Description of the Puparium of Eumerus vestitus Bezzi, 1912 (Diptera: Syrphidae) Reared from Supermarket Plums in Israel
Abstract
1. Introduction
2. Materials and Methods
3. Results
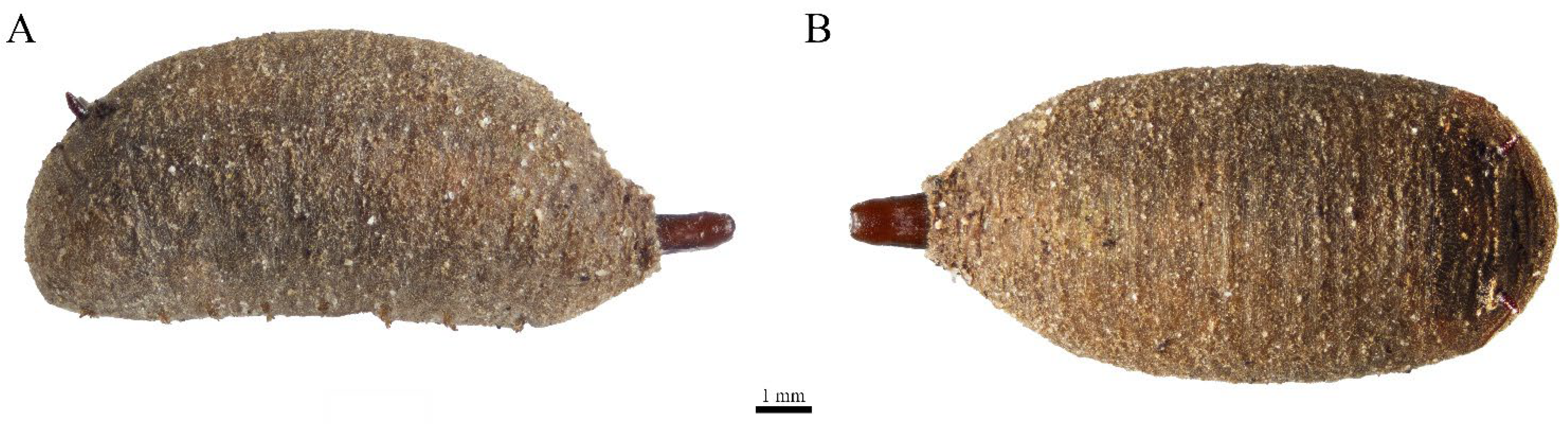
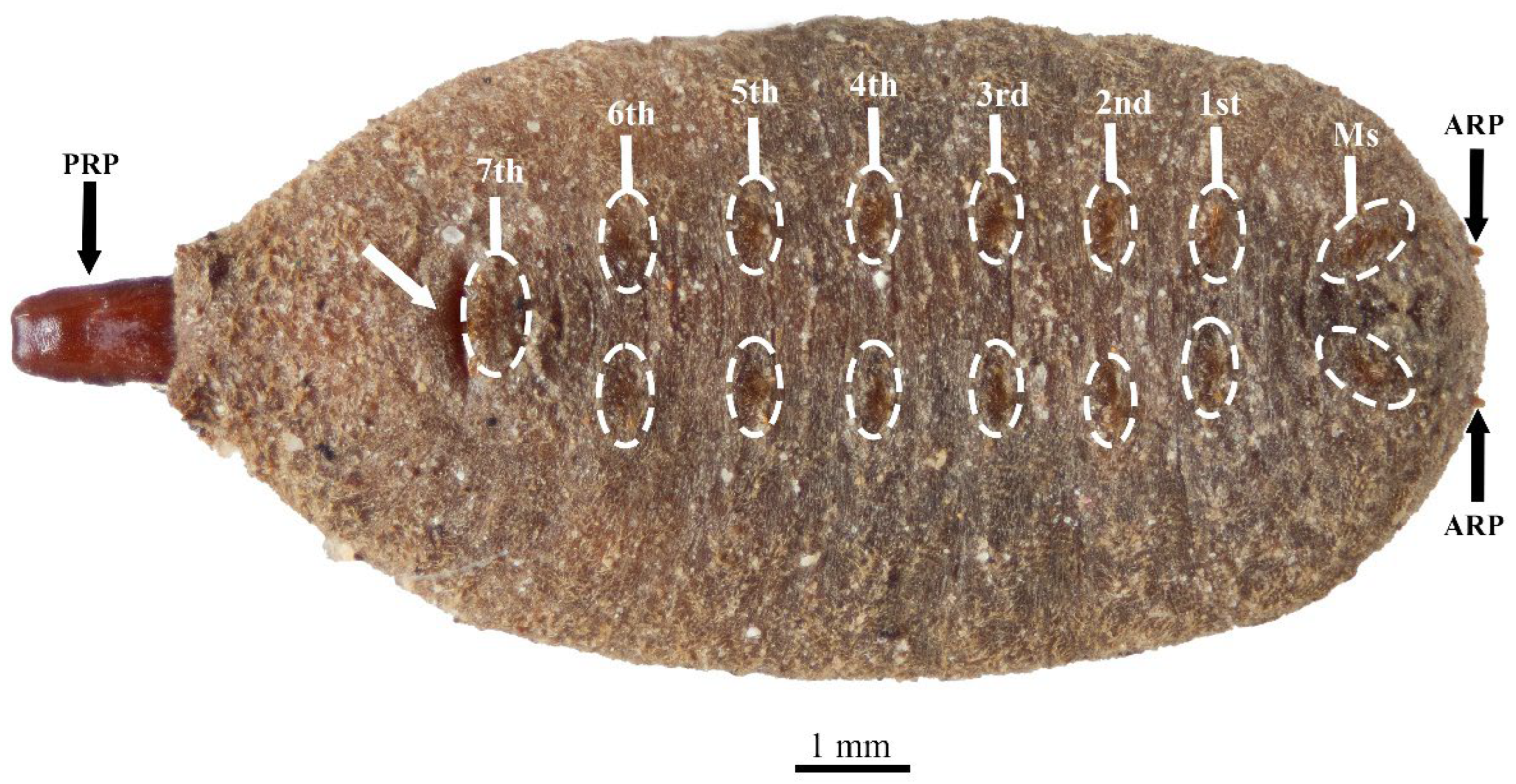
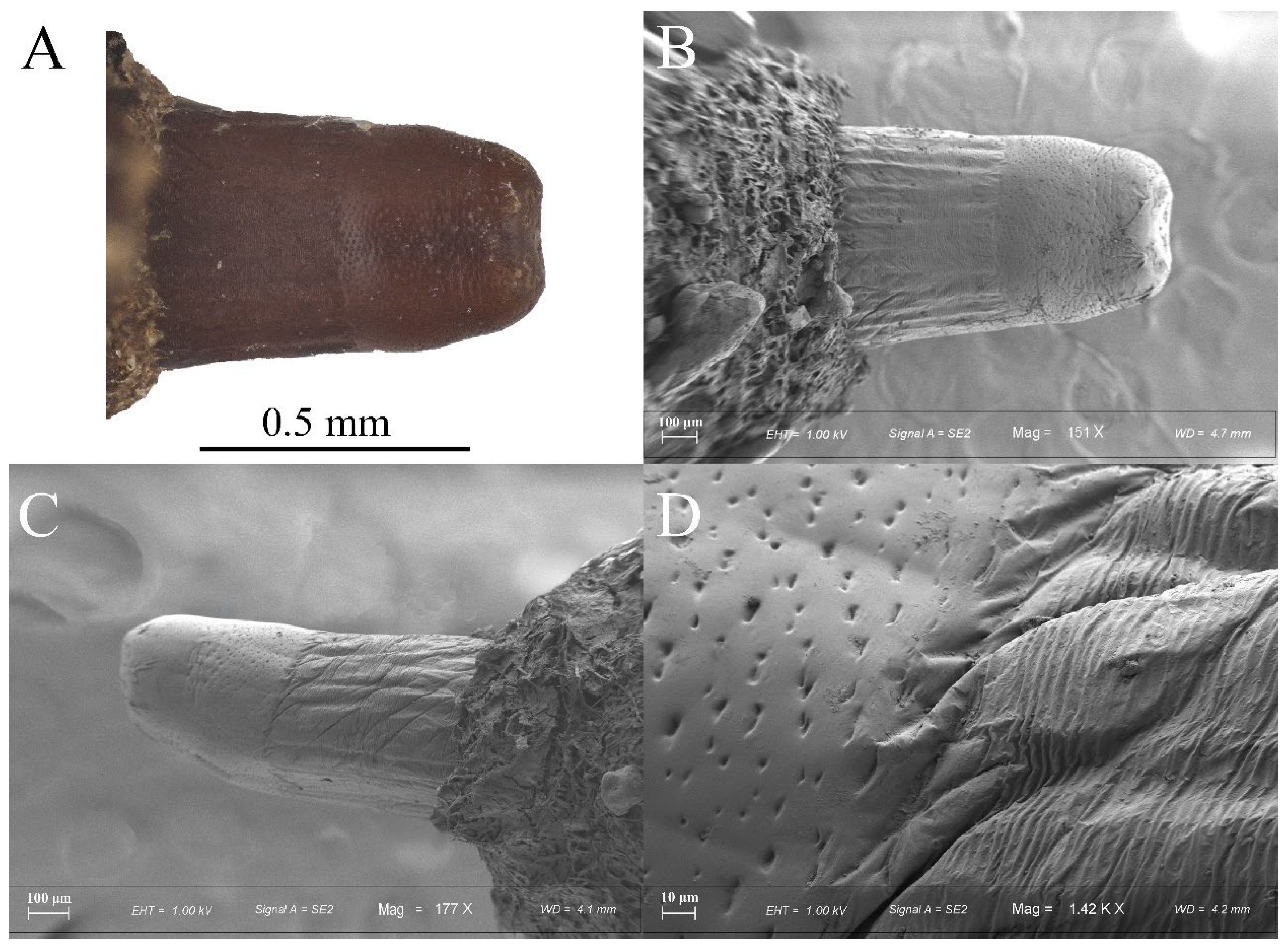
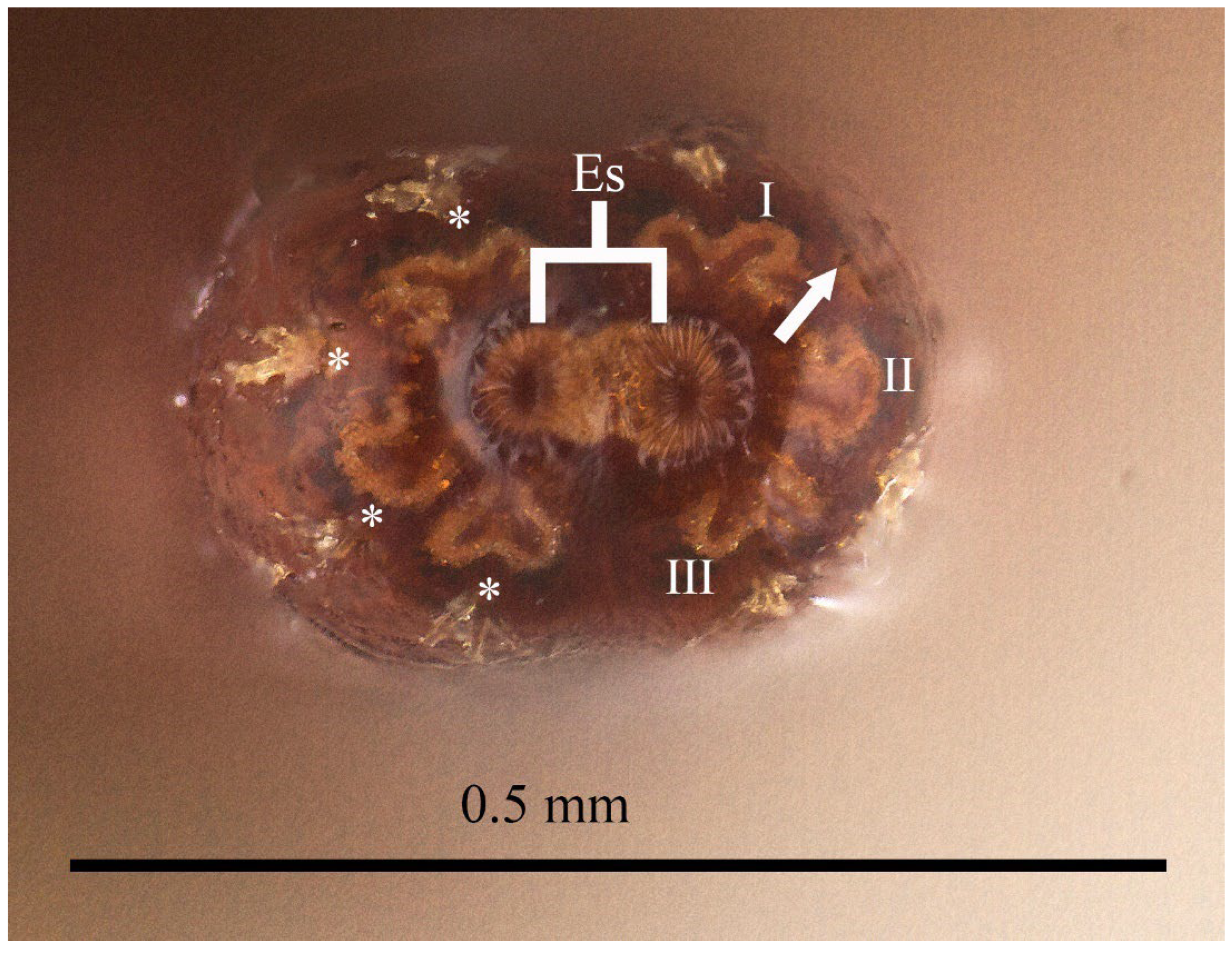
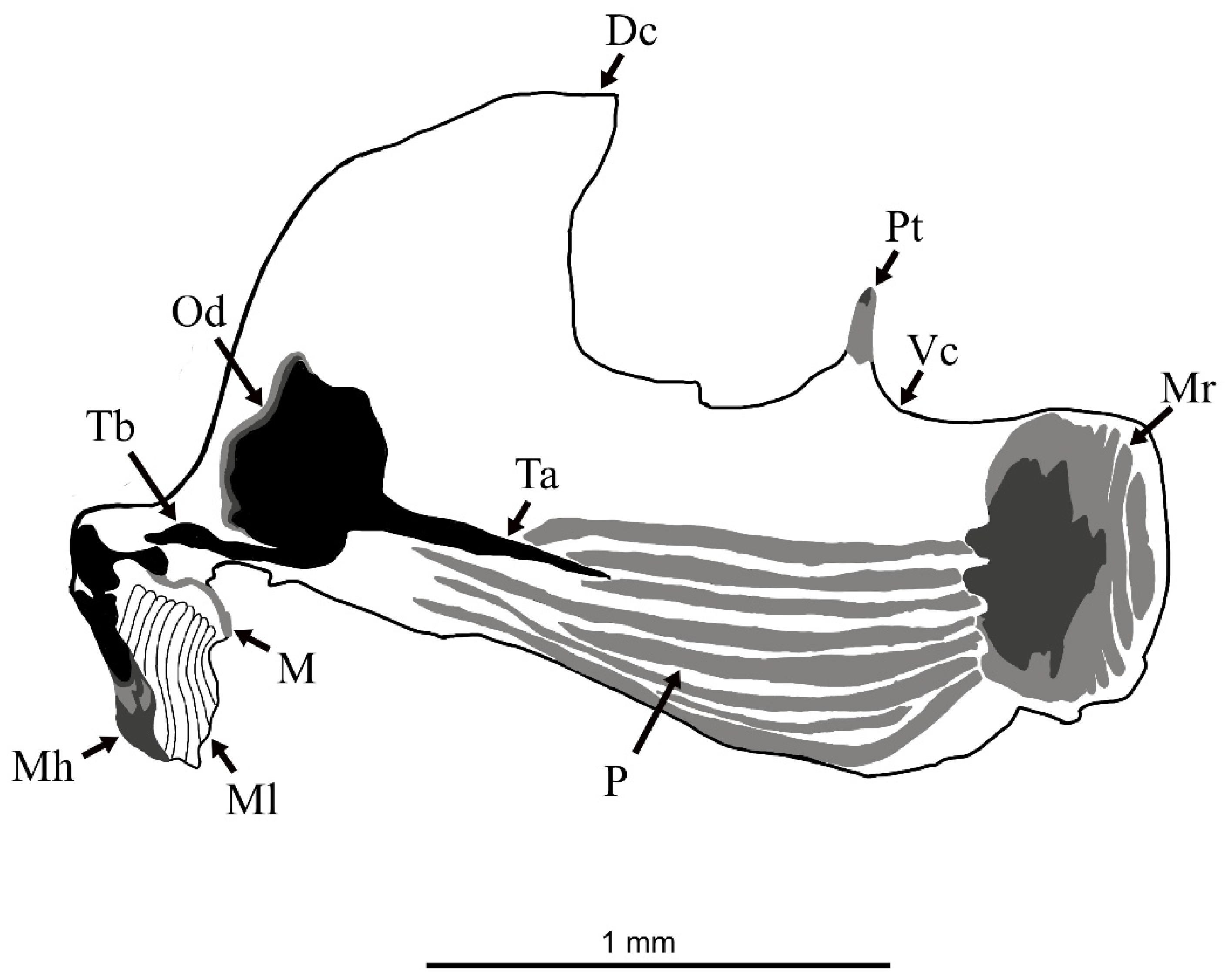
3.1. Eumerus vestitus Puparium (Figure 2)
3.2. Eumerus vestitus Development Sites
3.3. Update of the L3/Puparia Taxonomic Key of Eumerus Species
- 1a. Mesothoracic prolegs present………………………………………………………………..2
- 1b. Mesothoracic prolegs absent…………………………………………………………………5
- 2a. Head skeleton: mandibular hook serrated apically……………………………………….3
- 2b. Head skeleton: mandibular hook not serrated…………………………………………….4
- 3a. Head skeleton: ventral cornu without pestle………..Eumerus etnensis van der Goot, 1964
- 3b. Head skeleton: ventral cornu with a slightly sclerotized pestle………………E. rufotibialis
- 4a. Prolegs of the seventh abdominal segment united in a medial position of the segment. Head skeleton: ventral cornu with a slightly sclerotized pestle…………..……………………………………………………………….……………..E. vestitus
- 4b. Prolegs of the seventh abdominal segment separated. Head skeleton: ventral cornu without pestle…………………………………………………………………………..E. obliquus
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marinoni, L.; Morales, M. The second record of the Genus Eumerus Meigen, 1822 (Diptera: Syrphidae) for the neotropical region and the first for Brazil. Proc. Entomol. Soc. Wash. 2007, 109, 493–495. [Google Scholar]
- Speight, M.C.D.; Hauser, M.; Withers, P. Eumerus narcissi Smith (Diptera, Syrphidae), presence in Europe confirmed, with a redescription of the species. Dipter. Dig. 2013, 20, 17–32. [Google Scholar]
