Abstract
A new species of Bauhinia from the Brazilian Cerrado is described. Based on floral morphology, it can be assigned to Bauhinia sect. Pauletia ser. Cansenia, but its vegetative characters distinguish it from all other known members of the group. To elucidate its taxonomic position, we conducted detailed morphological comparisons, and molecular phylogenetic analyses using the nuclear ribosomal ITS region and two plastid markers (matK and trnL–F). Bayesian Inference (BI) and Maximum Likelihood (ML) analyses were performed. Our results support (both PP from BI and BP from ML) the placement of this new species within Bauhinia ser. Cansenia. The new species named B. latistipulata sp. nov. is easily distinguished from other species of Bauhinia ser. Cansenia by its reniform stipules and cymbiform bracts. The species shows morphological similarities to B. ungulata, sharing elliptic to ovate leaf blades with obtuse apices, tomentose ovaries, and clavate stigmas. However, it differs by its hirsute abaxial leaf surface (vs. tomentose), tubular and ribbed flower buds (vs. clavate or subclavate and smooth), tomentose staminal tube (vs. pubescent), and distinct color of the ovary indumentum (white vs. ferruginous).
1. Introduction
The genus Bauhinia L. (Fabaceae) is the most diverse within the subfamily Cercidoideae, comprising approximately 230 species [,]. It has a pantropical distribution and is characterized by trees or shrubs with unifoliolate (occasionally bifoliolate), entire or bilobed leaves, pentamerous and hermaphroditic flowers, 1–10 stamens, and a stipitate ovary [,,]. Currently, the genus is divided into eight sections: Afrobauhinia Wunderlin, K.Larsen, & S.S.Larsen, Alvesia (Welw.) Wunderlin, K.Larsen & S.S. Larsen, Amaria (S. Mutis ex Caldas) Endl., Bauhinia, Micralvesia Wunderlin, K.Larsen, & S.S.Larsen, Pauletia (Cav.) DC., Pseudophanera Wunderlin, K.Larsen & S.S.Larsen e Telestria (Raf.) Wunderlin, K.Larsen & S.S.Larsen [].
Bauhinia sect. Pauletia contains about 80 species distributed across tropical America and Southeast Asia, making it the largest section of Bauhinia [,,,,]. It is particularly relevant in Brazil, where over 60 species are found, making the country the center of diversity for this section [,]. It is morphologically characterized by tubular, fusiform, or clavate flower buds, whitish petals, 5–10 fertile stamens, and filaments fused at the base into an irregular tube [,,,]. The sect. Pauletia was delimited by Wunderlin et al. [], with Brazilian species later revised by Vaz [], and a synopsis of Pauletia in Brazil was published by Vaz & Tozzi []. The section is organized in six series: Acuminatae Wunderlin, K.Larsen & S.S.Larsen; Aculeatae Vaz & A.M.G.Azevedo; Ariaria (Cuervo Márq.) Wunderlin, K.Larsen & S.S.Larsen; Cansenia (Raf.) Wunderlin, K. Larsen & S.S. Larsen; Pentandrae Wunderlin, K.Larsen & S.S.Larsen; and Perlebia (Mart.) Wunderlin, K.Larsen & S.S.Larsen.
Bauhinia sect. Pauletia ser. Cansenia includes approximately 60 species distributed throughout tropical America [,,,,,]; 43 occur in Brazil [], making it the largest series of the genus. Brazilian species of Bauhinia ser. Cansenia were revised by Vaz and Tozzi [], and they are characterized by the absence of prickles, paired extrafloral nectaries hidden by stipules, inflorescences in terminal pseudoraceme, a calyx with 2–5 lobes at anthesis, linear to lanceolate petals (rarely obovate), and a clavate stigma []. Recent studies have described several new Bauhinia species in Brazil, highlighting ongoing taxonomic discoveries [,,,,,] or tested species circumscriptions [].
Here, we describe a new species of Bauhinia discovered during a field expedition in the state of Mato Grosso, Midwest of Brazil. Floral features indicate it as a member of B. ser. Cansenia, but it exhibits distinct vegetative features if compared to other species of this series. Thus, we investigated its phylogenetic position, and we also provide taxonomic comments and diagnostic comparisons to closely related species.
2. Materials and Methods
2.1. Morphology, Distribution, and Conservation Status
Specimens were collected in the SESC Serra Azul Private Natural Heritage Reserve (RPPN). This reserve is located in the municipality of Nobres, and in Xingu State Park, municipality of Santa Cruz do Xingu, both in Mato Grosso State, Brazil. We also examined digitized specimens, used for morphological reference and occurrence mapping, available online from speciesLink [] and Reflora. [], from the herbaria HUEFS, IAN, K, MO, NY, RB [], and physical specimens from UFMT. Morphological descriptions were based on collected samples and deposited vouchers, following the terminology of Harris and Harris [] and Radford et al. []. For Bauhinia-specific terminology, we adhered to Vaz [] and Vaz and Tozzi []. QGIS (version 3.4.2) [] was used to generate distribution maps based on voucher coordinates. The extent of occurrence (EOO) and area of occupancy (AOO) were calculated using GeoCAT [], and the conservation status was assessed following IUCN guidelines, Criterion B [].
