Abstract
The Neotropical region is recognized as the center of diversification of Phalangopsidae. The true extent of this diversity, however, remains understudied, reinforcing the need for further taxonomic investigations. In this study, we describe Igatuia cavernicola gen. et sp. nov., a new genus and species from caves in Igatu village, Chapada Diamantina, central Bahia state, Brazil. The genus is distinguished by unique morphological traits, including ectophallic apodemes with an expanded apex, bearing a ring of minute pores and an inner canal that opens into the ectophallic arc. This description advances our knowledge of subterranean fauna in the region and contributes to a broader understanding of Phalangopsidae cricket diversity in the Neotropics. We also report new records of Sishiniheia, including the first occurrence of the genus in a cave.
1. Introduction
The Neotropical region harbors the highest ecological and phylogenetic diversity of phalangopsid crickets (Orthoptera: Grylloidea: Phalangopsidae) worldwide [1,2]. In Brazil alone, more than 40 distinct genera are currently recognized [3]. Approximately one-third of these occur in the northeastern portion of the country, including Adelosgryllus Mesa & Zefa, 2004 [4], Aracamby de Mello, 1992 [5], Cacruzia de Mello, 1992 [5], Dyscophogryllus Rehn, 1901 [6], Ectecous Saussure, 1878 [7], Eidmanacris Chopard, 1956 [8], Endecous Saussure, 1878 [7], Erebonyx de Mello, 2021 [9], Guabamima de Mello, 1992 [10], Izecksohniella de Mello, 1992 [5], Marcgraviella Souza-Dias & Desutter-Grandcolas, 2014 [11], Paragryllus Guérin-Méneville, 1844 [12], Peulerneca de Mello, D’Oran Souza & Tavares, 2023 [13], and Sishiniheia de Mello & Souza-Dias, 2016 [3,14,15].
Most of these genera are associated with humid habitats of the Atlantic Forest along the eastern coast of South America [3]. Only Adelosgryllus, Endecous, Erebonyx, and Sishiniheia have been recorded from the continent’s interior, where the semi-arid Caatinga biome predominates [3]. With the exception of Sishiniheia, all have been documented in subterranean environments [9,16,17,18,19,20]. Sishiniheia is monotypic and, until now, known exclusively from the deciduous seasonal forest of Lençóis, in the Chapada Diamantina, central Bahia [14].
Over the past two decades, the Chapada Diamantina region has yielded numerous species new to science from diverse taxonomic groups, including plants [21,22,23], lichens [24], fishes [25,26], amphibians [27,28], reptiles [29,30], birds [31,32], arachnids [33], and insects [34,35]. The region also harbors a high number of endemic and rare species [36]. The exceptional biodiversity of the Chapada Diamantina arises from a combination of biotic and abiotic factors, such as habitat heterogeneity, local and regional isolation [37], and the presence of diverse vegetation types linked to distinct edaphic conditions [38]. Despite this, the flora and fauna of the region remain poorly documented and insufficiently sampled, warranting further investigation.
Recent expeditions to the Chapada Diamantina region, in central Bahia State, northeastern Brazil, have revealed a new phalangopsid cricket associated with quartzite caves in an area that has been heavily impacted by diamond mining over the past two centuries. In this study, we formally describe the new genus and discuss the principal threats to the region’s subterranean ecosystems. We also report the first occurrence of Sishiniheia in a cave, thereby extending the known habitat range of this genus.
2. Materials and Methods
Study area—Situated in the central portion of Bahia State in northeastern Brazil (Figure 1), the Chapada Diamantina region constitutes a prominent geological and geomorphological province, encompassing an area of approximately 65,619 km2 and spanning 75 municipalities [39]. This region functions as a critical hydrological divide, directing drainage westward toward the São Francisco River basin and eastward into river systems that ultimately discharge into the Atlantic Ocean, notably via the Paraguaçu River.
Geologically, Chapada Diamantina is distinguished by its diverse landscape, comprising rugged mountain ranges, expansive plateaus, and well-developed karst systems. These landforms predominantly evolved within sedimentary and metasedimentary rock sequences of Proterozoic age [40,41]. The region is part of the broader Precambrian structural framework of the São Francisco Craton [42], whose stratigraphy encompasses lithological units from both the Mesoproterozoic Espinhaço Supergroup and the Neoproterozoic São Francisco Supergroup [43]. This complex geologic evolution has played a crucial role in shaping the topography, hydrology, and mineral resources of the area.
The region’s climate is characterized by pronounced variability, influenced by tropical atmospheric dynamics and substantial altitudinal gradients. According to the Köppen–Geiger classification, the study area is categorized as a tropical highland climate (Cwb), typified by moderate temperatures and marked seasonal rainfall variability. The region exhibits an average annual temperature of approximately 24 °C and a mean annual precipitation around 1060 mm [44]. Seasonal patterns are well defined, with a humid period concentrated between December and March and a distinctly drier interval from June to September, conditions that significantly influence local ecosystems and hydrological processes.
The village of Igatu is situated in the southeastern sector of the Chapada Diamantina region and is administratively part of the municipality of Andaraí. Located along the eastern boundary of the Chapada Diamantina National Park, Igatu possesses significant historical importance due to its association with diamond mining activities, which have profoundly shaped both the cultural identity and the external and subterranean landscapes of the area. The caves from which Igatuia cavernicola gen. et sp. nov. was collected are situated in the vicinity of Igatu village, in close proximity to human settlements (Figure 1D). Further details regarding the habitat characteristics and existing threats will be presented in the Section 4.4.
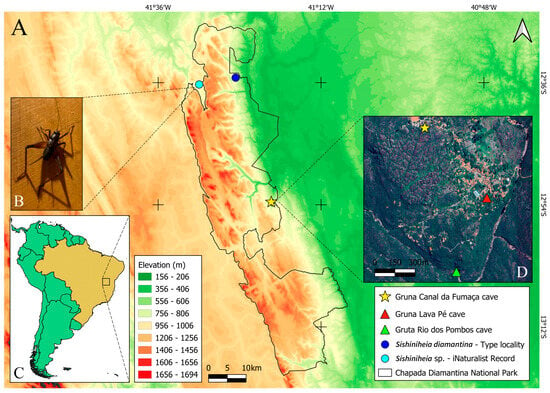
Figure 1.
(A) Topographic map of the Chapada Diamantina region, central Bahia state, Brazil; boundaries of Chapada Diamantina National Park highlighted with a gray line; blue circles indicate the type locality of Sishiniheia diamantina (Lençóis municipality) and a new record for the genus (Palmeiras municipality); yellow star marks the type locality of Igatuia cavernicola gen. et sp. nov; (B) new record of Sishiniheia (photograph from iNaturalist, observation ID = 151438210, user name = Renan Cassimiro); (C) map of South America, with Chapada Diamantina region indicated by a rectangle; (D) Entire Igatu village shown [45], yellow star and red and green triangles indicate caves where the new genus was collected.
Collection and Depository—Adult specimens of Igatuia cavernicola gen. et sp. nov. were collected through active search during daytime in three caves located in the Chapada Diamantina region: Gruna Canal da Fumaça (12°53′35.36″ S, 41°19′20.46″ W), Gruna Lava Pé (12°53′52.60″ S, 41°19′5.15″ W), and Gruta Rio dos Pombos (12°54′10.86″ S, 41°19′12.66″ W), all situated in Igatu Village, municipality of Andaraí, Bahia State, Brazil. Fieldwork was conducted from 8 June to 14 June 2024. The holotype, allotype and paratypes were preserved in 70% ethanol and deposited in the Collection of Subterranean Invertebrates of Lavras (ISLA), at the Center of Studies on Subterranean Biology (CEBS), Federal University of Lavras (UFLA).
The adult female of Sishiniheia sp. was collected on 9 June 2024, in Gruna Canal da Fumaça cave (12°53′35.36″ S, 41°19′20.46″ W) through active search. The specimen was also preserved in 70% ethanol and deposited in the ISLA collection.
Analyses—The general morphology of adult males and females was examined under a Stemi 2000 stereomicroscope (Zeiss, Oberkochen, Germany). Photographs were obtained using an Axiocam 506 color camera (Zeiss) mounted on an Axio Zoom V16 stereomicroscope (Zeiss), as well as a TM4000 scanning electron microscope (Hitachi, Tokyo, Japan). Male phallic complexes and female copulatory papillae were dissected and digested in a pancreatin solution for approximately 24 h, following Álvarez-Padilla & Hormiga [46]. After digestion, the genitalia were rinsed in 70% ethanol, analyzed, and photographed using the same imaging equipment.
The terminology used for the description of the male genitalia follows Desutter-Grandcolas [47], as updated by Souza-Dias [48] and modified by Carvalho & Ferreira [20].
