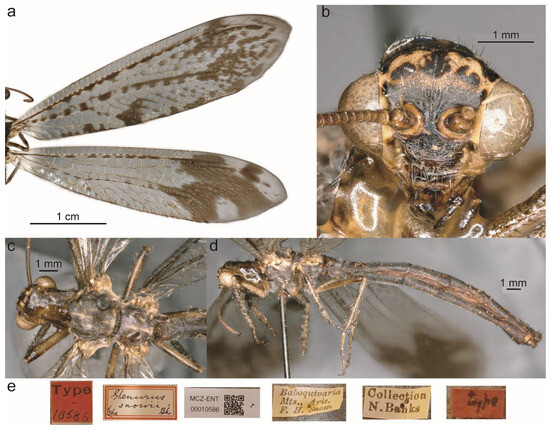
Figure 23.
Glenurus snowii. (a) Fore and hind wings. (b) Holotype frons, (c) thorax, dorsal view, (d) thorax, legs and abdomen, lateral view, and (e) type labels.
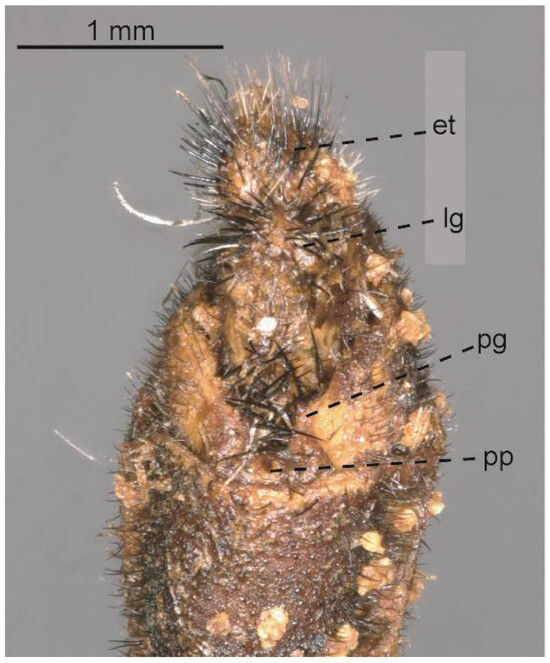
Figure 24.
Glenurus snowii holotype female terminalia. Abbreviations: et: ectoproct, lg: lateral gonapophysis, pg: posterior gonapophysis, pp: pregenital plate.
- Glenurus snowii Banks, 1907a: 100 [50] [Holotype female, Boboquivari Mountains, Arizona (MCZ)]. Banks, 1907b: 30 [51] [species list]. Banks, 1927: 69 [24] [distribution, redescription, taxonomy]. Stange, 1970: 23 [35] [taxonomy, type]. Penny et al., 1997: 77 [37] [distribution, taxonomy]. Stange, 2000: 228 [15] [biology, distribution]. Oswald et al., 2002: 581 [48] [distribution]. Stange, 2004: 179 [16] [distribution, taxonomy, type]. Machado et al., 2019: 425 [6] [phylogeny]. Oswald, 2025 [1] [species list].
- Distribution. Mexico (BC, JAL, NAY, SIN, SON (Oswald et al., 2002)), USA (AZ (Banks, 1907; Stange, 2000)).
- Diagnosis. Pronotum dark brown with a medial longitudinal pale stripe, and two sublateral pale brown bands. Forewing longer than hind wing; forewing subapical dark band broken into small spots; forewing Cubital area dark mark large; hind wing subcostal area suffused; hind wing posterior area similar in size to presectoral area.