2.2. Molecular Phylogenetics
This study includes 54 species of Bauhinia sensu Sinou et al. [], as well as two species of Phanera Lour., and one of Cheniella R.Clark & Mackinder. Adenolobus garipensis (E.Mey.) Torre & Hillc. was included as an outgroup based on Sinou et al. []. Our sampling includes species from all sections of the genus: Afrobauhinia (10 spp.), Alvesia (4 spp.), Amaria (5 spp.), Bauhinia (7 spp.), Micralvesia (4 spp.), Pauletia (21 spp.), Pseudophanera (1 spp.), and Telestria (2 spp.). The sequences of the nuclear ribosomal region ITS (nrITS; ITS1+5.8S+ITS2), the plastid trnL–F spacer region, and the plastid gene matK were retrieved from Genbank (NCBI). Sequences were generated in this study only for the new species. Samples of the new species were collected during fieldwork (the type specimen served as voucher for the sequences we generated) and were preserved in silica gel for DNA extraction []. Total DNA was extracted from 50 mg of silica-gel-dried tissue using the 2×CTAB method []. The primers C and F from Taberlet et al. [] were used to amplify the plastid trnL–F spacer region. For the analysis of nuclear ribosomal ITS (nrITS; ITS1+5.8S+ITS2), we used the primers of Sun et al. []. PCRs were carried out using a final volume of 25 μL: 12.5 μL of Dream TAq mix (Thermofisher, Waltham, MA, USA); 0.5 μL forward primer; 0.5 μL reverse primer; 10.5 μL H2O; and 1 μL total DNA. A specific program was used for each primer pair, with the following temperature changes: trnL–F: 94 °C for 1 min; 35 cycles of 94 °C for 30 sec., 53 °C for 40 sec., and 72 °C for 40 sec.; followed by a final extension at 72 °C for 5 min; nrITS: 94 °C for 3 min; 35 cycles of 94 °C for 45 sec., 56 °C for 1 min, 72 °C for 2 min; followed by a final extension at 72 °C for 5 min. The PCR products were then prepared for sequencing using a BigDye® Terminator v. 3.1 Cycle Sequencing kit (Applied Biosystems, Waltham, MA, USA) and sequenced with an ABI3730xl Genetic Analyzer (Applied Biosystems, Waltham, MA, USA).
Sequences were assembled and aligned using Geneious Prime v.2021.1.1 (Biomatters, Auckland, New Zealand). GenBank accessions are available in Appendix A. For alignment, we used the MUSCLE [] plugin implemented on Geneious. Substitution models for each region were selected using JModelTest v.2.1.10 [] with the Bayesian information criterion. We employed GTR+G for all regions. For calculating the Bayesian Inference (BI), we used MrBayes v.3.2.7a [], implemented at the CIPRES Science Gateway portal []. We used two independent runs with four chains each, with Markov chain Monte Carlo parameters (MCMC) defined for 40,000,000 generations, sampling every 4000 trees. The first 2500 trees were discarded as burn-in (25%). Convergence between the runs was verified using Tracer v.1.6, searching for ESS values above 200 []. Clades with ≥0.95 posterior probability (PP) were considered strongly supported, and those between 0.94 and 0.85 were classified as moderately supported. Maximum Likelihood (ML) analysis was performed using RAxML v.8.1.20 [], also implemented at the CIPRES Science Gateway portal, with 1000 pseudoreplicates of thorough bootstrap using the same models cited above. Clades with bootstrap percentages (BP) ≥ 85% were considered strongly supported, and between 0.84 and 0.70 moderately supported [,,]. The trees were edited using the software FigTree v.1.4.4 [].
3. Results
The Bayesian Inference results strongly support the division of Bauhinia into two clades (Clade A, PP = 1.00; Clade B, PP = 1.00), although the Maximum Likelihood analysis results provided only low to moderate support (Clade A, BP = 82; Clade B, BP = 81) (Figure 1 and Figure S1). Bauhinia sect. Pauletia was recovered as non-monophyletic, with representatives distributed across both Clades A and B. Within Clade B, species of B. sect. Pauletia further segregated into two strongly supported but unrelated subclades in both BI and ML analyses (Clade C, PP = 1.00, BP = 100; Clade D, PP = 1.00, BP = 75; Figure 1 and Figure S1). The new species was placed within Clade D, forming a well-supported subclade (Clade E, PP = 0.93, BP = 93) in both analyses. Although all species in Clade E belong to B. sect. Pauletia ser. Cansenia, their internal relationships lack support. The new species was found within a polytomy alongside B. longicuspis Spruce ex Benth., B. pulchella Benth., B. rufa (Bong.) Steud., B. subclavata Benth., and B. ungulata L. Other species of B. ser. Cansenia were placed in Clade C, rendering the series polyphyletic.