Suprageneric classification—This study follows the current Phalangopsidae taxonomic classification adopted by the Orthoptera Species File [3]. The phylogenetic relationships within this family remain poorly resolved [49,50], with subfamilies, tribes, and subtribes repeatedly erected, redefined, or abandoned over the past 35 years. In this context of instability, the new genus and species described herein are tentatively placed in the tribe Phalangopsini, subtribe Phalangopsina. This placement is based on comparisons with other Neotropical phalangopsid genera, using characters outlined in the major taxonomic and systematic contributions of Desutter-Grandcolas [51,52,53,54,55] and Gorochov [56,57,58,59,60].
Abbreviations—Male genitalia: Ps.db, pseudepiphallic dorsal branches; Ps. A, pseudepiphallic “A” sclerite; Ps.ib, pseudepiphallic inner bars; Ps.dp, pseudepiphallic dorsal parameres; Ps.vp, pseudepiphallic ventral parameres; Ect.arc, ectophallic arc; Ect.ap, ectophallic apodemes; Ect.c, ectophallic canal; Ect.co, ectophallic canal opening; Ect.f, ectophallic fold; Ect.vl, ectophallic ventrolateral projections; End.scl, endophallic sclerite; End.mp, endophallic medioposterior projection; End.ap, endophallic apodeme. Proventriculus: ld, lateral denticles; lt, lateral teeth; md, median denticles; mt, median teeth; sl, sclerotized lobes. Hind tibiae spurs: os, outer subapical spur; is, inner subapical spur; oa, outer apical spur; ia, inner apical spur.
3. Results
Igatuia gen. nov.
Classification—Order Orthoptera Olivier, 1789; Suborder Ensifera Chopard, 1921; Superfamily Grylloidea Laicharting, 1781; Family Phalangopsidae Blanchard, 1845; Subfamily Phalangopsinae Blanchard, 1845; Tribe Phalangopsini Blanchard, 1845; Subtribe Phalangopsina Blanchard, 1845.
Etymology—The genus name refers to Igatu village, the locality where the specimens were collected.
Type species—Igatuia cavernicola sp. nov.
Diagnosis—The new genus shares key traits with members of the subfamily Phalangopsinae, tribe Phalangopsini, such as maxillary palpi elongated relative to head length, equidistant ocelli arranged in an approximate equilateral triangle, reduced forewings not covering the entirety of the abdomen, hind tibiae bearing four dorsal subapical spurs on each side, and male genitalia with reduced median lophi and well-developed pseudepiphallic arms. Igatuia gen. nov. can be further distinguished from genera of the tribe Paragryllini by the morphology of the ovipositor: in Paragryllini, the apices of the dorsal valves possess a lamella that covers anteriorly the apices of the ventral valves, a modification absent in the new genus described herein.
Igatuia gen. nov. is tentatively assigned to the subtribe Phalangopsina, a decision supported by its long, slender legs; the inner median apical spur being markedly longer than the other inner apical spurs; an apical maxillary palpomere that is elongated, curved, and obliquely truncated; and dorsally positioned ocelli on the fastigium. In comparison with other Phalangopsina members (Eidmanacris, Phalangopsis Serville, 1831, and Philippopsis Desutter-Grandcolas, 1992), Igatuia gen. nov. can be distinguished by a unique combination of traits: a fastigium approximately as wide as the antennal scapes (see Table 1 and Table 2), a metasternite with an arched posterior margin, the hind basitarsi bearing a row of small subapical spines on the outer side and a single subapical spine on the inner side, the foretibiae with an oval tympanum on the inner face, mid tibiae with only two ventral apical spurs, and the hind tibiae bearing three inner and three outer apical spurs, the median outer apical spur being twice as long as the dorsal and three times longer than the ventral, while the median inner apical spur is the longest, contrasting with a ventral inner spur nearly one-third its length.

Table 1.
Igatuia cavernicola gen. et sp. nov., morphological measurements (mm) of the holotype and male paratypes (mean value (M); standard deviation (SD); (*) indicates cases where the right leg was measured instead of the left).

Table 2.
Igatuia cavernicola gen. et sp. nov., morphological measurements (mm) of the allotype and female paratypes (mean value (M); standard deviation (SD); (*) indicates cases where the right leg was measured instead of the left).
The adult females can be differentiated by their apterous condition, a medially emarginated subgenital plate, an ovipositor that is shorter than the cerci or approximately three-quarters the length of the hind femora, with an apex roughly as wide as the shaft of the ovipositor, tapering only toward the tip, and a copulatory papilla that is truncate at the apex and broadened at the base.
Specifically in the case of adult males, Igatuia gen. nov. is distinguished by a metanotum with a median pair of oval protrusions covered with modified setae, which are possibly glandular, a supra-anal plate lacking any projections, a right forewing with an inconspicuous mirror area marked by anastomotic crossveins, a harp with multiple complete crossveins, and a stridulatory file characterized by sparse, large, flattened teeth, as well as a lateral field outlined by two longitudinal veins.
The ectophallic apodemes of the male genitalia of Igatuia gen. nov. set it apart from all other known phalangopsids. These structures possess expanded, saddle-like apices with a ring of minute pores at their base, which are associated with an inner canal that opens at the broad and dorsally projected ectophallic arc. Additional distinctive features of the male genitalia of Igatuia gen. nov. include pseudepiphallic inner bars dorsally projected and deeply concave, absence of rami, pseudepiphallic dorsal parameres highly sclerotized and concave, and pseudepiphallic ventral parameres slightly concave, laterally surrounding the dorsal parameres and fused to them ventrally. Other noteworthy features are the V-shaped endophallic sclerite with a deep median ventral concavity and a short dorsoapical apodeme.
Description—In addition to the characters included in the “Diagnosis”: head pubescent and dorsoventrally elongated; compound eyes well-developed; fastigium covered with elongated, thick setae; maxillary palpomeres I and II short, III to V elongated; pronotum wider than long, arched; pubescent; anterior and posterior margins densely covered with elongated, thick setae; mesosternite roughly quadrangular, posterior margin arcuate; metasternite approximately hexagonal, lateral margins slightly curved inward; tergite I approximately three times longer than subsequent tergites; cerci covered with elongated setae, inner side bearing globose setae; coxae I with an outer spine and an inner rounded projection; femora III distally banded; basitarsi III bearing a pair of ventral apical spurs, the inner spur longer than the outer. Male body morphology: supra-anal plate posterior margin rounded and covered with elongated setae; subgenital plate broadly trapezoidal, with a constriction in the distal third, lateral margins curved inward, and posterior margin rounded; lateral field of the right forewing marked by two longitudinal veins connected to several secondary crossveins. Male genitalia: pseudepiphallic A sclerite projected inward, reaching the pseudepiphallic ventral parameres; ectophallic ventrolateral projections reaching the ventral portion of the pseudepiphallic ventral parameres; ectophallic fold dorsally sclerotized. Female body morphology: metanotum not differentiated; supra-anal plate subtriangular, lateral margins bearing a short triangular projection, posterior margin rounded and covered with elongated setae; ovipositor spear-shaped.
Igatuia cavernicola sp. nov.
(Table 1 and Table 2; Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9)
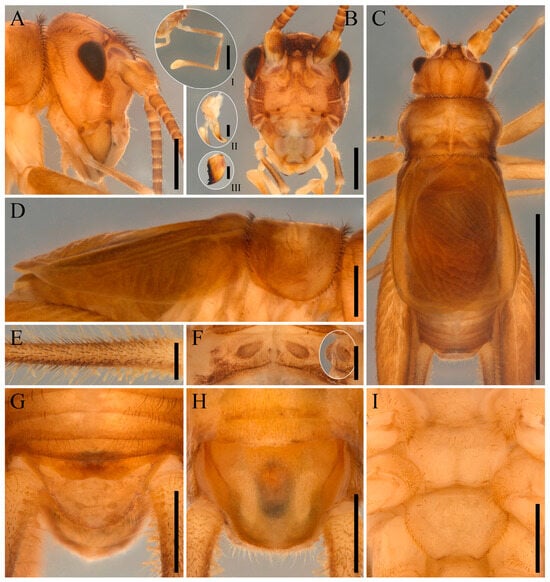
Figure 2.