- Grković, A.; Vujić, A.; Radenković, S.; Chroni, A.; Petanidou, T. Diversity of the genus Eumerus Meigen (Diptera, Syrphidae) on the eastern Mediterranean islands with description of three new species. Ann. Soc. Entomol. Fr. 2015, 51, 361–373. [Google Scholar] [CrossRef]
- Garcete-Barrett, B.R.; Morales, M.N.; Hauser, M.; Smit, J.T.; González, L.; Ramírez de López, M.B.; Arias, O.; Adomo, M.; Sormanti, G.; Mereles, A. New geographical records and key to the species of Eumerus Meigen, 1822 (Diptera: Syrphidae) introduced into the Americas and Hawaii. Rev. Bras. Entomol. 2020, 64, e20190016. [Google Scholar] [CrossRef]
- Moran, K.M. Systematics of Syrphidae, with an Emphasis on the Subtribe Criorhinina. Ph.D. Thesis, Carleton University, Ottawa, ON, Canada, 2023; pp. 1–504. [Google Scholar]
- Aguado-Aranda, P. Revisión Taxonómica y Sistemática del Género Eumerus Meigen, 1822 (Diptera: Syrphidae) en el Mediterráneo Occidental, Con Énfasis en el Área Ibero-Balear. Ph.D. Thesis, University of Alicante, Alicante, Spain, 2024; pp. 1–102. [Google Scholar]
- Aguado-Aranda, P.; Ricarte, A.; Nedeljković, Z.; Kelso, S.; Skevington, J.H.; Marcos-García, M.Á. Diversity and systematics of the Ibero-Balearic Eumerus (Diptera: Syrphidae): Providing tools for species identification. Eur. J. Entomol. 2025, 122, 13–24. [Google Scholar] [CrossRef]
- Radenković, S.; Veličković, N.; Jordaens, K.; Grković, A.S.; Djan, M.; Ståhls, G.; Smit, J.; Vujić, A. Revision of the Afrotropical endemic Eumerus triangularis group (Diptera: Syrphidae: Merodontini)—Species with glistering antennae. PLoS ONE 2025, 20, e0313829. [Google Scholar] [CrossRef] [PubMed]