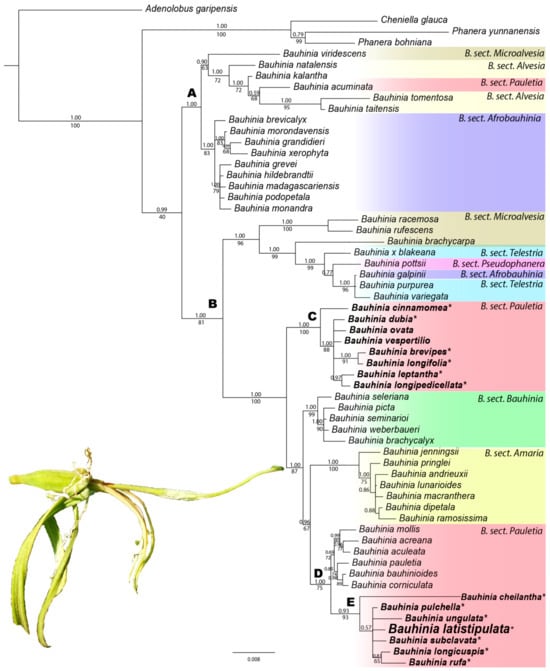
Figure 1.
Phylogenetic relationships of Bauhinia produced by Bayesian Inference based on nuclear nrITS, and plastids matK and trnL–F combined (50% majority rule consensus tree) showing the relationship of the new species. Posterior probabilities from Bayesian Inference (PP ≥ 0.5) are indicated above branches, and Maximum Likelihood bootstrap percentages (BP ≥ 50) are indicated below branches. Species in bold and marked with “*” belong to B. sect. Pauletia ser. Cansenia.
4. Discussion
As confirmed by our molecular phylogenetic analyses and following the current classification for Bauhinia [,,], the new species is placed within B. sect. Pauletia ser. Cansenia. Morphological characters suggest placement within B. sect. Pauletia, supported by its tubular or clavate flower buds, tubular hypanthium, white linear petals, and an irregularly fused staminal column. It belongs to ser. Cansenia due to its pseudoracemose inflorescences and calyces with five-lobed apices at anthesis. The species is distinguished from other members of ser. Cansenia by its reniform stipules (up to 2.0 cm long) with cuspidate apices and pilose abaxial surface, hirsute white abaxial leaf surfaces, and cymbiform bracts and bracteoles. Although morphology supports placement in Cansenia, our phylogeny reveals that both the section and series may be polyphyletic, challenging current classification systems. While B. sect. Pauletia had previously been identified as non-monophyletic by Sinou et al. [], their study included only five species of B. ser. Cansenia (corresponding to our Clade E), which formed a monophyletic group in their analyses, as in ours. Our results therefore provide the first evidence for the non-monophyly of this series when considering broader sampling. However, we acknowledge that our dataset includes a limited number of ITS sequences, which may reduce phylogenetic resolution and lead to some collapsed relationships within clades. This limited resolution could partially influence the inferred non-monophyly of ser. Cansenia. Nevertheless, its division into two well-supported clades is consistent across both Bayesian Inference and Maximum Likelihood analyses, suggesting that the observed topology is not solely an artifact of marker choice.
Among B. sect. Pauletia ser. Cansenia, the new species shows affinities with B. ungulata L. and B. cupulata Benth. (the latter not included in our molecular sampling) through shared elliptic to ovate leaf blades with obtuse apices, tomentose ovaries, and clavate stigmas [,]. However, B. ungulata differs by lanceolate or ovate stipules (vs. reniform), tomentellous abaxial leaf surfaces (vs. hirsute), ovate or lanceolate bracts (vs. cymbiform), and smooth clavate flower buds (vs. tubular and ribbed). Unlike B. cupulata, which shows ovate stipules (vs. reniform), villous abaxial leaf surfaces (vs. hirsute), ovate bracts (vs. cymbiform), smooth clavate flower buds (vs. tubular and ribbed), ferruginous-tomentose inner staminal tube (vs. white-tomentose), ferruginous-tomentose inner hypanthium (vs. glabrous), and ferruginous-tomentose ovary (vs. white-tomentose) (Table 1).

Table 1.
Comparative morphological analysis of Bauhinia latistipulata and related species.
A notable intraspecific variation was observed in the flower bud morphology (tubular to ribbed, smooth or striate, aristate or winged), in the staminal column indumentum (uniformly tomentose or with tufted trichomes).