Igatuia cavernicola gen. et sp. nov. male body morphology (holotype, ISLA144199; paratype, ISLA144205): (A) head, lateral view, scale bar–1 mm, holotype; (B) head, frontal view, scale bar–1 mm, paratype (insets: (I) maxillary palp, lateral view, scale bar–1 mm; (II) maxilla, ventral view, scale bar–0.5 mm; (III) mandible, frontal view, scale bar–0.5 mm); (C) body, dorsal view, scale bar–5 mm, holotype; (D) thorax and right tegmen, lateral view, scale bar–1 mm; (E) cercus, lateral view, scale bar–0.5 mm, paratype; (F) metanotum, dorsal view, scale bar–0.5 mm, paratype (inset: metanotal protrusions, lateral view); (G) supra-anal plate, dorsal view, scale bar–1 mm, holotype; (H) subgenital plate, ventral view, scale bar–1 mm, holotype; (I) meso and metasternites, ventral view, scale bar–1 mm, holotype.
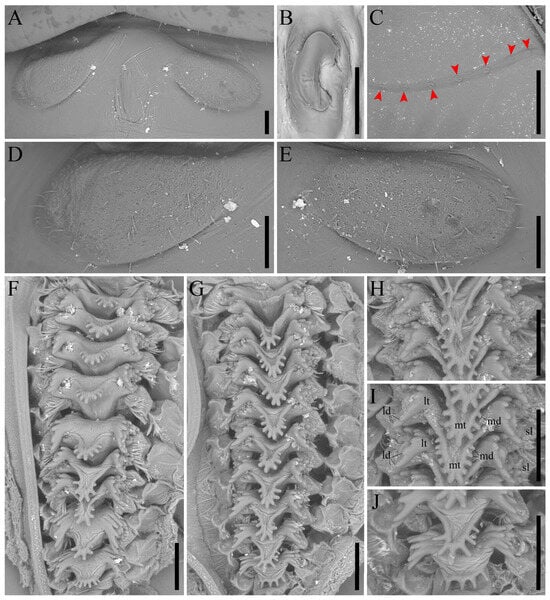
Figure 3.
Igatuia cavernicola gen. et sp. nov. metanotum protrusions, stridulatory file, and proventriculus (paratype, ISLA144205): (A) metanotum protrusions, dorsal view, scale bar–0.1 mm; (B) stridulatory file tooth, ventral view, scale bar–0.05 mm; (C) stridulatory file, ventral view, scale bar–0.5 mm, red arrows indicate stridulatory file teeth; (D) left metanotum protrusion, dorsal view, scale bar–0.1 mm; (E) right metanotum protrusion, dorsal view, scale bar–0.1 mm; (F) sclerotized appendices row, scale bar–0.1 mm; (G) sclerotized appendices row, scale bar–0.1 mm; (H) anterior sclerotized appendices, scale bar–0.1 mm; (I) median sclerotized appendices, scale bar–0.1 mm; (J) posterior sclerotized appendices, scale bar–0.1 mm. Abbreviations: lateral denticles (ld); lateral teeth (lt); median denticles (md); median teeth (mt); sclerotized lobes (sl).

Figure 4.
Igatuia cavernicola gen. et sp. nov. legs and right forewing (holotype, ISLA144199; paratype, ISLA144205): (A) leg I, outer view, and leg II, inner view, scale bar–2 mm; (B) leg I, inner view, and leg II, outer view, scale bar–2 mm; (C) tympanum, foretibia, inner view, scale bar–0.5 mm; (D) forecoxa, anterior view, scale bar–0.5 mm; (E) hind tibia, dorsal view, subapical spurs labelled, scale bar–2 mm; (F) leg III, outer view, scale bar–2 mm (inset: outer apical spurs, scale bar–0.2 mm); (G) leg III, inner view, scale bar–2 mm (inset: inner apical spurs, scale bar–0.2 mm); (H) right forewing, dorsal view, scale bar–1 mm; (I) right forewing, lateral view, scale bar–1 mm. Abbreviations: outer subapical spur (os); inner subapical spur (is); outer apical spur (oa); inner apical spur (ia).
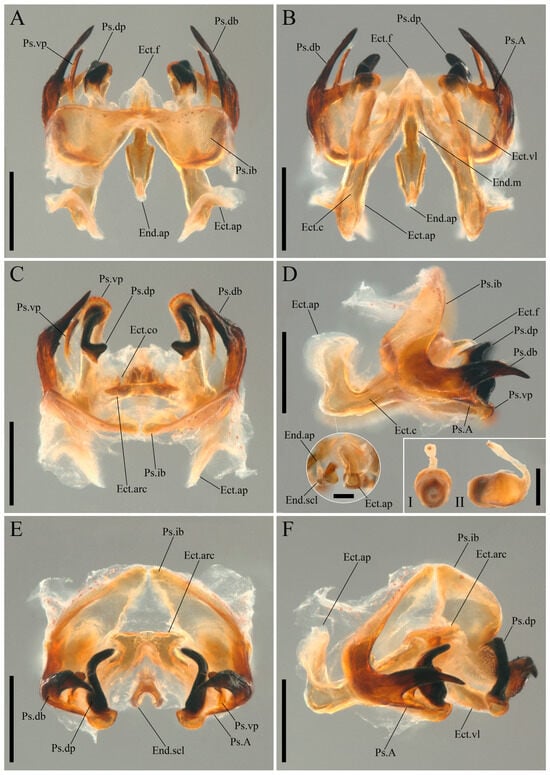
Figure 5.
Igatuia cavernicola gen. et sp. nov. male genitalia and spermatophore (paratype, ISLA144200): (A) dorsal view, scale bar–0.5 mm; (B) ventral view, scale bar–0.5 mm; (C) dorsoposterior view, scale bar–0.5 mm; (D) lateral view, scale bar–0.5 mm (ectophallic apodeme and endophallic sclerite, anterior view, scale bar–0.2 mm; spermatophore, frontal view (I), lateral view (II), scale bar–0.5 mm); (E) posterior view; (F) lateroposterior view, scale bar–0.5 mm.
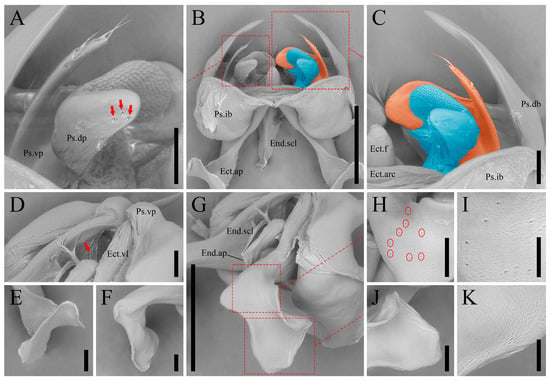
Figure 6.
Igatuia cavernicola gen. et sp. nov. scanning electron micrographs of male genitalia (paratypes, ISLA144202, ISLA144205): (A) dorsal and ventral pseudepiphallic parameres, dorsal view, red arrows indicate setae at the posterodorsal cusp, scale bar–0.1 mm; (B) phallic complex, dorsal view, with Ps.dp and Ps.vp highlighted in blue and orange, respectively, scale bar–0.5 mm; (C) Ps.dp (blue) and Ps.vp (orange), dorsal view, scale bar–0.1 mm; (D) median portion of the phallic complex, lateroventral view, red arrow indicates the Ps.dp inner surface bearing setae or their insertion points, scale bar–0.1 mm; (E) ectophallic apodeme apex, dorsal view, scale bar–0.1 mm; (F) Ect.ap apex, inner lateral view, scale bar–0.1 mm; (G) anterior portion of the phallic complex, lateroventral view, scale bar–0.5 mm; (H) Ect.ap apex, proximal portion, red circles mark pores, scale bar–0.1 mm; (I) Ect.ap apex, proximal portion, ring of pores in detail, scale bar–0.05 mm; (J) Ect.ap apex, distal portion, scale bar–0.1 mm; (K) Ect.ap apex, distal portion, surface texture in detail, scale bar–0.05 mm.
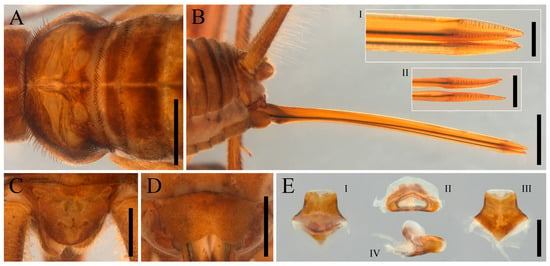
Figure 7.
Igatuia cavernicola gen. et sp. nov. female body morphology and copulatory papilla (allotype ISLA144206, paratype ISLA144207): (A) pronotum, first and second tergites, dorsal view, scale bar–2 mm; (B) ovipositor, lateral view, scale bar–0.2 mm (ovipositor apex in detail, lateral view, scale bar–0.5 mm (I); ovipositor apex in detail, dorsal view, scale bar–0.5 mm (II)); (C) supra-anal plate, dorsal view, scale bar–1 mm; (D) subgenital plate, ventral view, scale bar–1 mm; (E) copulatory papilla, scale bar–0.5 mm (dorsal view (I), axial view (II), ventral view (III), lateral view (IV)).