- Chroni, A.; Grković, A.; Ačanski, J.; Vujić, A.; Radenković, S.; Veličković, N.; Djan, M.; Petanidou, T. Disentangling a cryptic species complex and defining new species within the Eumerus minotaurus group (Diptera: Syrphidae), based on integrative taxonomy and Aegean palaeogeography. Contrib. Zool. 2018, 87, 197–225. [Google Scholar] [CrossRef]
- Ricarte, A.; Souba-Dols, G.J.; Hauser, M.; Marcos-García, M.Á. A review of the early stages and host plants of the genera Eumerus and Merodon (Diptera: Syrphidae), with new data on four species. PLoS ONE 2017, 12, e0189852. [Google Scholar] [CrossRef]
- Souba-Dols, G.J.; Ricarte, A.; Hauser, M.; Speight, M.; Marcos-García, M.Á. What do Eumerus Meigen larvae feed on? New immature stages of three species (Diptera: Syrphidae) breeding in different plants. Org. Divers. Evol. 2020, 20, 267–284. [Google Scholar] [CrossRef]
- Aracil, A.; Radenković, S.; Pérez-Bañón, C.; Campoy, A.; Vujić, A.; Rojo, S. Preimaginal morphology and notes on the natural history of some Afrotropical flower flies of genus Eumerus Meigen 1822 (Diptera Syrphidae) including description of a new species. Bull. Insectol. 2024, 77, 137–154. [Google Scholar]
- Orengo-Green, J.J.; Ricarte, A.; Hauser, M.; Langlois, D.; Marcos-García, M.Á. On the immature stages of some Merodontini hoverflies (Diptera: Syrphidae) from Europe and Africa. Arth. Struct. Dev. 2024, 78, 101328. [Google Scholar] [CrossRef] [PubMed]
- Krivosheina, N.P.; Krivosheina, M.G. New data on the larvae of the hover-fly genus Eumerus Meigen, 1822 (Diptera: Syrphidae). Entomol. Rev. 2021, 101, 162–173. [Google Scholar] [CrossRef]
- Rotheray, G.E.; Gilbert, F. Phylogeny of Palaearctic Syrphidae (Diptera): Evidence from larval stages. Zool. J. Linnean. Soc. 1999, 127, 1–112. [Google Scholar] [CrossRef]