Taxonomic Treatment
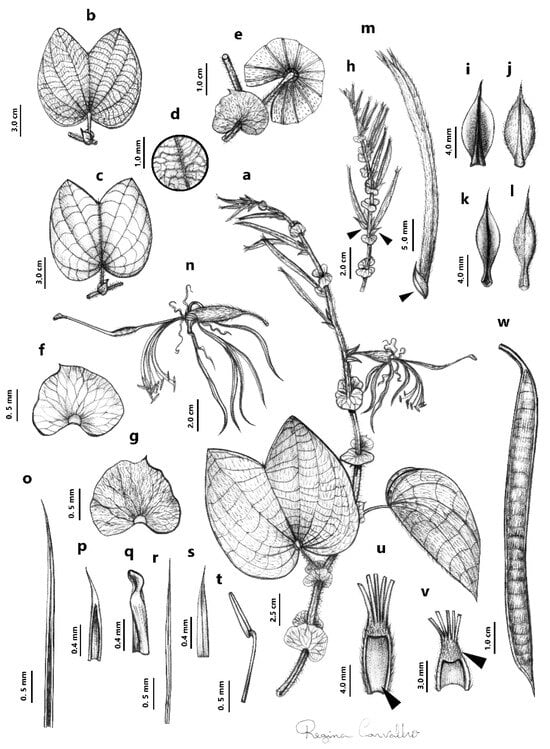 Figure 2. Bauhinia latistipulata D.S. Amorim & E. Pessoa. (a) Habit. (b) Abaxial surface leaf. (c) Adaxial surface leaf. (d) Detail of the abaxial surface leaf, illustrating venation pattern and trichome distribution. (e) Stipule position on the branch. (f) Adaxial surface stipule. (g) Abaxial surface stipule. (h) Inflorescence, showing bracts at the base of the pedicel (arrow). (i,j) Adaxial and abaxial surface bracteole, respectively. (k,l) Adaxial and abaxial surface bracts, respectively. (m) Flower bud, showing bracteole at the base of the flower bud (arrow). (n) Flower. (o) Sepal. (p) Aristate sepal apex. The extended midvein of the sepal forms an aristate apex. (q) Variation in the sepal apex, showing its winged form. (r) Petal. (s) Apex petal. (t) Stamen. (u) Longitudinal section of the hypanthium showing the glabrous internal structure (arrow). Above is the staminal tube. (v) Tomentose inner staminal tube (arrow). (w) Fruit. Illustrated by Regina Carvalho, based on the holotype and paratype.
Figure 2. Bauhinia latistipulata D.S. Amorim & E. Pessoa. (a) Habit. (b) Abaxial surface leaf. (c) Adaxial surface leaf. (d) Detail of the abaxial surface leaf, illustrating venation pattern and trichome distribution. (e) Stipule position on the branch. (f) Adaxial surface stipule. (g) Abaxial surface stipule. (h) Inflorescence, showing bracts at the base of the pedicel (arrow). (i,j) Adaxial and abaxial surface bracteole, respectively. (k,l) Adaxial and abaxial surface bracts, respectively. (m) Flower bud, showing bracteole at the base of the flower bud (arrow). (n) Flower. (o) Sepal. (p) Aristate sepal apex. The extended midvein of the sepal forms an aristate apex. (q) Variation in the sepal apex, showing its winged form. (r) Petal. (s) Apex petal. (t) Stamen. (u) Longitudinal section of the hypanthium showing the glabrous internal structure (arrow). Above is the staminal tube. (v) Tomentose inner staminal tube (arrow). (w) Fruit. Illustrated by Regina Carvalho, based on the holotype and paratype.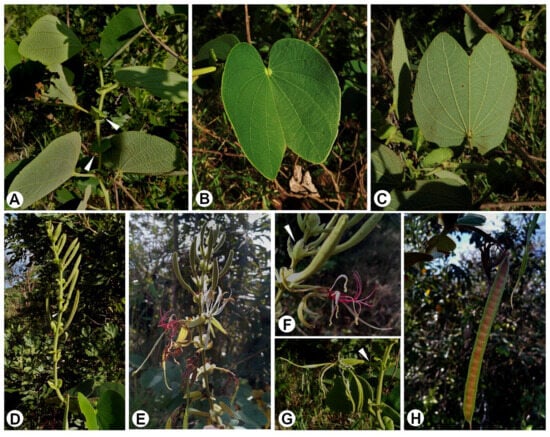 Figure 3. General of aspects of Bauhinia latistipulata. (A) Habit. Stipules highlighted by the arrow. (B) Adaxial surface leaf. (C) Abaxial surface leaf. (D,E) Inflorescence variation. (F,G) Post-pollination flowers: filaments shift to pink tones after pollination. The arrows indicate the position of bracts and bracteoles at the base of the pedicel. (H) Fruit.
Figure 3. General of aspects of Bauhinia latistipulata. (A) Habit. Stipules highlighted by the arrow. (B) Adaxial surface leaf. (C) Abaxial surface leaf. (D,E) Inflorescence variation. (F,G) Post-pollination flowers: filaments shift to pink tones after pollination. The arrows indicate the position of bracts and bracteoles at the base of the pedicel. (H) Fruit.
- Type. BRAZIL. Mato Grosso: Nobres, Reserva Particular do Patrimônio Natural SESC Serra Azul, 14°29′16.3″ S 55°42′26.7″ W, 07 November 2023, (bud, fl.), D.S. Amorim 225 et al. (holotype: UFMT 49428!; isotypes: RB!, HUEFS!, CEN!, BMA!, HUFABC!, UB!, BOTU!, BHCB!, HERBAM!).
- Diagnosis. Bauhinia latistipulata is distinguished from other members of B. ser. Cansenia by its reniform stipules and cymbiform bracts, as well as its hirsute abaxial leaf surface, tubular and ribbed flower buds, and white-tomentose ovary. The most similar species is B. ungulata, but the new species differs by its hirsute abaxial leaf surface (vs. tomentellous), tubular and ribbed flower buds (vs. clavate or subclavate and smooth), tomentose staminal tube (vs. pubescent), and distinct color of the ovary indumentum (white vs. ferruginous).