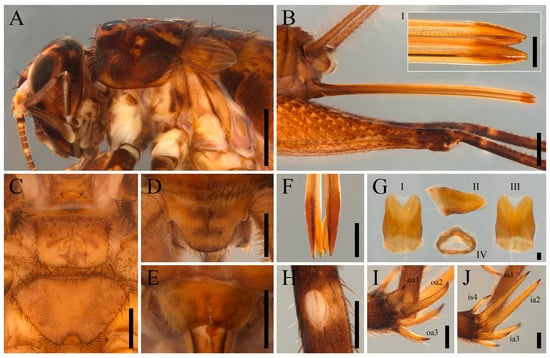
Figure 8.
Sishiniheia sp., adult female, body morphology and copulatory papilla (ISLA144212): (A) head, pronotum and forewings, lateral view, scale bar–2 mm; (B) ovipositor, lateral view, scale bar–2 mm (ovipositor, apex in detail, lateral view, scale bar–0.5 mm (I)); (C) meso and metasternites, ventral view (D) supra-anal plate, dorsal view, scale bar–1 mm; (E) subgenital plate, ventral view, scale bar–1 mm; (F) ovipositor, apex in detail, dorsal view, scale bar–0.5 mm; (G) copulatory papilla (I) dorsal view, (II) lateral view, (III) ventral view, (IV) frontal view; (H) foretibia, outer tympanum in detail, scale bar–0.5 mm; (I) hind tibia, outer apical spurs in detail, scale bar–0.5 mm; (J) hind tibia, inner apical spurs in detail, scale bar–0.5 mm. Abbreviations: inner subapical spur (is); outer apical spur (oa); inner apical spur (ia).

Figure 9.
Igatuia cavernicola gen. et sp. nov. habitat: (A) Igatu village, yellow arrow indicates Gruna Canal da Fumaça cave, white arrow indicates Gruna Lava Pé cave; (B) Gruna Canal da Fumaça cave, entrance; (C) stone wall within Gruna Canal da Fumaça cave; (D) accumulations of rock debris, evidence of excavation activities, Gruna Canal da Fumaça cave; (E) Igatuia cavernicola gen. et sp. nov. adult male.
Etymology—The specific epithet cavernicola derives from the Latin caverna (“cave”) and -cola (“inhabitant”, “dweller”), referring to the subterranean habitats where all specimens were found. It is to be treated as a noun in apposition.
Material examined–Holotype, ♂, ISLA144199, Brazil, Bahia, municipality of Andaraí, Igatu village, Gruna Canal da Fumaça cave (12°53′35.36″ S, 41°19′20.46″ W), 09.VI.2024, R.L. Ferreira; condition: left legs II detached and stored alongside the specimen, left hind femur slightly damaged. Allotype, ♀, ISLA144206, same data as the holotype; condition: left hind leg missing, copulatory papilla dissected and stored alongside the specimen. Paratypes, ♂♂, ISLA144200, ISLA144201, ISLA144202, ISLA144203, ISLA144205, ISLA144209, ISLA144210, ISLA144211, same data as the holotype. Paratype, ♂, ISLA144204, and paratype, ♀, ISLA144207, Brazil, Bahia, municipality of Andaraí, Igatu village, Gruna Lava Pé cave (12°53′52.60″ S, 41°19′5.15″ W), 14.VI.2024, R.L. Ferreira. Paratype, ♀, ISLA144208, Brazil, Bahia, municipality of Andaraí, Igatu village, Gruta Rio dos Pombos cave (12°54′10.86″ S, 41°19′12.66″ W), 08.VI.2024, R.L.Ferreira.
Diagnosis–As for the genus.
Male body morphology (holotype and paratypes, Figure 2, Figure 3 and Figure 4)–Head (Figure 2A–C): dorsoventrally elongated in lateral view; predominantly yellowish brown; genae and frons separated by a brown stripe; frons with two short brown stripes curved outwardly; fastigium brown, covered with elongated, thick setae; vertex displaying four faded brown stripes, each covered by a row of elongated, thick setae—the two lateral stripes extending from the occiput to the compound eyes, and the two median stripes from the occiput to the fastigium and apically bifurcated; median ocellus rounded, situated in a heart-shaped, light-colored concavity on the dorsal surface of the fastigium, approximately half the size of the oval lateral ocelli, which are located on a droplet-shaped, light-colored area at the apex of the vertex; fastigium not projected; antennae yellowish-brown; antennal socket dorsally brown; mandibles and maxillary laciniae yellow, with dark brown, sclerotized apices; laciniae bearing two apical pointed teeth; clypeus white with yellow and brown spots; labrum, galea, and labial palps white; maxillary palpomeres I and II short and white, III-V elongated and yellowish brown; palpomere V the longest, with a rounded and dorsally curved apex; holotype’s left maxillary palp teratological, with palpomeres IV and V underdeveloped relative to those of the right palp.
Thorax (Figure 2C,D,F,I and Figure 3A,D,E): pronotum yellowish-brown with brown spots and sparsely distributed elongated setae; lateral lobes rounded and directed anteriorly, mostly brown, with yellowish-brown areas on the apex and anterior margin; metanotum posterior margin elevated and forming an inverted V-shape.
Right forewing (Figure 2C,D, Figure 3B,C and Figure 4H,I): brown, slightly sclerotized; roughly oval, covering tergite IV; harp with one incomplete and seven complete crossveins, the most distal one bifurcated; lateral field with two longitudinal veins—dorsal vein well marked, ventral vein connected to numerous secondary crossveins; stridulatory file with five to eight widely spaced, large, flattened teeth (mean = 6.5, n = 7).
Abdomen (Figure 2G,H): pubescent; coloration varying from light yellowish-brown to brown; supra-anal plate divided into two distinct regions—a roughly W-shaped, smooth, and dark yellowish-brown basal region, and a light brown with irregular brown spots and a pair of pale oval patches near the base medioapical region; posterior margin brown; subgenital plate lateral portion yellowish-brown; central portion partially translucent, with a hourglass-shaped, whitish-brown spot; cerci yellowish-brown.
Legs (Figure 4A–G): pubescent; yellowish-brown with darker areas; femora III well developed; tibiae III median outer apical spur twice as long as the dorsal and ventral outer apical spurs (oa2 > oa3 > oa1); median inner apical spur the longest, ventral inner apical spur approximately three times shorter (ia2 > ia1 > ia3); holotype’s right tibia III with four outer and three inner subapical spurs; inner subapical spurs generally shorter than the outer ones, except for the most distal outer subapical spur, which is of comparable length.
Male genitalia (holotype and paratypes, Figure 5A–F and Figure 6A–K)–Pseudepiphallus: inner bars (Ps.ib) dorsally projected, deeply concave, and tapered toward the apex; rami absent; dorsal branches (Ps.db) sclerotized, elongated, apically tapered, and curved inward and ventrally; A sclerites (A) sclerotized, projected inward almost perpendicularly to the dorsal branches, with a tapered apex reaching the ventrolateral portion of the pseudepiphallic ventral parameres; dorsal parameres (Ps.dp) highly sclerotized, concave, consisting of two cusps; posteroventral cusp broad, short, and knob-like, posterior inner surface formed by small overlapping scale-like protrusions, anterior inner surface covered with several short setae with conspicuous insertion points; posterodorsal cusp curved inward, tapered, with a rounded apex, bearing three dorsal, thick setae; ventral parameres (Ps.vp) sclerotized, slightly concave, laterally surrounding the dorsal parameres and fused to them ventrally, posteroventral cusp broad and subquadrangular, posterodorsal cusp forming an elongated projection that follows the curvature of the dorsal branches, tapering toward the apex, which bears multiple elongated setae.
Ectophallic invagination: ventrolateral projections (Ect.vl) sclerotized, inclined inward and reaching the ventral portion of the posteroventral cusp of the pseudepiphallic ventral parameres; fold (Ect.f) dorsally sclerotized, with a finger-like apex; apodemes (Ect.ap) sinuous, sclerotized, with an inner canal (Ect.c) that opens at the ectophallic arc, apodeme apex dorsally expanded, forming a saddle-like structure with a ring of minute pores at the base; arc (Ect.arc) sclerotized, dorsally projected and π-shaped, bearing the continuation of the ectophallic apodemes canals and their openings.
Endophallus: medioposterior projection (End.mp) sclerotized, as long as the ectophallic lateroventral, with a median bulge; sclerite (End.scl) V-shaped, with a median furrow and a short dorsoapical apodeme (End.ap).