- Aracil, A.; Grković, A.; Pérez-Bañón, C.; Tubić, N.K.; Juan, A.; Radenković, S.; Vujić, A.; Rojo, S. A new species of phytophagous flower fly (Diptera, Syrphidae), feeding on holoparasitic broomrape plants (Orobanchaceae) for the first time in Europe. Arth. Plant Int. 2023, 17, 401–418. [Google Scholar] [CrossRef]
- Rotheray, G.E. Colour Guide to Hoverfly Larvae (Diptera, Syrphidae) in Britain and Europe. In Dipterist Digest No. 9; Derek Whitely: Sheffield, UK, 1993. [Google Scholar]
- Gilasian, E.; van Steenis, J.; Parchami-Araghi, M. Six new species of the genus Eumerus Meigen, 1822 from Iran (Diptera, Syrphidae). J. Insect Biodivers. Syst. 2022, 8, 483–512. [Google Scholar] [CrossRef]
- Smit, J.T.; van Harten, A.; Ketelaar, R. Order Diptera, family Syrphidae. The hoverflies of the Arabian Peninsula. In Arthropod Fauna of the UAE; Van Harten, A., Ed.; Department of the President’s Affairs: Ras al Akhdar peninsula, Abu Dhabi, 2017; Volume 6, pp. 572–612. [Google Scholar]
- Ricarte, A.; Hauser, M.; Kinnee, S.; Marcos-García, M.Á. A new Eumerus hoverfly (Diptera: Syrphidae) from Namibia and South Africa, with notes on similar especies. Zootaxa 2020, 4890, 493–508. [Google Scholar] [CrossRef] [PubMed]
- de Moor, F.C. Notes on a syrphid fly, Eumerus obliquus (Fabricius) (Diptera: Syrphidae). Arnoldia 1973, 6, 7. [Google Scholar]
- Ricarte, A.; Marcos-García, M.Á.; Rotheray, G.E. The early stages and life history of three Eumerus and two Merodon species (Diptera: Syrphidae) from the Mediterranean region. Entomol. Fenn. 2008, 19, 129–141. [Google Scholar] [CrossRef]