- Redescription. Head: Antennae clubbed with 38–43 flagellomeres, dark brown, slightly lighter at the club. Flagellomeres dark brown, with short dark setae. Vertex slightly raised, pale or almost yellowish brown; anterior row with three large dark brown patches and middle row as a dark brown transversal irregular stripe; posterior row with three large semicircular dark brown patches; anterior, middle, and posterior rows connected by a longitudinal dark brown patch. Vertex with short brown, anteriorly oriented decumbent setae. Epicranial mark, interantennal area and frons entirely dark brown, with a few scattered white setae. Interocular distance shorter than pedicel diameter. Genae pale brown. Clypeus dark brown, with rows of long white setae at frontoclypeal margin. Labrum brown, with a subtriangular dark brown patch medially, and with a row of setae at distal margin. Mandible well developed, dark brown. Palpi pale brown, dark brown near joints, with distal palpomere entirely dark brown and fusiform. Palpimacula round. Thorax: Thorax dark with pale lines. Pronotum longer than wide, dark brown, with a longitudinal pale stripe medially, and two incomplete sublateral pale bands. Pronotum with some short dark setae on anterior margin, anteriorly curved white and dark setae at lateral margins, and erect dark setae on posterior margin. Meso and metathorax dark brown, with a longitudinal pale brown medially. Mesoprescutum with erect and decumbent brown setae. Mesoscutum dark brown, with two pale brown marks submedially, and some scattered short dark setae. Mesoscutellum dark brown, pale brown on posterior margin. Mesoscutellum with a few decumbent white setae near posterolateral margin. Postmesoscutellum dark brown. Metaprescutum with some scattered, decumbent dark setae. Metascutum dark brown with two large and darker velvety areas submedially. Metascutellum dark brown, pale brown on posterior margin, with white setae posteriorly oriented. Thorax laterally dark brown, with many scattered white setae, and with dark setae on dorsal area of mesepisternum. Wings: Wings broad, lanceolate. Forewing slightly longer than hind wing, in repose the apex of both wings coincides, or hind wing slightly extends after forewing. Fore and hind wing veins dark brown dashed with pale brown, beset with short black setae. Costal vein dark brown. Forewing: membrane hyaline before subapical dark band, except on subcostal area which is highly suffused with dark brown; on mediocubital area densely suffused with dark brown around crossveins; on a large dark brown patch at cubital area around and above the end of posterior branch of CuA fork; a patch of dark brown suffusion on posterior area just before origin of CuA fork, and a few scattered crossveins that are surrounded by a dark brown tint. Seven to eight presectoral crossveins, simple. Crossveins at prefork area simple. Pterostigma whitish. Apex veinlets white, brown, or dashed, and membrane white or hyaline. Subapical dark band incomplete, mostly formed by or broken into small dark brown spots on gradates at apex, poststigmal area and posterior margin. Apex and poststigmal area whitish or hyaline. Anterior banksian line present but weakly developed, posterior banksian line absent. Hind wing: Membrane hyaline before subapical dark band. Subcostal area densely suffused with dark brown. Pterostigma whitish, and poststigmal area white. Hind wing subapical dark band complete, with a jagged margin, with one white mark on posterior margin, and a whitish spot just below rhegma. Hind wing presectoral area as wide as posterior area. Hind wing posterior area wide, with cells higher than long. Legs: Coxae dark brown with many white setae. Femora pale brown with an apical dark band, and dark spots at setal bases which sometimes combine forming a dark suffusion, mostly on mid and hind femora. Femora with many decumbent setae, mostly dark, and shorter than femoral width, and a few erect dark setae on femora closing face, which are around to or subequal to femoral width. Forefemoral sense hair short, much shorter than femur, and midfemoral sense hair similar in size to forefemoral sense hair. Tibia pale, with an apical and a subbasal dark band, and small dark spots at setal bases. Tibia with many short, decumbent dark setae slightly shorter than tibial width, and a few long dark bristles. Fore and mid tibiae roughly same length as femora. Hind tibia longer than femur. Tibial spurs well developed, reaching tarsomere II. Tarsomeres pale, with distal margin dark. Hind leg basitarsus shorter than distal tarsomere, but longer than remaining tarsomeres. Hind leg basitarsus short, around the length of tarsomeres II and III. Pretarsal claws well developed, roughly half to two thirds the length of distal tarsomere. Abdomen: Abdominal tergites and sternites dark brown with basal and distal margins pale brown. Abdominal sclerites covered with short dark and white setae, which are longer nearing terminalia. Female terminalia: tergite IX dark beset with many short dark setae. Ectoproct with many delicate setae dorsally and posteriorly, and many long, slender digging setae on ectoproct sub ventral and ventral regions. Pregenital plate very small, sclerotized, and subtriangular. Anterior gonapophysis inconspicuous. Posterior gonapophysis very short, posterior margin rounded, with many long digging setae. Lateral gonapophysis shorter than ectoproct in lateral view, with many digging setae, slightly thicker than those in ectoproct. Gonapophyseal plates elongate and parallel.
- Larva. Unknown.
- Remarks. This species was restricted to Arizona (US), until Oswald et al. [48] expanded its distribution to western Mexico. Curiously, this species is the only one treated in this work whose distributional patterns are very different between literature and iNaturalist records (Figure 4 and Figure 5). Despite this species widespread occurrence on western Mexico as reported by Oswald et al. [48], iNaturalist observations for G. snowii are still restricted to Arizona. Whether this means this species is scarcely seen on Mexico despite its wide distribution, or if somehow the Mexican specimens were misidentified, remains to be determined.
This species looks somewhat similar to the Nearctic forms of G. luniger, but can be readily differentiated by the forewings which have much more suffusion across its length, the subapical dark band, which is always broken down into spots, and by the hind wing subcostal area which is suffused with dark brown. The latter is very diagnostic, as the only other Mexican Glenurus with this character is G. maya sp. n., which differs from G. snowii in many other morphological traits.
Larva was previously reared by Stange [15] and stated to live in dry wood debris in a tree stump. However, it remains undescribed, as well as the males.
- Examined material. USA: Arizona: Holotype, Boboquivari Mountains (1♀, MCZ); Pinal Co., Jct Devil’s Cyn. and US Hwy. 60, vii-26-1989, at UV light, W. B. Warner (1?, FSCA).
3.2. Taxonomic Key to Adult Glenurus Species
- 1.
- Thorax laterally pale brown contrasting with pterothoracic pleura dorsally dark brown (Figure 20d). 2
- —
- Thorax laterally dark brown, not contrasting with pterothoracic pleura. 3
- 2.