Proventriculus (paratype ISLA144205, Figure 3F–J)–consisting of six rows of nine or ten overlapping sclerotized appendices, divided into a pair of sclerotized lobes (sl) bearing a cluster of setae, a pair of lateral teeth (lt), and a median tooth (mt); lateral teeth with up to 12 lateral denticles (ld); median tooth with at least five and up to 11 median denticles (md); denticles short or elongated but always rounded at the apex; the most anterior and posterior sclerotized appendices may be regular or differentiated; the anterior sclerotized appendix either lacking a median tooth or bearing one with fewer median denticles; the posterior sclerotized appendix bearing a broadened median tooth with almost parallel median denticles.
Female body morphology (allotype ISLA144206, paratype ISLA144207, Figure 7A–D)–head, thorax, abdomen, legs and color pattern similar to the male; supra-anal plate pubescent, brown posterior margin covered with elongated setae; subgenital plate pubescent, yellowish brown; ovipositor slightly curved downward, sclerotized, yellowish-brown.
Copulatory papilla (allotype ISLA144206, ISLA144207, Figure 7E)–sclerotized; approximately pentagonal, broader basally than apically; anterior portion ventrally triangular; dorsal surface with an anterior pair of irregular, sclerotized short projections covered by a hard, translucent, but opaque mass; lateral margins curved outward; apex membranous and truncated.
Sishiniheia sp.
Material examined: adult female, ♀, (ISLA 144212), Brazil, Bahia, municipality of Andaraí, Igatu village, Gruna Canal da Fumaça cave (12°53′35.36″ S, 41°19′20.46″ W), 09.VI.2024, R.L. Ferreira; copulatory papilla dissected and stored alongside the specimen.
Female body morphology (Figure 8A–F,H–J): vertex dark brown with four yellowish-brown stripes; antennae annulated; pronotum dark-brown with several irregular yellowish-brown spots; abdomen brown with small dark brown spots; legs I and II dark brown with light-colored rings and irregular spots; femur III dark brown posteriorly and brown anteriorly, but almost entirely masked by dense yellowish-brown spotting, especially on the ventral and lateral surfaces; left forewing reduced and scale-like, right forewing missing (likely due to damage); metasternite posterior margin deeply emarginated, forming a triangular notch; supra-anal plate yellowish-brown, subtriangular, posterior margin covered with elongated, thick setae and lateral margins bearing short outward projections; subgenital plate emarginated, posterior margin roughly W-shaped; ovipositor spear-shaped, apex as wide as the rest of the ovipositor, slightly curved downward, and almost as long as the cerci; tibia I with inner and outer tympana; tibia II with four apical spurs; tibia III with four outer and four inner subapical spurs, and three inner and three outer apical spurs; dorsal and median inner apical spurs nearly equal in length and twice as long as the ventral inner apical spur (ia1 = ia2 > ia3); median outer apical spur twice as long as the dorsal and ventral outer apical spurs (oa2 > oa3 > oa1).
Copulatory papilla (Figure 8G): sclerotized; anterior and posterior portions similarly broadened; lateral margins with an anteromedial bulge; anterodorsal margin longer than ventrodorsal; posterior margin ventrally emarginated; medioapical region membranous and roughly heart-shaped; dorsal surface with a medial longitudinal bulge.
4. Discussion
4.1. First Record of Sishiniheia in Caves
We report the first record of Sishiniheia in a cave environment and only the second occurrence of the genus since its original description in 2016. This record extends its known distribution approximately 38 km southward. Notably, the copulatory papilla of the specimen differs from that illustrated by Souza-Dias et al. [14], a variation that may result from differences in preparation protocols or indicate a distinct species. However, species-level identification is not possible because the specimen is an adult female, and reliable comparisons require examination of male genitalia. Targeted surveys aimed at collecting adult males in the Igatu village region are therefore necessary to clarify its taxonomic status.
In addition, a search on the citizen science platform iNaturalist yielded a potential third record for the genus: an adult male photographed on 17 March 2023 near a forested area in the district of Caeté-Açu (12°36′16.1″ S, 41°29′57.3″ W), municipality of Palmeiras, Bahia State, Brazil (observation ID = 151438210, user ID = 3524286, user name = Renan Cassimiro; see <https://www.inaturalist.org/observations/151438210, accessed on 16 July 2025> or Figure 1B). This observation may represent a westward range extension of the genus to the opposite side of the mountains and plateaus of the Chapada Diamantina, approximately 13 km from the type locality. Unfortunately, confirmation at the species level remains uncertain due to the absence of detailed morphological information, particularly on male genitalia.
4.2. Is Igatuia Cavernicola gen. et sp. nov. a Troglobitic Species?
The ecological and evolutionary status of I. cavernicola gen. et sp. nov. remains uncertain. The species lacks most morphological traits typically associated with strictly subterranean organisms, such as eye reduction or tegument depigmentation [61,62]. However, it is important to note that, according to the Schiner–Racovitza classification, troglobites are defined as organisms whose entire life cycle occurs in subterranean environments, regardless of the presence or absence of troglomorphic features [62,63]. The presence of both juvenile and adult specimens in the sampled caves during daytime indicates that the species is, at minimum, cavicolous, and possibly troglophilic. Nevertheless, additional surveys in surrounding epigean habitats are necessary to determine whether surface populations exist and, consequently, whether the species is truly cave-restricted.
One notable feature of I. cavernicola gen. et sp. nov. is its elongated legs, a trait often regarded as an indicator of cave adaptation [61,62]. However, long legs are also common among epigean Phalangopsidae. As emphasized by Heads [49], traits shared by both epigean and hypogean taxa should not be interpreted as definitive adaptations to a strictly subterranean lifestyle. Conversely, comparative studies within the genus Endecous have shown that some troglobitic species possess significantly longer legs than their surface-dwelling congeners [19,20,64]. Therefore, the identification of troglomorphic traits requires broad comparative analyses with congeners to determine the evolutionary state of a given character [65]. Unfortunately, as I. cavernicola gen. et sp. nov. is currently monotypic, it is not yet possible to establish with confidence which of its features, if any, represent true troglomorphisms.
4.3. Male Genitalia, Glandular Structures and Systematic Relationships
Igatuia cavernicola gen. et sp. nov. is distinguished from all other South American Phalangopsidae primarily by the unique morphology of its male genitalia (see Igatuia gen. nov., “Diagnosis”). One of the most distinctive features of its phallic complex is the channeled structure of the ectophallic arch and apodemes, which is an unprecedented configuration within the family. The presence of a whitish substance at the apex of the ectophallic arch, together with the ring of pores at the apices of the ectophallic apodemes, may indicate the transport of secretions through these channels, although no well-defined glandular structures were identified in association with the genitalia. Furthermore, another noteworthy diagnostic trait can be found in the ectophallic apodemes apices, which are dorsally expanded, forming saddle-shaped lamellae that borders the anterior openings of the ectophallic channels. These structures may serve as anchoring sites for muscles or, potentially, glandular tissues.
Phallic glands have been reported in other members of Phalangopsidae [1,54,55,66]. Such glands are often associated with the pseudepiphallic arms [5,11,67,68,69] and may be involved in various reproductive functions, including the production of mating plugs [70]. The potential glandular structures of I. cavernicola gen. et sp. nov are internal and could produces substances that do not require direct transfer to the female genitalia, like female stimulants or attractants during courtship. Nonetheless, these interpretations remain speculative, and further studies with live specimens are necessary to clarify their function.
Following the suprageneric classification of Phalangopsidae proposed by Gorochov [58,60] and currently adopted by the Orthoptera Species File [3], we tentatively assign I. cavernicola gen. et sp. nov. to the subtribe Phalangopsina. This placement is supported by several morphological traits outlined in the “Diagnosis” section. However, given the current gaps in our understanding of Phalangopsidae systematics, integrative approaches that combine detailed morphological studies with molecular data are essential to resolve the phylogenetic relationships within the group [49,50], including those of the new genus described herein. The description of Igatuia cavernicola gen. et sp. nov. represents a valuable contribution to the growing body of knowledge on the diversity, evolution and ecology of Neotropical phalangopsid crickets.
4.4. Habitat and Threats
Igatu village underwent significant development during the mid-19th century, emerging as a prominent center of artisanal “garimpo” mining fueled by intensive diamond extraction between approximately 1846 and 1871. Mining activities continued into the early 20th century, propelling the village’s population to a peak of around 4600 inhabitants [71]. The subsequent decline of diamond mining, however, resulted in substantial depopulation and economic contraction, leaving the current population at fewer than 500 residents. Architectural remnants, such as abandoned stone buildings and mining infrastructure, persist as tangible evidence of this historical period.