- Description. Shrubs to small trees, 0.5–2.0 m tall. Branches unarmed, cylindrical and sulcate, villous, covered with reddish glandular trichomes; extrafloral nectaries present, ovoid, solitary, intrapetiolar, 0.5–4 mm long. Stipules persistent, 0.6–2.0 × 0.6–2.6 cm, reniform, apex cuspidate, adaxial surface glabrous, abaxial surface pilose, primary and secondary veins prominent. Leaves unifoliolate; petiole 1.5–3.5 × 0.1–0.2 cm, villous to hirsute, canaliculate; blade 6.8–12.6 × 6.5–12.3 cm, elliptic to ovate, base cordate, bilobed, leaflets fused ca. 3/4 of their total length, apex lobes obtuse, 1.0–3.1 cm long, adaxial surface pilose on main vein, impressed, abaxial surface hirsute, whitish, with sparse glandular trichomes; primary veins 11–13(–15), prominent. Inflorescence terminal, pseudoraceme; peduncle 2.6–6.2 cm long; rachis 8.0–18 cm long, villous to hirsute, with reddish glandular trichomes, 8–16 flowers per inflorescence, arranged in pairs along the rachis; bracts (at flower base) and bracteoles 2 (alternate on pedicel), cymbiform, apex aristate, persistent, 0.5–1.0 × 0.2–0.4 cm; pedicel 1.0–3.0 cm long. Flower buds developed, 6.0–8.0 cm long, apex aristate or winged, tubular or subclavate, ribbed or smooth, villous, with abundant glandular trichomes; hypanthium tubular, 1.3–1.8 cm long, internally glabrous, externally villous. Sepals 4.1–6.5 × 0.2–0.3 cm, villous externally, glabrous internally, apex aristate or winged. Petals 2.9–3.3 × 0.1–0.2 cm, glabrous on both surfaces, white, unguiculate, linear, apex acuminate. Fertile stamens 10, heterodynamous; filaments 2.0–4.1 cm long, connate at base; staminal tube 0.1–0.25 cm high, internally white-tomentose or barbate between filaments, externally glabrous; anthers 0.7–1.5 × 0.1 cm. Gynoecium 4.0–4.5 cm long; stipe 2.2–3.2 cm long; ovary 1.1–1.2 cm long, white-tomentose; style 1.6–1.8 cm long; stigma clavate. Fruits ca. 11 × 1.0 cm, oblong, apex acuminate, base attenuate, valves woody, pilose; stipe ca. 4.2 cm long.
- Etymology. The specific epithet “latistipulata” refers to the conspicuous, broad stipules. This distinctive feature of the new species enables its identification even in a vegetative state.
- Distribution and Ecology. Bauhinia latistipulata occurs in the states of Mato Grosso and Goiás, Brazil (Figure 4). It is found in the Cerrado phytogeographic domain, as well as in transitional zones between the Cerrado and Amazon domains in northern Mato Grosso. The species inhabits cerradão vegetation and gallery forest margins, typically growing in lateritic soils at elevations ranging from 300 to 500 m. Most species of ser. Cansenia occur in the Cerrado, a biodiversity hotspot that includes some of the main centers of diversity for the genus []. Flowering and fruiting specimens were collected between November and May.
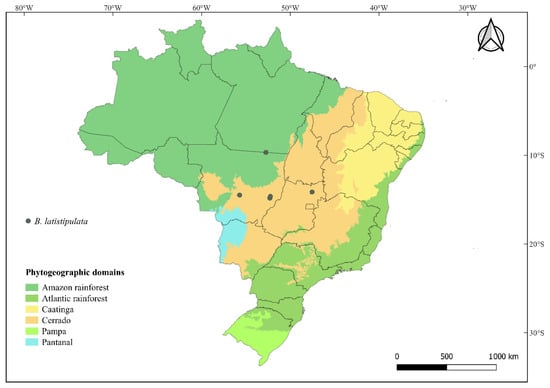 Figure 4. Distribution map for Bauhinia latistipulata D.S. Amorim & E. Pessoa. Note: QGIS (version 3.4.2) [] was used to generate distribution maps based on voucher coordinates.
Figure 4. Distribution map for Bauhinia latistipulata D.S. Amorim & E. Pessoa. Note: QGIS (version 3.4.2) [] was used to generate distribution maps based on voucher coordinates.
- Conservation Status. Bauhinia latistipulata is currently known from five recorded populations in Alto Paraíso de Goiás, Barra do Garças, Nobres (SESC Serra Azul Private Reserve), Santa Cruz do Xingu (Xingu State Park), and Nova Xavantina. It has an Area of Occupancy (AOO) = 254.16 km2 and Extent of Occurrence (EOO) = 20.0 km2. Although some populations occur within protected areas (Xingu State Park and SESC Serra Azul Private Reserve), the species qualifies as Endangered (EN) under IUCN (2024) criteria B2ab(ii, iii, iv). Furthermore, since it is primarily distributed in the Cerrado, it faces ongoing threats from deforestation due to agricultural expansion and urban development.