- —
- Forewing subapical dark band as a solid blackish band (fig. 4 in [20]); forewing C vein dark; forewing posterior area with dark spots at crossveins base; hind basitarsus shorter than distal tarsomere (Argentina). Glenurus penningtoni (Navás)
- 3.
- Forewing without subapical dark band (Figure 3) (Antilles, Panama, to Ecuador and North Brazil). 4
- —
- Forewing with a subapical dark band (Figure 13). 6
- 4.
- —
- Hindwing subapical dark band not broken (Figure 3b,d). 5
- 5.
- Forewing with dark marks on the subcostal area, apex speckled with many dark spots; hindwing slightly longer than forewing; head with frons, scape and pedicel dark brown (Figure 10) (Nicaragua to Ecuador). Glenurus heteropteryx Gerstaecker
- —
- Forewing without dark marks on the subcostal area, apex with two dark spots near rhegma; hindwing same length as forewing; head with frons, scape and pedicel light brown (Figure 8b) (Brazil, Colombia, French Guyana, Martinique, Panama). Glenurus discors Navás
- 6.
- —
- Forewing subapical dark band as a continuous band from stigma to posterior margin (Figure 13); hind wing subcostal area membrane before subapical dark band mostly hyaline. 8
- 7.
- Fore and hind wing apexes mostly whitish; forefemoral sense hair two times longer than femoral width; space between the antennae subequal to the scape diameter; clypeus and labrum pale brown (Figure 16) (Southern Mexico and Guatemala). Glenurus maya sp. n.
- —
- Fore and hind wing apexes mostly dark brown with whitish or hyaline areas; forefemoral sense hair about as long as femoral width; space between the antennae less than scape diameter; clypeus and labrum dark brown (Figure 23) (USA (Arizona) and western Mexico). Glenurus snowii Banks
- 8.
- South America. 9
- —
- Central and North America. 11
- 9.
- Forewing subapical dark band encompasses rhegma completely (fig. 3 in [20]); forewing membrane before subapical dark band mostly covered by dark brown spots around crossveins; forewing cubital area with a large dark brown spot at RP origin; legs yellowish brown (Bolivia to Argentina). Glenurus croesus Banks
- —
- Forewing rhegmal area with a dark brown spot, connecting to or completely separated from the subapical dark band; forewing membrane before subapical dark band mostly hyaline, except for a few areas; forewing cubital area with a dark brown stripe at RP origin; legs pale brown. 10
- 10.
- Hind wing subapical dark band encompassing two whitish marks on posterior margin (fig. 2c in [10]) (Brazil to Argentina). Glenurus peculiaris (Walker)
- —
- Hind wing subapical dark band encompassing a single whitish mark on posterior margin (fig. 1 in [10]) (Peru and Brazil). Glenurus incalis Banks
- 11.
- Femora dark or reddish brown; whitish areas on forewing apex frequently pinkish (fig. 1 in [11]) (Eastern USA). Glenurus gratus (Say)
- —
- Femora pale brown or with a brownish tint on external face (Figure 12); whitish areas on forewing rarely pinkish, if so, only near pterostigma (Western USA to Panama). Glenurus luniger Gerstaecker
4. Discussion
4.1. Biogeography
The Glenurus species are distributed in the New World, and seven of the twelve species of the genus are registered from Mexico and Central America so far. Glenurus maya sp. n. is restricted to the Neotropical region, with altitudes not exceeding 1500 m above sea level, discovered in Chiapas, Mexico, and Huehuete, Guatemala on the Chiapas Highlands, Pacific lowlands, and the Veracruzan provinces (Figure 4 and Figure 5). All provinces are characterized by the presence of various types of tropical, temperate, and deciduous forests. There are also savannas and scrublands, where Glenurus species are commonly encountered (Figure 4 [30]). Furthermore, Glenurus oswaldi sp. n. also has a Neotropical affinity but is restricted to the Lesser Antilles in the British West Indies (Figure 4 and Figure 5). Most likely the preferred types of vegetation for this species are rainforests, dry tropical, dry forests, and scrublands, due those three types of vegetation being reported for the Lesser Antilles and other areas of the Caribbean subregion [30]. Nonetheless, further studies need to be carried out in order to learn more about the biology, behavior, and habits of these species.