The siliciclastic caves located in the vicinity of Igatu village have undergone substantial anthropogenic alterations resulting from historical diamond extraction activities [40]. These interventions led to significant modifications, including the enlargement of pre-existing conduits and the excavation of entirely new passages, as documented in Gruna Canal da Fumaça (Figure 9B) and Gruna Lava Pé caves, habitats of I. cavernicola gen. et sp. nov. Among the visible alterations are the construction of low stone walls (Figure 9C), accumulations of rock debris derived from excavation activities along the cave floors and side walls (Figure 9D), and the presence of artificial galleries carved into the rock.
Although other caves in the region have previously been the focus of biological inventories, specimens of I. cavernicola gen. et sp. nov. have so far been recorded exclusively in Gruna Canal da Fumaça, Gruna Lava Pé, and Gruta Rio dos Pombos caves. In all localities, population densities were relatively low, with individuals observed only sporadically within the aphotic zones of the caves (Figure 9E), contrasting with most Brazilian cave-dwelling phalangopsid crickets, which are often found in considerably higher abundances [72,73,74]. As there are no historical records of this species prior to anthropogenic disturbances, it remains impossible to determine whether the observed low population densities reflect the species’ natural condition or result from significant habitat alterations that may have led to population declines. Notably, no specimens were observed near cave entrances, indicating a marked preference for hypogean conditions, at least during daytime
The primary organic resources observed in these caves, which could potentially serve as food sources for the species, consist of plant detritus transported and deposited by water flows throughout the cave passages, along with sparse small accumulations of guano. In this context, I. cavernicola gen. et sp. nov. likely plays an important role in nutrient cycling by consuming decomposing organic matter, thus contributing to the functioning of the cave ecosystems in which it occurs. Additionally, as observed for other phalangopsid crickets [75], the species may serve as a food source for cave predators, further highlighting its ecological significance in subterranean habitats.
It is also important to emphasize that no collections were conducted in external environments, leaving the true extent of the species’ distribution unknown. Thus, it is presently not possible to infer the precise degree of this species’ dependence on subterranean habitats, although its occurrence exclusively in aphotic zones suggests at least a degree of preadaptation to cave environments (as discussed previously in the section “Is I. cavernicola gen. et sp. nov. a troglobitic species?”).
Finally, considering I. cavernicola gen. et sp. nov. currently known distribution, which is limited to three caves that are subject to significant human impacts, it is strongly recommended that future research efforts focus on expanding surveys to additional caves and potential surface habitats. Such studies should also consider implementing monitoring programs for the known populations to detect potential fluctuations that might pose further risks to the species’ survival.
Author Contributions
Conceptualization, P.H.M.-C.; Methodology, P.H.M.-C. and R.L.F.; Investigation, P.H.M.-C.; Writing—original draft, P.H.M.-C.; Writing—review and editing, M.S.-S. and R.L.F.; Supervision, M.S.-S. and R.L.F.; Funding acquisition, M.S.-S. and R.L.F.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), which granted a scholarship to Pedro H. Mendes Carvalho; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), which provided a research productivity grant to Rodrigo L. Ferreira (Grant No. 302925/2022-8) and Marconi Souza Silva (CNPq n. 303434/2025-2); and by the Centro Nacional de Pesquisa e Conservação de Cavernas (CECAV) and the Instituto Brasileiro de Desenvolvimento e Sustentabilidade (IABS), which provided financial support through the Termo de Compromisso de Compensação Espeleológica (TCCE ICMBio/VALE 1/2022).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author. Specimens were deposited in the Collection of Subterranean Invertebrates of Lavras (ISLA), Lavras, at the Center of Studies on Subterranean Biology (CEBS), Federal University of Lavras (UFLA), Minas Gerais State, Brazil.
Acknowledgments
We thank Renan Cassimiro for kindly allowing us to use his photograph of the Sishiniheia specimen observed in Caeté-Açu. We also acknowledge João Taramba, Chiquinho de Igatu (Raimundo Cruz dos Santos), and the team from the Center for Subterranean Biology Studies (CEBS/UFLA), with particular thanks to Paulo Reis-Venâncio, Gabriel Augusto Silva Vaz, and Lucas Guimarães de Souza for their valuable assistance in the field.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| Ps.db | Pseudepiphallic dorsal branches |
| Ps.A | Pseudepiphallic “A” sclerite |
| Ps.ib | Pseudepiphallic inner bars |
| Ps.dp | Pseudepiphallic dorsal parameres |
| Ps.vp | Pseudepiphallic ventral parameres |
| Ect.arc | Ectophallic arc |
| Ect.ap | Ectophallic apodemes |
| Ect.c | Ectophallic canal |
| Ect.co | Ectophallic canal opening |
| Ect.f | Ectophallic fold |
| Ect.vl | Ectophallic ventrolateral projections |
| End.scl | Endophallic sclerite |
| End.mp | Endophallic medioposterior projection |
| End.ap | Endophallic apodeme |
| ld | Lateral denticles |
| lt | Lateral teeth |
| md | Median denticles |
| mt | Median teeth |
| sl | Sclerotized lobes |
| os | Outer subapical spur |
| is | Inner subapical spur |
| oa | Outer apical spur |
| ia | Inner apical spur |
References
- Desutter-Grandcolas, L. Toward the knowledge of the evolutionary biology of phalangopsid crickets (Orthoptera: Grylloidea: Phalangopsidae): Data, questions and evolutionary scenarios. J. Orthoptera Res. 1995, 4, 163–175. [Google Scholar] [CrossRef]
- Desutter-Grandcolas, L.; Faberon, L. Phalangopsidae crickets (Orthoptera, Grylloidea) from the Mitaraka biological survey, French Guiana. Zoosystema 2020, 42, 739–797. [Google Scholar] [CrossRef]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File, Version 5.0/5.0. Available online: http://orthoptera.speciesfile.org/ (accessed on 25 July 2025).
- Mesa, A.; Zefa, E. Adelosgryllus rubricephalus: A new genus and species of cricket (Orthoptera: Phalangopsidae). Neotrop. Entomol. 2004, 33, 327–332. [Google Scholar] [CrossRef]
- de Mello, F.A.G. Aracamby, Cacruzia and Izecksohniella: Three new genera of phalangopsid crickets from the Brazilian coastal forests (Orthoptera: Grylloidea). J. Orthoptera Res. 1992, 1, 50–58. [Google Scholar] [CrossRef]
- Rehn, J.A.G. Some necessary changes and corrections in names of Orthoptera. Can. Entomol. 1901, 33, 271–272. [Google Scholar] [CrossRef][Green Version]
- Saussure, H. Mélanges orthoptérologiques. VI. fascicule Gryllides. Mém. Soc. Phys. Hist. Nat. Genève 1878, 25, 369–834. [Google Scholar][Green Version]
- Chopard, L. Some crickets from South America (Grylloidea and Tricatyloidea). Proc. U.S. Natl. Mus. 1956, 106, 241–293. [Google Scholar] [CrossRef]
- De Mello, F.A.G.; Ferreira, R.L. Erebonyx catacumbae, n. gen. et sp.: A blind, troglobitic cricket from Brazil (Orthoptera, Grylloidea, Phalangopsidae). Zootaxa 2021, 4975, 343–356. [Google Scholar] [CrossRef]
- de Mello, F.A.G. A new genus of long-legged crickets from the forests of eastern Brazil (Orthoptera: Grylloidea: Phalangopsidae). Rev. Agric. 1992, 67, 125–131. [Google Scholar] [CrossRef]
- Souza-Dias, P.G.B.; Desutter-Grandcolas, L. A new genus and two new species of Luzarinae cricket from the Atlantic Forest of Northeast Brazil (Orthoptera, Grylloidea). Zootaxa 2014, 3872, 498–512. [Google Scholar] [CrossRef][Green Version]