- Anooj, S.; Kalia, V.; Krishna, G.K.; Ghopade, K. New biogeographic distribution record of phytophagous syrphid, Eumerus vestitus Bezzi, its biosystematics, host preferences and association behavior. Int. J. Tropic. Insect Sci. 2020, 40, 527–538. [Google Scholar] [CrossRef]
- Efflatoun, H.C. A Monograph of Egyptian Diptera (Part 1, Fam. Syrphidae). In Mémoires de la Société Entomologique D’Égypte; Biodiversity Heritage Library: Washington, DC, USA, 1922; Volume 2, pp. 1–123. [Google Scholar] [CrossRef]
- Miličić, M.; Grković, A. Eumerus vestitus (Europe assessment). IUCN Red. List. Threat. Species 2021, 2021, e.T149166921A149166923. [Google Scholar] [CrossRef]
- Hassan, M.A.; Shezad, A.; Dyola, U.; Qasim, M.; Fatima, N.; Maryam, Z. Two species of the hoverfly genus Eumerus Meigen (Diptera: Syrphidae) new record for Pakistan. Papéis Avulsos de Zool. 2022, 62, e202262067. [Google Scholar] [CrossRef]
- Speight, M.C.D. Species Accounts of European Syrphidae, 2024. In Syrph the Net, the Database of European Syrphidae (Diptera); Syrph the Net publications: Dublin, Ireland, 2024; Volume 115, pp. 1–381. [Google Scholar]
- Shaumar, N.; Kamal, S. The Syrphidae of Egypt. Bull. Mens. de la Société Linnéenne de Lyon 1978, 47, 79–84. [Google Scholar] [CrossRef]
- El-Hawagry, M.S.; Gilbert, F. Catalogue of the Syrphidae of Egypt (Diptera). Zootaxa 2019, 4577, 201–248. [Google Scholar] [CrossRef]
- Dirickx, H.G. Atlas des Diptères Syrphides de la Région Méditerranéenne; Institut Royal des Sciences Naturelles de Belgique: Brussels, Belgium, 1994; pp. 1–127. [Google Scholar]
- OEC [Observatory of Economic Complexity]. Plums & Sloes (fresh) in Israel. 2025. Available online: https://oec.world/en/profile/bilateral-product/plums-and-sloes-fresh/reporter/isr (accessed on 23 September 2025).