Meanwhile, another three species have both Nearctic and Neotropical distribution (G. luniger, G. proi and G. snowii) (Figure 4 [16,48]). Thus, G. luniger has the widest distribution, ranging from the United States to Panama and only Nicaragua lacked records of this species until now (Figure 4). Most records of G. luniger from Mexico are located in low to medium altitudes and in many different biogeographic provinces, ranging from provinces with arid zones to provinces with tropical, deciduous, and rainforests (Figure 4 and Figure 5 [30]). Glenurus proi has widespread presence throughout Mexico and Central America, herein with new records from Guatemala, El Salvador, and Nicaragua (Figure 4). Regarding biogeographical provinces, this species in Mexico is present in arid and semiarid zones such as the Sonoran Desert, Pacific Lowlands provinces, and tropical zones such as Balsas Basin, Sierra Madre del Sur provinces, with elevation ranging from sea level to 2000 m above sea level approx. (Figure 4 and Figure 5). Glenurus snowii is registered only in Mexico and the United States and occurs in arid zones such as the Sonora Desert, Balsas Dry Forest, Tamaulipan Thorn Scrub, and Sierra Madre Oriental, among others (Figure 4). Among the species treated in this work, this species was the only in which its distributional range was not expanded by iNaturalist records. In fact, iNaturalist observations of this species were restricted to the Arizona state (USA) (Figure 4), despite its widespread distribution throughout Mexico’s western coast (Figure 5). Whether this suggests its rarity or to a possible misidentification of the Mexican records, it is unknown.
Glenurus heteropteryx and G. discors are the only two species in this work that are registered to both part of Central America and South America as well, with presence in several countries of the latter (Figure 4 and Figure 5). Tropical affinities and also suggested for those species and types of vegetation are similar as in G. luniger, G. proi, and G. snowii. Regarding the altitude preferred or tolerated for these species probably correspond to low sea levels, but we do not have enough information from known localities.
4.2. Taxonomic Overview
Morphologically, Glenurus is somewhat a uniform genus, and its species show consistent morphological characters [13,35]. Additionally, the genus had been recovered as monophyletic by previous analyses [5,6]. There is a certain similarity between Glenurus and Eremoleon regarding morphological features [13,35,52], but, from another perspective, this is more likely due to Eremoleon’s lack of circumscribing and unifying characters than to a proximity between these two genera, as Eremoleon is a highly heterogeneous genus and probably paraphyletic, as recovered in a previous phylogenetic analysis [6].
Among the species covered here, G. luniger and G. proi males bear a medially split sternite IX (Figure 14 and Figure 21), a unique feature not previously seem in any other Glenurini genera. Although the male genitalia of G. gratus have never been formally described in the literature, we had the opportunity to examine and dissect a specimen of this species, which revealed that G. gratus also possesses a medially split sternite IX. Marquez-López et al. [27] described this feature as an autapomorphy of Glenurus, but as the new species G. maya sp. n. possesses a non-split sternite IX (Figure 17), and it is unclear whether this is a reversion state unique to this species, or if the condition observed in G. luniger, G. gratus, and G. proi represents a synapomorphy for these species and not the whole genus, especially considering that Glenurus male specimens appear to be extremely rare.
In fact, prior to this study, Glenurus male genitalia and terminalia were described only recently, depicted for a single species, G. proi [27]. For all Neotropical species, including those that were not treated in this work (G. croesus, G. incalis, G. peculiaris, and G. penningtoni), all known specimens are females. Glenurus penningtoni holotype is stated to be a male specimen, but so were G. discors and G. proi type specimens, which were here confirmed to be female specimens. This ultimately raises doubts on the sex of the G. penningtoni type specimen especially since these types were described by the same author. Moreover, the type specimen of G. penningtoni is also stated to be missing [16], providing additional grounds for its reevaluation. Additionally, the absolute majority of the Glenurus specimens photographed and submitted as observations through iNaturalist platform are also females. It is unclear as whether this happens because the female specimens are more attracted to lights, more abundant, live longer or for an unknown reason altogether.
Nonetheless, Glenurus taxonomic consistency is further reinforced by its larval morphology and behavior, as its larvae only bear two mandibular teeth and a three segmented labial palpi [13,52] (Figure 15 and Figure 22). Their biology is also unique among Glenurini genera, as the larvae inhabit particulate organic matter instead of sand or loose earth, and are seemingly attracted to dry wood debris, tree stumps, tree holes or animal burrows [15]. This departure from the psammophile lifestyle is reflected in some of their remarkable morphological traits, which are observed in many different lineages within Myrmeleontidae, such as the loss of rastra (Nemoleontinae: Glenurini: Navasoleon [8]; Dendroleontinae: Dendroleontini [53,54,55,56,57]) and the presence of developed scoli-like setiferous processes on thorax and abdomen (Nemoleontinae: Glenurini: Navasoleon [8]; Dendroleontinae: Acanthoplectrini: Layahima Navás, 1912 [58]; Dendroleontinae: Dendroleontini: Nepsalus Navás, 1914 and Gatzara Navás, 1915 [56,57]; Myrmeleontinae: Brachynemurini: Gnopholeon Stange, 1970 [26], Jaffuelia, Navás, 1918 [26], Neulatus Navás, 1918 [26]; Ascalaphinae: Ascalaphini [59], Haplogleniini [60], Ululodini [61]), whose larvae occur on the bare surfaces of rock, bark, leaves, etc. The Glenurus larvae described here also lack the odontoid processes in the sternite VIII, as occurs in some other Glenurini genera (e.g., Ripalda [5] and Navasoleon [8]) and its related tribe Megistopini genera from Eurasia [17,53]. Instead, they curiously bear a pair of conspicuous thick setae on the sternite VIII posterior margin (Figure 25).