Additional material examined (Paratypes). Brazil. Goiás: Alto Paraíso de Goiás, 22 March 1968, (bud), H.S. Irwin 21624 et al. (MO0100423165, MO0100425294, NY00958450 [image!]); ibidem, (bud), H.S. Irwin 21618-a et al. (NY00958449 [image]!). Mato Grosso: Barra do Garças, Fazenda Carvalinho, 14°59′56.8″ S, 52°26′25.3″ W, 26 April 2009, (bud, fl.), F.P. Athayde Filho 1936 et al. (RB00515936 [image!]); Nova Xavantina, estrada para a Ilha do Côco, 02 February 2001, (fr.), D.P.S. Campos 13 (RB00515938 [image!]); ca. 32 km de Nova Xavantina, 14°49′46″ S, 52°17′49″ W, 17 April 2005, (fl., fr.), L.P. Queiroz 10377 et al. (HUEFS0095179 [image!]); 5 km from Xavantina near village of Olaria, 14°38′ S, 52°14′ W, 21 November 1967, (bud, fl.), D. Philcox 3185 et al. (IAN155657, K000807912, RB01493719 [image!]); Santa Cruz do Xingu, Parque Estadual do Xingu, 9°40′43″ S, 52°44′15″ W, 13 May 2022, (bud, fl., fr.), T.S. Coutinho 524 & M. Zappani (UFMT 49404!).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/taxonomy5040065/s1, Figure S1: Phylogenetic relationships of Bauhinia produced by Maximum Likelihood analysis based on nuclear nrITS, and plastids matK and trnL–F combined (50% majority rule consensus tree) showing the relationship of the new species.
Author Contributions
Conceptualization: D.d.S.A.; Funding acquisition: E.M.P.; Investigation: D.d.S.A. and T.S.C.; Methodology: D.d.S.A., T.S.C. and E.M.P.; Project administration: E.M.P.; Writing—original draft: D.d.S.A.; Writing—review and editing: T.S.C. and E.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (Process 303556/2022-6) and Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão—FAPEMA (Process BM-06273/22).
Data Availability Statement
All DNA sequences used in this study are deposited in GenBank: https://www.ncbi.nlm.nih.gov (accessed 7 October 2025).
Acknowledgments
We thank FAPEMA (Fundação de Amparo à Pesquisa e Desenvolvimento Científico e Tecnológico do Maranhão) for the scholarship granted to the first author (process BM-06273/22). EMP thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico (Process 303556/2022-6) for the productivity grant.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. Taxa and GenBank Accession Numbers (nrITS, matK, trnL–F) Used in the Analyses. New Sequences Generated in This Study Are Marked with an Asterisk “*”
| Species | nrITS | matK | trnL–F |
| Adenolobus garipensis (E. Mey.) Torre & Hillc. | KY306484 | EU361844 | FJ801157 |
| Bauhinia acreana Harms | - | JN881357 | FJ801100 |
| Bauhinia aculeata L. | - | JN881358 | FJ801051 |
| Bauhinia acuminata L. | - | - | KT461938 |
| Bauhinia andrieuxii Hemsl. | - | JN881359 | - |
| Bauhinia bauhinioides (Mart.) J.F.Macbr. | - | JN881360 | FJ801107 |
| Bauhinia × blakeana Dunn | - | JN881361 | FJ801115 |
| Bauhinia brachycarpa Wall. ex Benth. | FJ432276 | JN881433 | KT461947 |
| Bauhinia brachycalyx Ducke | - | KY046063 | - |
| Bauhinia brevicalyx Du Puy & R.Rabev. | - | JN881362 | FJ801126 |
| Bauhinia brevipes Vogel | - | KY118256 | - |
| Bauhinia cheilantha (Bong.) Steud. | DQ787410 | - | - |
| Bauhinia cinnamomea DC. | - | KY176802 | - |
| Bauhinia corniculata Benth. | - | JN881363 | FJ801105 |
| Bauhinia dipetala Hemsl. | - | JN881364 | FJ801078 |