- Orengo-Green, J.J.; Quinto, J.; Ricarte, A.; Marcos-García, M.Á. Combined stereomicroscope and SEM disentangle the fine morphology of the undescribed larva and puparium of the hoverfly Milesia crabroniformis (Fabricius, 1775) (Diptera: Syrphidae). Micron 2023, 165, 103397. [Google Scholar] [CrossRef] [PubMed]
- Rotheray, G.E. Ecomorphology of Cyclorrhaphan Larvae (Diptera). In Zoological Monograph 4; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Courtney, G.W.; Sinclar, B.J.; Meier, R. Morphology and Terminology of Diptera larvae. In Contributions to a Manual of Palaearctic Diptera; Papp, L., Darvàs, B., Eds.; Science Herald: Budapest, Hungary, 2000; Volume 1, pp. 85–161. [Google Scholar]
- Campoy, A.; Aracil, A.; Pérez-Bañón, C.; Rojo, S. An in-depth study of the larval head skeleton and the external feeding structures related with the ingestions of food particles by the eristaline flower flies Eristalis tenax and Eristalinus aeneus. Entomol. Exp. Appl. 2020, 168, 783–798. [Google Scholar] [CrossRef]
- Young, A.D.; Lemmon, A.L.; Skevington, J.H.; Mengual, X.; Ståhls, G.; Reemer, M.; Jordaens, K.; Kelso, S.; Lemmon, E.M.; Hauser, M.; et al. Anchored enrichment dataset for true flies (order Diptera) reveals insights into the phylogeny of flower flies (family Syrphidae). BMC Evol. Biol. 2016, 16, 143. [Google Scholar] [CrossRef]
- Moran, K.M.; Skevington, J.H.; Kelson, S.; Mengual, X.; Jordaens, K.; Young, A.D.; Ståhls, G.; Mutin, V.; Bot, S.; van Zuijen, M.; et al. A multigene phylogeny of the eristaline flower flies (Diptera: Syrphidae), with emphasis on the subtribe Criorhinina. Zool. J. Linn. Soc. 2022, 194, 120–135. [Google Scholar] [CrossRef]
- Pérez-Bañón, C.; Marcos-García, M.Á. Life history and description of the immature stages of Eumerus purpurariae (Diptera: Syrphidae) developing in Opuntia maxima. Eur. J. Entomol. 1998, 95, 373–382. [Google Scholar]