- Guérin-Méneville, F.E. Iconographie du Règne Animal de G. Cuvier; Baillère, J.B., Ed.; Libraire de L’Académie Royale de Médecine: Paris, France, 1844; Volume 7, 576p. [Google Scholar][Green Version]
- de Mello, F.A.G.; D’Oran Souza, M.; Tavares, G.C. Peulerneca ubaira gen. et sp. nov.: A new Lernecina cricket with white and hyaline forewings from the Brazilian Atlantic Forest (Phalangopsidae, Luzarinae, Lernecina). Zootaxa 2023, 5271, 366–374. [Google Scholar] [CrossRef]
- Souza-Dias, P.G.B.; de Mello, F.A.G.; Vieira, L.B. A new genus and species of cricket from the Chapada Diamantina National Park, northeastern Brazil (Grylloidea: Phalangopsidae; Luzarinae). Zootaxa 2016, 4121, 258–266. [Google Scholar] [CrossRef]
- Zefa, E.; Martins, L.P.; Demari, C.P.; Acosta, R.C.; Centeno, E.; Castro-Souza, R.A.; de Oliveira, G.L.; Miyoshi, A.R.; Fianco, M.; Redü, D.R.; et al. Singing crickets from Brazil (Orthoptera: Gryllidea), an illustrated checklist with access to the sounds produced. Zootaxa 2022, 5209, 211–237. [Google Scholar] [CrossRef]
- Castro-Souza, R.A.; Zefa, E.; Ferreira, R.L. Two new species of cave crickets Endecous (Notoendecous) Gorochov, 2014 (Orthoptera: Grylloidea: Phalangopsidae) from northeastern Brazil. Zootaxa 2017, 4318, 474–498. [Google Scholar] [CrossRef]
- Merlo, R.L.S.; Castro-Souza, R.A.; Junta, V.G.P.; Ferreira, R.L. The first record of Adelosgryllus Mesa & Zefa, 2004 (Orthoptera: Grylloidea: Phalangopsidae) from caves, with the description of a new species from Brazil. Zootaxa 2021, 4933, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Merlo, R.L.S.; Castro-Souza, R.A.; Bento, D.M.; Ferreira, R.L. A new troglophilic species of Erebonyx (Orthoptera: Grylloidea: Phalangopsidae) from Brazilian caves. Zootaxa 2022, 5222, 83–93. [Google Scholar] [CrossRef]
- Carvalho, P.H.M.; Junta, V.G.P.; Castro-Souza, R.A.; Ferreira, R.L. Three new cricket species and a new subgenus of Endecous Saussure, 1878 (Grylloidea: Phalangopsidae) from caves in northeastern Brazil. Zootaxa 2023, 5263, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.H.M.; Ferreira, R.L. No need to jump in the darkness: A new troglobitic species of Endecous Saussure, 1878 (Orthoptera: Grylloidea: Phalangopsidae) from the Brazilian Semiarid region. Zootaxa 2025, 5642, 317–330. [Google Scholar] [CrossRef]
- Pataro, L.; Romero, R.; Roque, N. Four new species of Microlicia (Melastomataceae) from Chapada Diamantina, Bahia, Brazil. Kew Bull. 2013, 68, 285–293. [Google Scholar] [CrossRef]
- Cavalcante, B.P.; Silva, K.R.; de Souza, E.H.; Versieux, L.M.; Martinelli, A.P. Hohenbergia erecta (Bromeliaceae: Bromelioideae), a new once-branched species from Chapada Diamantina, Bahia State, Brazil. Trop. Plant Biol. 2022, 15, 319–325. [Google Scholar] [CrossRef]
- Romero, R.; Pataro, L.; Versiane, A.F.A. Three new species of Microlicia (Melastomataceae) with yellow corollas from Chapada Diamantina, Bahia, Brazil. Plant Ecol. Evol. 2025, 158, 157–170. [Google Scholar] [CrossRef]
- Aptroot, A.; da Silva Cáceres, M.E. New lichen species from Chapada Diamantina, Bahia, Brazil. Bryologist 2018, 121, 67–79. [Google Scholar] [CrossRef]
- Zanata, A.M.; Burger, R.; Camelier, P. Two new species of Astyanax Baird & Girard (Characiformes: Characidae) from the upper rio Paraguaçu basin, Chapada Diamantina, Bahia, Brazil. Zootaxa 2018, 4438, 471–490. [Google Scholar] [CrossRef]
- Costa, W.J.; Feltrin, C.R.; Mattos, J.L.; Katz, A.M. Relationships and description of a new catfish species from Chapada Diamantina, the northernmost record of Trichomycterus s. s. (Siluriformes: Trichomycteridae). Zoosyst. Evol. 2024, 100, 223–231. [Google Scholar] [CrossRef]
- Napoli, M.F.; Junca, F.A. A new species of the Bokermannohyla circumdata group (Amphibia: Anura: Hylidae) from Chapada Diamantina, State of Bahia, Brazil. Zootaxa 2006, 1244, 57–68. [Google Scholar] [CrossRef]
- Carvalho, T.D.; Leite, F.S.F.; Pezzuti, T.L. A new species of Leptodactylus Fitzinger (Anura: Leptodactylidae: Leptodactylinae) from montane rock fields of the Chapada Diamantina, northeastern Brazil. Zootaxa 2013, 3701, 349–364. [Google Scholar] [CrossRef]
- Rodrigues, M.T.; Freitas, M.A.; Silva, T.F.S. New species of earless lizard genus Heterodactylus (Squamata: Gymnophthalmidae) from the highlands of Chapada Diamantina, State of Bahia, Brazil. J. Herpetol. 2009, 43, 605–611. [Google Scholar] [CrossRef]
- Fernandes, D.S.; Hamdan, B. A new species of Chironius Fitzinger, 1826 from the state of Bahia, Northeastern Brazil (Serpentes: Colubridae). Zootaxa 2014, 3881, 563–575. [Google Scholar] [CrossRef]
- Bornschein, M.R.; Maurício, G.N.; Belmonte-Lopes, R.; Mata, H.; Bonatto, S.L. Diamantina tapaculo, a new Scytalopus endemic to the Chapada Diamantina, northeastern Brazil (Passeriformes: Rhinocryptidae). Rev. Bras. Ornitol. 2007, 15, 151–174. [Google Scholar]
- Gonzaga, L.P.; Carvalhaes, A.M.; Buzzetti, D.R. A new species of Formicivora antwren from the Chapada Diamantina, eastern Brazil (Aves: Passeriformes: Thamnophilidae). Zootaxa 2007, 1473, 25–44. [Google Scholar] [CrossRef]
- Araujo-da-Silva, L.P.; Desouza, A.M.; Dasilva, M.B. Paragoniosomatinae, a new subfamily of Gonyleptidae (Arachnida: Opiliones), based on a new species from the Chapada Diamantina relict cloud forests, Brazil. Zootaxa 2020, 4808, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Silva-Neto, A.M.D.; Aldrete, A.N.G. A new genus in the family Ptiloneuridae (Psocodea: ‘Psocoptera’: Psocomorpha: Epipsocetae) from Brazil. Zootaxa 2015, 3914, 168–174. [Google Scholar] [CrossRef]
- Salles, F.F.; Nascimento, J.M.C.; Monjardim, M.; Paresque, R.; Hamada, N.; Dominguez, E. Diamantina: An endemic new genus of Neotropical Atalophlebiinae (Ephemeroptera: Leptophlebiidae) evidenced by morphological and molecular data. Zool. Anz. 2020, 284, 30–42. [Google Scholar] [CrossRef]
- Santos, A.C.; Caiola, N. Environmental typology of rivers from the Brazilian semiarid as a first step for the application of the index of biotic integrity: The case of the Chapada Diamantina. River Res. Appl. 2020, 36, 1151–1159. [Google Scholar] [CrossRef]
- Conceição, A.A.; Pirani, J.R. Delimitação de habitats em campos rupestres na Chapada Diamantina, Bahia: Substratos, composição florística e aspectos estruturais. Bol. Bot. Univ. São Paulo 2005, 23, 85–111. [Google Scholar] [CrossRef]
- Neves, S.P.S.; Funch, R.; Conceição, A.A.; Miranda, L.A.P.; Funch, L.S. What are the most important factors determining different vegetation types in the Chapada Diamantina, Brazil? Braz. J. Biol. 2016, 76, 315–333. [Google Scholar] [CrossRef]
- Parra, R.; Pereira, R.G.A.; Vieira, L.F.; Maia, R.P. Siliciclastic cave or diamond mine? Multiapproach investigation in Igatu Village, Chapada Diamantina, northeastern Brazil. Int. J. Speleol. 2025, 54, 6. [Google Scholar] [CrossRef]
- Pedreira, A.J. Sistemas deposicionais da Chapada Diamantina centro-oriental, Bahia. Rev. Bras. Geociênc. 1997, 27, 229–240. [Google Scholar] [CrossRef]
- Pereira, R.G.F.A. Geoconservação e Desenvolvimento Sustentável na Chapada Diamantina (Bahia, Brasil). Ph.D. Thesis, Universidade do Minho, Braga, Portugal, 2010; 295p. [Google Scholar]
- Almeida, F.F.M. O Cráton do São Francisco. Rev. Bras. Geociênc. 1977, 7, 349–364. [Google Scholar]
- Guimarães, J.T.; Alkmin, F.F.; Cruz, S.C.P. Supergrupos Espinhaço e São Francisco. In Geologia da Bahia: Pesquisa e Atualização, Série Publicações Especiais 2; Barbosa, J.S.F., Mascarenhas, J.F., Correa-Gomes, L.C., Dominguez, L.M., Santos de Souza, J., Eds.; CBPM: Salvador, Brazil, 2012; pp. 33–85. [Google Scholar]
- INMET. Normal Climatológica do Brasil (1991–2020); Instituto Nacional de Meteorologia, Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brazil, 2022; 27p. [Google Scholar]
- Google Earth. Satellite Imagery; Maxar Technologies: Westminster, CO, USA, 2025; Available online: https://earth.google.com/web/ (accessed on 30 July 2025).