| Bauhinia dubia G.Don | - | KY045931 | - |
| Bauhinia galpinii N.E.Br. | - | EU361875 | FJ801055 |
| Bauhinia grandidieri Baill. | - | JN881368 | FJ801080 |
| Bauhinia grevei Drake | - | JN881369 | FJ801147 |
| Bauhinia hildebrandtii Vatke | - | JN881370 | FJ801060 |
| Bauhinia jenningsii P.Wilson | AY258411 | JN881371 | FJ801062 |
| Bauhinia kalantha Harms | - | JN881372 | FJ801148 |
| Bauhinia latistipulata D.S. Amorim & E. Pessoa * | PV770934 | - | PV769134 |
| Bauhinia leptantha Malme | - | KY118265 | - |
| Bauhinia longicuspis Spruce ex Benth. | - | - | FJ801095 |
| Bauhinia longifolia (Bong.) Steud. | - | KY118257 | - |
| Bauhinia longipedicellata Ducke | - | KY118258 | - |
| Bauhinia lunarioides A.Gray ex S.Watson | - | JN881374 | FJ801099 |
| Bauhinia macranthera Benth. ex Hemsl. | JN942381 | JN881375 | FJ801063 |
| Bauhinia madagascariensis Desv. | - | JN881376 | - |
| Bauhinia mollis (Bong.) D.Dietr. | - | JN881377 | FJ801096 |
| Bauhinia monandra Kurz | - | JN881387 | FJ801135 |
| Bauhinia morondavensis Du Puy & R.Rabev. | - | JN881379 | - |
| Bauhinia natalensis Oliv. | - | JN881380 | FJ801064 |
| Bauhinia ovata (Bong.) Vogel | - | KY118266 | - |
| Bauhinia pauletia Pers. | - | JN881381 | FJ801087 |
| Bauhinia picta (Kunth) DC. | - | JN881385 | FJ801068 |
| Bauhinia podopetala Baker | - | JN881386 | FJ801129 |
| Bauhinia pottsii G.Don | - | JN881388 | FJ801077 |
| Bauhinia pringlei S.Watson | - | JN881389 | FJ801140 |
| Bauhinia pulchella Benth. | - | JN881390 | FJ801097 |
| Bauhinia purpurea L. | - | JN881391 | FJ801069 |
| Bauhinia ramosissima Benth. ex Hemsl. | - | JN881393 | FJ801101 |
| Bauhinia racemosa Lam. | OP467012 | JN881392 | - |
| Bauhinia rufa (Bong.) Steud. | - | - | FJ801098 |
| Bauhinia rufescens Lam. | KX057837 | JN881395 | FJ801082 |
| Bauhinia seleriana Harms | - | JN881397 | KT461943 |
| Bauhinia seminarioi Harms ex Eggers | - | JN881398 | FJ801102 |
| Bauhinia subclavata Benth. | - | - | FJ801109 |
| Bauhinia taitensis Taub. | - | - | FJ801142 |
| Bauhinia tomentosa L. | KX057838 | JX517621 | FJ801071 |
| Bauhinia ungulata L. | FJ009818 | JN881404 | FJ801110 |
| Bauhinia variegata L. | JX856408 | GU135033 | FJ801081 |
| Bauhinia vespertilio | - | KY118267 | - |
| Bauhinia viridescens Desv. | - | JN881406 | KT461945 |
| Bauhinia weberbaueri Harms | - | JN881407 | - |
| Bauhinia xerophyta Du Puy & R.Rabev. | - | JN881408 | FJ801128 |
| Cheniella glauca (Benth.) R.Clark & Mackinder | AY258384 | KX146237 | - |
| Phanera bohniana (H.Y.Chen) K.W.Jiang, S.R.Gu & T.Y.Tu | AY258403 | - | FJ801052 |
| Phanera yunnanensis (Franch.) Wunderlin | AF286360 | JN881453 | - |
References
- Sinou, C.; Cardinal-McTeague, W.; Bruneau, A. Testing generic limits in Cercidoideae (Leguminosae): Insights from plastid and duplicated nuclear gene sequences. Taxon 2020, 69, 67–86. [Google Scholar] [CrossRef]
- Legume Data Portal. Available online: https://www.legumedata.org/ (accessed on 11 April 2024).
- Vaz, A.M.S.F.; Tozzi, A.M.G.A. Bauhinia ser. Cansenia (Leguminosae—Caesalpinioideae) no Brasil. Rodriguesia 2003, 54, 55–143. [Google Scholar] [CrossRef]
- Vaz, A.M.S.F.; Santos, A.C.B. Bauhinia in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB22811 (accessed on 24 August 2025).