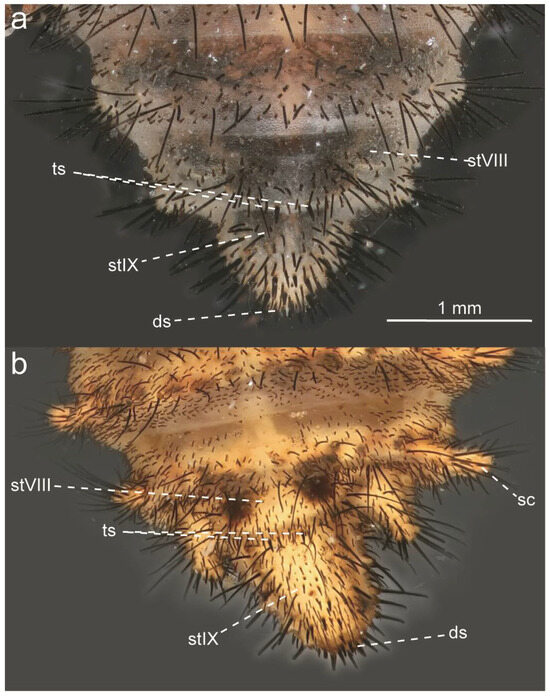
Figure 25.
Glenurus larvae, last abdominal sternites. (a) Glenurus luniger, (b) G. proi. Abbreviations: ds: digging setae, sc: scoli-like process, stIX: ninth sternite, stVIII: eighth sternite, ts: thick setae.
Stange [16] mentions that five species larvae are known, but none of them were properly described in the literature. Glenurus larva mandible was illustrated and compared with other genera [62], and G. gratus larva had photographic images published [11,19], but never described. This species also has had its habitats and biology described a few times [11,14,15,19], but the remaining species mentioned as having known larvae (G. luniger, G. penningtoni, G. proi, and G. snowii) had only their biology described [15], and G. gratus, G penningtoni, and G. snowii larval stage still remains to be described, while the latter two species larvae morphology is still unknown to the literature.
Furthermore, despite Glenurus apparent monophyletic state and uniform morphology, some species are still in need of taxonomic treatment. Glenurus gratus, the type species, has its holotype specimen stated as missing [16]. Additionally, G. croesus, once synonymized under G. penningtoni [40], later had its species status reinstated [16]. Although a taxonomic key was provided with a character distinguishing both species [20] (Petko et al., 2016), no images or illustrations for such character were provided and these two species still lack an updated taxonomic treatment.
Author Contributions
L.G.M.T. conceptualization—equal, investigation—equal, methodology—equal, visualization—equal, writing—original draft, writing—review and editing—equal. Y.M.-L. investigation—equal, methodology—equal, validation—equal, visualization—equal, writing—review and editing—equal. R.J.P.M. investigation—equal, methodology—equal, validation—equal, visualization—equal, writing—review and editing—equal. Y.Z. investigation—equal, methodology—equal, validation—equal, visualization—equal, writing—review and editing—equal. A.C.-R. funding acquisition—equal, supervision—equal, validation—equal, writing—review and editing—equal. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by project “Biodiversidad de Diptera y Neuropteroidea de la Reserva de la Biósfera Tehuacán-Cuicatlán con herramientas novedosas”, UNAM-DGAPA-PAPIIT IN216824.
Data Availability Statement
All relevant data are contained within the article.
Acknowledgments
We would like to thank all museums and collections that made specimens available through loan or pictures: André Nel (MNHN), Lara Lopardo, and Peter Michalik (ZIMG), Charles Farnum (MCZ), Elijah J. Talamas (FSCA), and Robert Jones (CIUAQ). Special thanks to Cristy Mayorga, technical curator at CNIN, for general support. L.G.M.T. thanks Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES, proc. 88887.569801/2020) and CAPES-PRINT (PRINT—Programa Institucional de Internacionalização, proc. 88887.750120/2022-00). R.J.P.M. thanks the Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq) (grant number 402785/2021-5). Y.M.-L. thanks SECIHTI-Mexico for a doctoral studies fellowship number 718709.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CNIN | Colección Nacional de Insectos, Instituto de Biología, UNAM, Mexico City, Mexico |
| CIUAQ | Colección de Insectos, Universidad Autónoma de Querétaro, Querétaro, Mexico |
| FSCA | Florida State Collection of Arthropods, Gainesville, USA |
| MCZ | Museum of Comparative Zoology, Cambridge, USA |
| MEL | Museo Entomológico de León, León, Nicaragua |
| MNHN | Muséum National d’Histoire Naturelle, Paris, France |
| ZIMG | Zoological Institute and Museum Greifswald, Greifswald, Germany |
References
- Oswald, J.D. Neuropterida Species of the World. Lacewing Digital Library, Research Publication No. 1. Available online: http://lacewing.tamu.edu/SpeciesCatalog/Main (accessed on 5 May 2024).