- Álvarez-Padilla, F.; Hormiga, G. A protocol for digesting internal soft tissues and mounting spiders for scanning electron microscopy. J. Arachnol. 2007, 35, 538–542. [Google Scholar] [CrossRef]
- Desutter-Grandcolas, L. Phylogeny and the evolution of acoustic communication in extant Ensifera (Insecta, Orthoptera). Zool. Scr. 2003, 32, 525–561. [Google Scholar] [CrossRef]
- Souza-Dias, P.G.B. Análise Cladística e Morfologia do Complexo Fálico de Phalangopsidae, Com ênfase em Luzarinae (Orthoptera, Ensifera, Grylloidea). Ph.D. Thesis, Instituto de Biociências da Universidade de São Paulo, São Paulo, Brazil, 2015; 183p. [Google Scholar]
- Heads, S.W. The first fossil spider cricket (Orthoptera: Gryllidae: Phalangopsinae): 20 million years of troglobiomorphosis or exaptation in the dark? Zool. J. Linn. Soc. 2010, 158, 56–65. [Google Scholar] [CrossRef]
- Chintauan-Marquier, I.C.; Legendre, F.; Hugel, S.; Robillard, T.; Grandcolas, P.; Nel, A.; Zuccon, D.; Desutter-Grandcolas, L. Laying the foundations of evolutionary and systematic studies in crickets (Insecta, Orthoptera): A multilocus phylogenetic analysis. Cladistics 2016, 32, 54–81. [Google Scholar] [CrossRef]
- Desutter, L. Structure et evolution du complexe phallique des Gryllidea (Orthopteres) et classification des genres néotropicaux de grylloidea. Première Partie. Ann. Soc. Entomol. Fr. 1987, 23, 213–239. [Google Scholar] [CrossRef]
- Desutter, L. Structure et evolution du complexe phallique des Gryllidea (Orthopteres) et classification des genres néotropicaux de grylloidea. Deuxième Partie. Ann. Soc. Entomol. Fr. 1988, 24, 343–373. [Google Scholar] [CrossRef]
- Desutter-Grandcolas, L. Etude Phylogénétique Biogéographique et Écologique des Grylloidea Néotropicaux (Insectes Orthoptères); Université de Paris: Paris, France, 1990; p. 347. [Google Scholar]
- Desutter-Grandcolas, L. The cricket fauna of Chiapanecan caves (Mexico): Systematics, phylogeny and the evolution of troglobitic life (Orthoptera, Grylloidea, Phalangopsidae, Luzarinae). Int. J. Speleol. 1993, 22, 1–82. [Google Scholar] [CrossRef]
- Desutter-Grandcolas, L. New taxa and data for Neotropical Phalangopsidae (Orthoptera, Grylloidea). Zootaxa 2014, 3866, 398–420. [Google Scholar] [CrossRef] [PubMed]
- Gorochov, A.V. Taxonomic study of Mexican Phalangopsinae (Orthoptera: Gryllidae). Zoosyst. Rossica 2007, 16, 177–200. [Google Scholar] [CrossRef]
- Gorochov, A.V. New and little known Phalangopsinae (Orthoptera, Gryllidae): 7. Neotropical taxa of the tribes Paragryllini and Luzarini. Entomol. Rev. 2012, 92, 68–82. [Google Scholar]
- Gorochov, A.V. Classification of the Phalangopsinae subfamily group, and new taxa from the subfamilies Phalangopsinae and Phaloriinae (Orthoptera: Gryllidae). Zoosyst. Rossica 2014, 23, 7–88. [Google Scholar] [CrossRef]
- Gorochov, A.V. New and little-known crickets of the subfamily Phalangopsinae (Orthoptera, Gryllidae). 8. The genus Paragryllodes and notes on the subfamily classification. Entomol. Rev. 2015, 95, 600–611. [Google Scholar] [CrossRef]
- Gorochov, A.V. The cricket subfamily Phalangopsinae (Orthoptera: Gryllidae) in Peru. Zoosyst. Rossica 2019, 28, 51–87. [Google Scholar] [CrossRef]
- Christiansen, K.A. Morphological adaptations. In Encyclopedia of Caves, 2nd ed.; White, W.B., Culver, D.C., Eds.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 517–528. [Google Scholar]
- Howarth, F.G.; Moldovan, O.T. The ecological classification of cave animals and their adaptations. In Cave Ecology; Ecological Studies; Moldovan, O.T., Kováč, Ľ., Halse, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 235, pp. 41–67. [Google Scholar] [CrossRef]
- Sket, B. Can we agree on an ecological classification of subterranean animals? J. Nat. Hist. 2008, 42, 1549–1563. [Google Scholar] [CrossRef]
- Campos, L.D.D.; Bolfarini, M.P.; Suemitsu, M.M.; Borghezan, L.M.C.; Souza-Dias, P.G.B. Living in the darkness: A new cave cricket species of the genus Endecous Saussure (Orthoptera, Grylloidea, Phalangopsidae) from Serra da Bodoquena in Mato Grosso do Sul state, Brazil. Zootaxa 2023, 5285, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Junta, V.G.P.; Castro-Souza, R.A.; Ferreira, R.L. A new species of Arachnopsita (Orthoptera: Grylloidea: Phalangopsidae) from caves in Guatemala. Zootaxa 2022, 5094, 409–434. [Google Scholar] [CrossRef]
- Desutter-Grandcolas, L. Les Phalangopsidae de Guyane française (Orthoptères, Grylloidea): Systématique, éléments de phylogénie et de biologie. Bull. Mus. Natl. Hist. Nat. 1992, 14, 93–177. [Google Scholar] [CrossRef]
- de Mello, F.A.G.; dos Reis, J.C. Substrate drumming and wing stridulation performed during courtship by a new Brazilian cricket (Orthoptera: Grylloidea). J. Orthoptera Res. 1994, 3, 21–24. [Google Scholar] [CrossRef]
- Mews, C.M.; Mól, A.P.; Sperber, C.F. Crickets with phallic glands: Two new genera and one new species of Brazilian Luzarinae (Grylloidea: Phalangopsidae). Zootaxa 2009, 2292, 34–48. [Google Scholar] [CrossRef]
- Souza-Dias, P.G.B.; Campos, L.D.D.; de Mello, F.A.G. Desutterella n. gen., a new genus of Luzarinae (Orthoptera: Grylloidea: Phalangopsidae) and the first report of the Aracambiae group Souza-Dias & Desutter-Grandcolas, 2014 in the Amazon. Zootaxa 2017, 4350, 136–150. [Google Scholar] [CrossRef]
- de Mello, F.A.G. Female monopolization and paternity assurance in South American crickets (Orthoptera, Grylloidea): Mating plugs, extra claspers and forced copulation. Pap. Avulsos Zool. 2007, 47, 245–257. [Google Scholar] [CrossRef]
- Jesus, D.S.S. Garimpo de Silêncios: Experiências do Trabalho de Mulheres nas Lavras Diamantinas (Igatu/Andaraí-BA, Décadas de 1930 a 1970). Master’s Thesis, Universidade Federal de Sergipe, São Cristóvão, Brazil, 2019; 178p. [Google Scholar]
- Castro-Souza, R.A.; Zefa, E.; Ferreira, R.L. New troglobitic and troglophilic syntopic species of Endecous (Orthoptera, Grylloidea, Phalangopsidae) from a Brazilian cave: A case of sympatric speciation? Zootaxa 2020, 4810, 271–304. [Google Scholar] [CrossRef] [PubMed]
- Junta, V.G.P.; Castro-Souza, R.A.; Ferreira, R.L. Five new species of Phalangopsis Serville, 1831 (Orthoptera: Phalangopsidae) from Brazilian caves in the Amazon Forest. Zootaxa 2020, 4859, 151–194. [Google Scholar] [CrossRef] [PubMed]
- Bolfarini, M.P.; de Campos, L.D.; Souza-Dias, P.G.B. Orthoptera. In Fauna Cavernícola do Brasil, 1st ed.; Zampaulo, R.A., Prous, X., Eds.; Editora Rupestre: Belo Horizonte, Brazil, 2022; pp. 418–433. [Google Scholar]
- Lavoie, K.H.; Helf, K.L.; Poulson, T.L. The biology and ecology of North American cave crickets. J. Cave Karst Stud. 2007, 69, 114–134. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).