- Dowding, V.M. The function and ecological significance of the pharyngeal ridges occurring in the larvae of some cyclorrhaphaous Diptera. Parasitology 1967, 57, 371–388. [Google Scholar] [CrossRef]
- Orengo-Green, J.J.; Kanturski, M.; Ricarte, A.; Marcos-García, M.Á. A great little ally: Revealing the morphology of the immature stages of the aphid pest predator Sphaerophoria rueppellii (Wiedemann, 1830) (Diptera: Syrphidae). Eur. Zool. J. 2022, 89, 625–640. [Google Scholar] [CrossRef]
- Rosace, M.C.; Cendoya, M.; Mattion, G.; Vicent, A.; Battisti, A.; Cavaletto, G.; Marini, L.; Rossi, V. A spatio-temporal dataset of plant pests’ first introductions across the EU and potential entry pathways. Sci. Data 2023, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Szyniszewska, A.M.; Bieszczak, H.; Kozyra, K.; Papadopoulos, N.T.; De Meyer, M.; Nowosad, J.; Ota, N.; Kriticos, D.J. Evidence that recent climatic changes have expanded the potential geographical range of the Mediterranean fruit fly. Sci. Rep. 2024, 14, 2515. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; Rocha, J.; Sousa, C.A.; Capinha, C. Wide and increasing suitability for Aedes albopictus in Europe is congruent across distribution models. Sci. Rep. 2021, 11, 9916. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D. Indirect effects of invasive Burmese pythons on ecosystems in southern Florida. J. App. Ecol. 2017, 54, 1251–1258. [Google Scholar] [CrossRef]
- Tal, A. Israeli Agriculture-Innovation and Advancement. In From Food Scarcity to Surplus; Springer: Singapore, 2021; pp. 299–358. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and livestock products. In Food and Agriculture Organization of the United Nations; FAOSTAT Statistics Database: Rome, Italy, 2025; Available online: https://www.fao.org/faostat (accessed on 17 June 2025).