- Mansell, M.W. Evolution and success of antlions (Neuropterida: Neuroptera: Myrmeleontidae). Stapfia 1999, 60, 49–58. [Google Scholar]
- Miller, R.B. Reproductive characteristics of some western hemisphere ant-lions (Insecta: Neuroptera: Myrmeleontidae). In Advances in Neuropterology: Proceedings of the Third International Symposium on Neuropterology, Berg en Dal, Kruger National Park, South Africa, 3–4 February 1988; Mansell, M.W., Aspöck, H., Eds.; South African Department of Agricultural Development: Pretoria, South Africa, 1990; pp. 171–179. [Google Scholar]
- Miller, R.B.; Stange, L.A. A revision of the genus Eremoleon Banks (Neuroptera: Myrmeleontidae: Nemoleontini). Insecta Mundi 2016, 495, 1–111. [Google Scholar]
- Badano, D.; Miller, R.; Stange, L.A. Rediscovery and revision of the antlion genus Ripalda Navás within a phylogeny of Nemoleontini (Neuroptera, Myrmeleontidae). Invertebr. Syst. 2018, 32, 933–949. [Google Scholar] [CrossRef]
- Machado, R.J.P.; Gillung, J.P.; Winterton, S.L.; Garzón-Orduña, I.J.; Lemmon, A.R.; Lemmon, E.M.; Oswald, J.D. Owlflies are derived antlions: Anchored phylogenomics supports a new phylogeny and classification of Myrmeleontidae (Neuroptera). Syst. Entomol. 2019, 44, 418–450. [Google Scholar] [CrossRef]
- Miller, R.B.; Stange, L.A. Revision of the genus Dimarella Banks (Neuroptera: Myrmeleontidae). Insecta Mundi 1989, 3, 11–40. [Google Scholar]
- Stange, L.A.; Miller, R.B. A revision of the genus Navasoleon Banks (Neuroptera: Myrmeleontidae: Nemoleontini). Insecta Mundi 2018, 619, 1–25. [Google Scholar]
- Ardila Camacho, A.; Arango Díaz, C.J.; Noriega, J.A. First record of Glenurus heteropterix [sic; for heteropteryx] Gerstaecker, 1885 (Neuroptera: Myrmeleontidae) from Colombia. Check List 2014, 10, 692–693. [Google Scholar] [CrossRef]
- Machado, R.J.P. Rediscovery of Glenurus incalis Banks (Neuroptera: Myrmeleontidae), and notes on the Brazilian species of Glenurus Hagen. Zootaxa 2020, 4858, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Stange, L.A. An antlion, Glenurus gratus (Say) (Insecta: Neuroptera: Myrmeleontidae): EENY-393/IN708, 10/2006. In Entomology and Nematology Publication Series; Institute of Food and Agricultural Sciences Extension, University of Florida: Gainesville, FL, USA, 2015; Volume 393, pp. 1–3. [Google Scholar]
- Stange, L.A. The ant-lions of Florida. I. Genera. In Entomology Circular; Division of Plant Industry, Florida Department of Agriculture and Consumer Services: Tallahassee, FL, USA, 1980; Volume 215, pp. 1–4. [Google Scholar]
- Stange, L.A. Family Myrmeleontidae. In A Guide to the Lacewings (Neuroptera) of Costa Rica; Proceedings of the California Academy of Sciences; Penny, N.D., Ed.; Department of Entomology, Iowa State University: Ames, IA, USA, 2002; Volume 53, pp. 275–289. [Google Scholar]
- Davis, D.R.; Milstrey, E.G. Description and biology of Acrolophus pholeter (Lepidoptera: Tineidae), a new moth commensal from Gopher Tortoise burrows in Florida. Proc. Entomol. Soc. Wash. 1988, 90, 164–178. [Google Scholar]
- Stange, L.A. Observations on the biology of the antlion genus Glenurus Hagen (Neuroptera: Myrmeleontidae). Insecta Mundi 2000, 14, 228. [Google Scholar]
- Stange, L.A. A systematic catalog, bibliography and classification of the world antlions (Insecta: Neuroptera: Myrmeleontidae). Mem. Am. Entomol. Inst. 2004, 74, 1–565. [Google Scholar]
- Zheng, Y.; Liu, X. A revision of the Oriental antlion genera Indoleon Banks, 1913, Indophanes Banks, 1940 and Yunleon Yang, 1986 (Neuroptera: Myrmeleontidae: Nemoleontinae). Zootaxa 2023, 5239, 41–78. [Google Scholar] [CrossRef] [PubMed]
- Giacomino, M. Contribution à la connaissance des Neuroptera des Antilles françaises: L. Les Myrmeleontinae de Guadeloupe (Neuroptera Myrmeleontidae). Entomologiste 2015, 71, 153–156. [Google Scholar]
- Miller, R.B.; Stange, L.A. The ant-lions of Florida. Glenurus gratus (Say) (Neuroptera: Myrmeleontidae). In Entomology Circular; Division of Plant Industry, Florida Department of Agriculture and Consumer Services: Tallahassee, FL, USA, 1983; Volume 251, pp. 1–2. [Google Scholar]