- Wunderlin, R.P.; Larsen, K.; Larsen, S.S. Reorganization of the Tribe Cercideae (Fabaceae: Caesalpinioideae); Biologiske Skrifter: Copenhagen, Denmark, 1987; pp. 1–38. [Google Scholar]
- Vaz, A.M.S.F.; Tozzi, A.M.G.A. Sinopse de Bauhinia sect. Pauletia (Cav.) DC. (Leguminosae—Caesalpinioideae: Cercideae) no Brasil. Braz. J. Bot. 2005, 28, 477–491. [Google Scholar] [CrossRef]
- Vaz, A.M.S.F.; Lewis, G.P. Four new species of Bauhinia sect. Pauletia and a new description of Bauhinia burchellii Bentham (Leguminosae) from Brazil. Phytotaxa 2015, 239, 264–272. [Google Scholar] [CrossRef]
- Casas-Restrepo, L.C.; Fonseca-Cortés, A.; Queiroz, L.P. Bauhinia angelae (Leguminosae: Cercidoideae): An overlooked new species from drylands of Northeastern Brazil. Phytotaxa 2025, 691, 271–281. [Google Scholar] [CrossRef]
- Vaz, A.M.S.F.; Bortoluzzi, R.L.C.; Silva, L.A.E. Checklist of Bauhinia sensu stricto (Caesalpiniaceae) in Brazil. Plant Ecol. Evol. 2010, 143, 212–221. [Google Scholar] [CrossRef]
- Vaz, A.M.S.F. Taxonomia de Bauhinia sect. Pauletia (Leguminosae: Caesalpinioideae: Cercideae) No Brasil. Ph.D. Thesis, Universidade Estadual de Campinas, São Paulo, Brazil, 2001. [Google Scholar] [CrossRef]
- Queiroz, L.P.; Oliveira, F.P.; Cedraz, B.; Melchor-Castro, R.B.; Fernandes, M.F. A new species of from coastal areas in Northeastern Brazil. Phytotaxa 2020, 435, 293–300. [Google Scholar] [CrossRef]
- Santos, A.C.B.; Queiroz, L.P.; Paula, A.P.O.; Carvalho, R. A new species of Bauhinia ser. Cansenia (Cercidoideae, Leguminosae) endemic to the Atlantic Forest in the state of Bahia, Brazil. Phytotaxa 2022, 568, 213–220. [Google Scholar] [CrossRef]
- Santos, A.C.B.; Vaz, A.M.S.F.; Silva, M.A.P.; Paula, A.P.O.; Feitoza, L.L.; Carvalho, R. A new species of Bauhinia (Cercidoideae, Leguminosae) from the states of Pernambuco and Alagoas, Brazil. Phytotaxa 2022, 576, 289–296. [Google Scholar] [CrossRef]
- Santos, A.C.B.; Queiroz, L.P.; Silva, M.A.P.; Paula, A.P.O.; Feitoza, L.L.; Carvalho, R. Bauhinia orbiculata (Cercidoideae, Leguminosae), a new species from Chapada Diamantina, Bahia, Brazil. Phytotaxa 2023, 584, 285–292. [Google Scholar] [CrossRef]
- Amorim, D.S.; Pessoa, E. Geometric morphometrics of leaves help distinguishing species from the Bauhinia fusconervis complex (Fabaceae–Cercidoideae). Acta Bot. Bras. 2025, 39, e20240133. [Google Scholar] [CrossRef]
- SpeciesLink Network. Available online: https://specieslink.net/search (accessed on 24 August 2025).
- Reflora—Herbário Virtual. Available online: https://reflora.jbrj.gov.br/reflora/herbarioVirtual/ (accessed on 24 August 2025).
- Thiers, B.M. (Updated Continuously). Index Herbariorum. Available online: https://sweetgum.nybg.org/science/ih/ (accessed on 17 June 2025).
- Harris, J.G.; Harris, M.W. Plant Identification Terminology: An Illustrated Glossary; Spring Lake Publishing: Spring Lake, UT, USA, 2001; pp. 1–206. [Google Scholar]
- Radford, A.E.; Dickison, W.C.; Massey, J.R.; Bell, C.R. Vascular Plant Systematics; Harper & Row: New York, NY, USA, 1976; Available online: https://www.ibiblio.org/botnet/glossary/ (accessed on 17 June 2025).
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 15 February 2024).
- Bachman, S.; Moat, J.; Hill, A.W.; de la Torre, J.; Scott, B. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef]
- IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria. Prepared by the Standards and Petitions Committee; Version 16; IUCN: Gland, Switzerland, 2024; pp. 1–122. Available online: https://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 6 February 2025).
- Chase, M.W.; Hills, H.H. Silica gel: An ideal material for field preservation of leaf samples for DNA studies. Taxon 1991, 40, 215–220. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- Sun, Y.; Skinner, D.Z.; Liang, G.H.; Hulbert, S.H. Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theor. Appl. Genet. 1994, 89, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Posada, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Miller, M.; Holder, M.; Vos, R.; Midford, P.; Liebowitz, T.; Chan, L.; Hoover, P.; Warnow, T. The CIPRES Portals. 2010. Available online: http://www.phylo.org/sub_sections/portal (accessed on 18 June 2025).
- Rambaut, A.; Suchard, M.A.; Xie, D.; Drummond, A.J. Tracer 1.6. 2013. Available online: http://tree.bio.ed.ac.uk/software/tracer/ (accessed on 18 June 2025).
- Stamatakis, A. Raxml version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.P.; Handley, S.A.; Myers, D.S.; Reed, D.L.; Rokas-Winka, A.K. Comparing bootstrap and posterior probability values in the four-taxon case. Syst. Biol. 2003, 52, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Erixon, P.; Svennblad, B.; Britton, T.; Oxelman, B. Reliability of Bayesian posterior probabilities and bootstrap frequencies in phylogenetics. Syst. Biol. 2003, 52, 665–673. [Google Scholar] [CrossRef]
- Simmons, M.P.; Pickett, K.M.; Miya, M. How meaningful are Bayesian support values? Mol. Biol. Evol. 2004, 21, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1.4.4. 2018. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 18 June 2025).
- Amorim, D.S.; Pessoa, E. Bauhinia s.s. (Fabaceae−Cercidoideae) from Baixada Cuiabana, Mato Grosso, Brazil: A neglected center of diversity for the genus. Phytotaxa 2025, 681, 146–166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).