| Host Plant | Locality of Origin |
|---|---|
| Banana | India [23] |
| Grape | Mex (Alexandria, Egypt) [24] |
| Plum | Unknown (probably Israel) |
| Potato tuber | Cyprus, Greece, Israel/Palestinian Authority [24], and Israel |
| Paw-paw (rotten stems) | Tel-el-Kebir (Egypt) [24] |
| Tomato | India [23] |
| Watermelon | Tul-Karam [Tulkarm] (Israel/Palestinian Authority) [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orengo-Green, J.J.; Aguado-Aranda, P.; Almodóvar, J.R.; Mostovski, M.; Ricarte, A. Description of the Puparium of Eumerus vestitus Bezzi, 1912 (Diptera: Syrphidae) Reared from Supermarket Plums in Israel. Taxonomy 2025, 5, 64. https://doi.org/10.3390/taxonomy5040064
Orengo-Green JJ, Aguado-Aranda P, Almodóvar JR, Mostovski M, Ricarte A. Description of the Puparium of Eumerus vestitus Bezzi, 1912 (Diptera: Syrphidae) Reared from Supermarket Plums in Israel. Taxonomy. 2025; 5(4):64. https://doi.org/10.3390/taxonomy5040064
Chicago/Turabian StyleOrengo-Green, José J., Pablo Aguado-Aranda, José R. Almodóvar, Mike Mostovski, and Antonio Ricarte. 2025. "Description of the Puparium of Eumerus vestitus Bezzi, 1912 (Diptera: Syrphidae) Reared from Supermarket Plums in Israel" Taxonomy 5, no. 4: 64. https://doi.org/10.3390/taxonomy5040064
APA StyleOrengo-Green, J. J., Aguado-Aranda, P., Almodóvar, J. R., Mostovski, M., & Ricarte, A. (2025). Description of the Puparium of Eumerus vestitus Bezzi, 1912 (Diptera: Syrphidae) Reared from Supermarket Plums in Israel. Taxonomy, 5(4), 64. https://doi.org/10.3390/taxonomy5040064