- Petko, O.; Smith, P.; Stange, L.A.; Rios, S.D. New antlion records (Neuroptera: Myrmeleontidae) from Paraguay. Insecta Mundi 2016, 487, 1–8. [Google Scholar]
- Banks, N. South America Glenurus and some other Myrmeleonidae. Can. Entomol. 1922, 54, 58–60. [Google Scholar] [CrossRef]
- Banks, N. Notes on native Myrmeleonidae. Ann. Entomol. Soc. Am. 1938, 31, 413–421. [Google Scholar] [CrossRef]
- Banks, N. Neuroptera of northern South America. Part II. Myrmeleonidae. Bol. Entomol. Venez. 1943, 2, 161–173. [Google Scholar]
- Banks, N. Revision of the Nearctic Myrmeleonidae [sic]. Bull. Mus. Comp. Zool. 1927, 68, 1–84. [Google Scholar]
- Cummings, J.M. Lactic acid as an agent for macerating Diptera specimens. Fly Times 1992, 8, 7. [Google Scholar]
- Stange, L.A. Reclassification of the New World antlion genera formerly included in the tribe Brachynemurini (Neuroptera: Myrmeleontidae). Insecta Mundi 1994, 8, 67–119. [Google Scholar]
- Marquez-López, Y.; Martins, C.C.; Guevara-Chumacero, L.M.; Ramírez-Ponce, A.; Contreras-Ramos, A. Comparative morphology of male genitalia in antlions (Insecta, Neuroptera, Myrmeleontidae), with emphasis on owlflies (Ascalaphinae) and a possible structural evolutionary scenario. J. Morphol. 2024, 285, e21701. [Google Scholar] [CrossRef]
- Breitkreuz, L.C.V.; Winterton, S.L.; Engel, M.S. Wing tracheation in Chrysopidae and other Neuropterida (Insecta): A resolution of the confusion about vein fusion. Am. Mus. Novit. 2017, 3890, 1–44. [Google Scholar] [CrossRef]
- Machado, R.J.P.; Oswald, J.D. Morphological phylogeny and taxonomic revision of the former antlion subtribe Periclystina (Neuroptera: Myrmeleontidae: Dendroleontinae). Zootaxa 2020, 4796, 1–322. [Google Scholar] [CrossRef] [PubMed]
- Morrone, J.J. Biogeografía de América Latina y el Caribe, 1st ed.; M&T–Manuales & Tesis SEA; Piera, F.M., Ed.; Sociedad Entomológica Aragonesa: Zaragoza, Spain, 2001; Volume 3. [Google Scholar]
- Morrone, J.J.; Escalante, T.; Rodríguez-Tapia, G.; Carmona, A.; Arana, M.; Mercado-Gómez, J.D. Biogeographic regionalization of the Neotropical region: New map and shapefile. An. Acad. Bras. Ciênc. 2022, 94, e20211167. [Google Scholar] [CrossRef] [PubMed]
- GBIF.org (May, 2025) GBIF Occurrence Download. Available online: https://www.gbif.org/occurrence/download/0032492-251120083545085 (accessed on 29 November 2025).
- Hagen, H.A. Hemerobidarum synopsis synonymica. Stett. Entomol. Ztg. 1866, 27, 369–462. [Google Scholar]
- Hagen, H.A. Die Larven von Myrmeleon. Stett. Entomol. Ztg. 1873, 34, 249–295, 377–398. [Google Scholar]
- Stange, L.A. A generic revision and catalog of the western Hemisphere Glenurini with the description of a new genus and species from Brazil (Neuroptera: Myrmeleontidae). Contrib. Sci. 1970, 186, 1–28. [Google Scholar] [CrossRef]
- Penny, N.D. Lista de Megaloptera, Neuroptera e Raphidioptera do México, América Central, ilhas Caraíbas e América do Sul. Acta Amaz. 1977, 7, 5–61. [Google Scholar] [CrossRef]
- Penny, N.D.; Adams, P.A.; Stange, L.A. Species catalog of the Neuroptera, Megaloptera, and Raphidioptera of America north of Mexico. Proc. Calif. Acad. Sci. 1997, 50, 39–114. [Google Scholar]
- Machado, R.J.P.; Califre Martins, C. The extant fauna of Neuroptera (Insecta) from Brazil: Diversity, distribution and history. Rev. Bras. Entomol. 2023, 66, 1–12. [Google Scholar] [CrossRef]
- Navás, L. Algunos insectos de la República Argentina. Rev. Real Acad. Cienc. Exact. Fís. Nat. Madr. 1918, 16, 491–504. [Google Scholar]
- Navás, L. Algunos insectos del Museo de París. 2.a serie [IIa]. Brotéria (Zool.) 1924, 21, 99–114. [Google Scholar]
- Navás, L. Sur des Névroptères nouveaux ou critiques. Deuxième [II] série. Ann. Soc. Sci. Brux. 1920, 39 Pt 2, 189–203. [Google Scholar]
- Navás, L. Estudis sobre Neuròpters (Insectes). Arxius Inst. Ciències (Secció Ciències) 1923, 7, 179–203. [Google Scholar]
- Gerstaecker, C.E. Vier Decaden von Neuropteren aus der Familie Megaloptera Burm. Mitt. Naturwiss. Ver. Neu-Vorpommern Rügen 1885, 16, 1–49. [Google Scholar]
- Navás, L. Insectos suramericanos. Décima [X] serie. Rev. Real Acad. Cienc. Exact. Fís. Nat. Madr. 1935, 32, 360–375. [Google Scholar]
- Whittington, A.E. Resources in Scottish Neuropterology. Acta Zool. Acad. Sci. Hung. 2002, 48 (Suppl. 2), 371–387. [Google Scholar]
- Gerstaecker, C.E. Ueber neue und weniger gekannte Neuropteren aus der Familie Megaloptera Burm. Mitt. Naturwiss. Ver. Neu-Vorpommern Rügen 1893, 25, 93–173. [Google Scholar]
- Henry, C.S.; Penny, N.D.; Adams, P.A. The neuropteroid orders of Central America (Neuroptera and Megaloptera). In Insects of Panama and Mesoamerica: Selected Studies; Quintero, D., Aiello, A., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 432–458. [Google Scholar]
- Oswald, J.D.; Contreras-Ramos, A.; Penny, N.D. Neuroptera (Neuropterida). In Biodiversidad, Taxonomía y Biogeografía de Artrópodos de México: Hacia Una Síntesis de Su Conocimiento; Llorente Bousquets, J., Morrone, J.J., Eds.; Universidad Nacional Autónoma de México: México, México, 2002; Volume 3, pp. 559–581. [Google Scholar]
- Navás, L. Insectos neotropicos. 5.a serie. Rev. Chil. Hist. Nat. 1929, 33, 17–24. [Google Scholar]
- Banks, N. A new antlion-fly from Arizona. Entomol. News 1907, 18, 100–101. [Google Scholar]
- Banks, N. Catalogue of the Neuropteroid Insects (Except Odonata) of the United States; American Entomological Society: Philadelphia, PA, USA, 1907; 53p. [Google Scholar]
- Stange, L.A.; Miller, R.B. Classification of the Myrmeleontidae based on larvae (Insecta: Neuroptera). In Advances in Neuropterology: Third International Symposium on Neuropterology; Proceedings; Mansell, M.W., Aspöck, H., Eds.; Department of Agricultural Development: Pretoria, South Africa, 1990; pp. 151–169. [Google Scholar]
- Badano, D.; Pantaleoni, R.A. The larvae of European Myrmeleontidae (Neuroptera). Zootaxa 2014, 3762, 1–71. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, X. First description of immature stages of the antlion Bullanga florida (Navás, 1913) (Neuroptera, Myrmeleontidae, Dendroleontini). Zootaxa 2020, 4858, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hayashi, F.; Matsumoto, R.; Liu, X. The antlions of the Dendroleon pupillaris group (Neuroptera, Myrmeleontidae, Dendroleontinae), with description of three new species from China. J. Asia-Pac. Entomol. 2024, 27, 102181. [Google Scholar] [CrossRef]
- Zheng, Y.; Hayashi, F.; Price, B.W.; Liu, X. Unveiling the evolutionary history of a puzzling antlion genus Gatzara Navás (Neuroptera: Myrmeleontidae: Dendroleontinae) based on systematic revision, molecular phylogenetics, and biogeographic inference. Insect Syst. Divers. 2022, 6, 4. [Google Scholar] [CrossRef]
- Zheng, Y.; Tu, Y.; Mai, Z.; Badano, D.; Liu, X. The Asian rock-dwelling antlions Gatzara Navás, 1915 and Nepsalus Navás, 1914 (Neuroptera: Myrmeleontidae): New advancements in systematics, biogeography and life history. Invertebr. Syst. 2024, 38, IS24010. [Google Scholar] [CrossRef]
- Gravely, F.H.; Maulik, S. Notes on the development of some Indian Ascalaphidae and Myrmeleonidae. Rec. Indian Mus. Calcutt 1911, 6, 101–110. [Google Scholar]
- Badano, D.; Pantaleoni, R.A. The larvae of European Ascalaphidae (Neuroptera). Zootaxa 2014, 3796, 287–319. [Google Scholar] [CrossRef] [PubMed]
- Ardila-Camacho, A.; Jones, J.R. A new species of Haploglenius Burmeister, 1839 (Neuroptera: Ascalaphidae) from the Colombian Orinoquía. Zootaxa 2012, 3268, 40–46. [Google Scholar] [CrossRef]
- Pires Machado, R.J.; Siqueira Oliveira, S.; Ribamar Lopes, W.; Pujol Luz, J.R. Description of the larva and updated distribution of Albardia furcata van der Weele (Neuroptera: Myrmeleontidae). Rev. Bras. De Entomol. 2021, 65, 1–8. [Google Scholar]
- Stange, L.A. The ant-lions of Florida. II. Genera based on larvae. In Entomology Circular; Division of Plant Industry, Florida Department of Agriculture and Consumer Services: Tallahassee, FL, USA, 1980; Volume 221, pp. 1–4. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).