Abstract
A new species of Choreutidae, Tebenna acanthophallus sp. nov., from the Huasco Province, Chile, is described. Diagnostic characters, photographs of adults and illustrations of wing venation and genital structures are provided. The new species is supported by morphological and molecular analyses. This finding provides evidence that the family Choreutidae has species native to Chile, and its presence is not the result of introductions from other countries.
1. Introduction
The genus Tebenna Billberg, 1820 (Choreutidae: Choreutinae) is a nearly cosmopolitan genus [1], currently comprising 31 described species [2]. The genus was originally described as belonging to the family Glyphipterigidae, transferred later to the family Choreutidae by [3] based on the articulation of the second abdominal sternite; Glyphipterigidae has tineoid apodemes, whereas Choreutidae has tortricoid apodemes.
Species of Tebenna have a wingspan between 10 and 20 mm, the head frons is smooth-scaled, labial palps are upcurved, with ventral tufts of scales on the second segment, filiform antennae with a length reaching half the length of the forewings, dark brown subtriangular forewings with different metallic spots and markings, veins CuA1 and CuA2 that converge towards the termen, the hind wing is rounded towards the tornus with acute apex, and the vein M3 is absent [2,4].
The presence of the family Choreutidae was first reported in Chile in 2025, based on a first record of the species Tebenna micalis in Central Chile [5]. However, the question whether the species (and by extension its family) represents a native taxon that had been so far overlooked or it corresponds to a recent introduction from another country remains unresolved.
In this study we describe the male of a new species of the genus Tebenna from the Atacama Region of Chile, helping to resolve the question regarding the native or introduced nature of the family Choreutidae in Chile.
2. Materials and Methods
This study is based on samples collected in Huasco (28°28′21.7″ S 71°08′40.4″ W) and Freirina (28°29′46.3″ S 71°04′21.9″ W), in the Atacama Region of Chile (Figure 1), captured in monitoring traps for Opogona sacchari (Bojer, 1856) (Lepidoptera: Tineidae) installed by the Servicio Agricola y Ganadero (SAG) between 2018 and 2020.
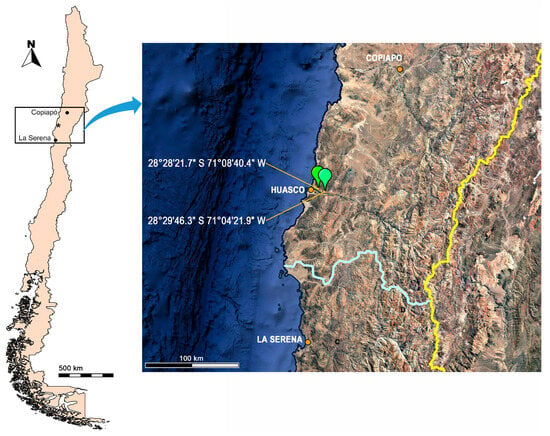
Figure 1.
Geographical distribution of Tebenna acanthophallus sp. nov. (Source: Google Earth Pro (2024), Maxar Technologies, used according to Google Earth Terms of Service). * Huasco.
The new species was described based on both the external morphological structures (Figure 2) and genital structures (Figure 3) of the males. This work was supported by phylogenetic analysis using a 658 base pair (bp) fragment of the Cytochrome Oxidase I (COI) from the mitochondrial DNA, which confirmed the phylogenetic placement of the species within the genus Tebenna. The general molecular protocol to obtain the COI sequencies followed the procedure described in [6]. Genital structures were studied following the procedure described in [7,8], and the nomenclature follows [9,10].
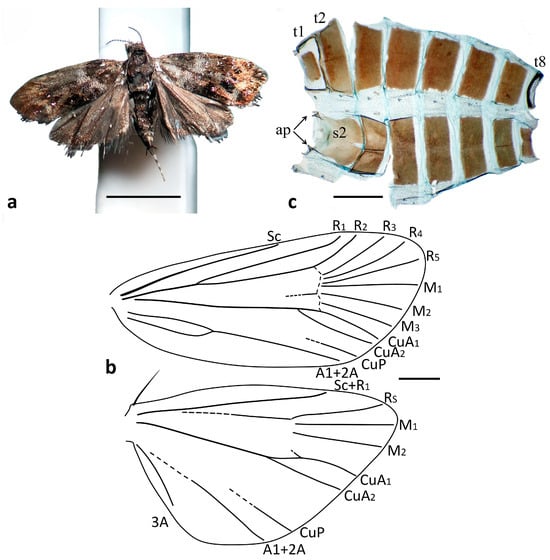
Figure 2.
Tebenna acanthophallus sp. nov. (a) Male in dorsal view (scale bar = 5.0 mm), (b) wing venation scheme (scale bar = 1.0 mm), (c) abdomen (scale bar = 1.0 mm). ap = apodeme, s2 = second abdominal sternite; t1, t2 and t8 = first, second and eighth abdominal tergites.
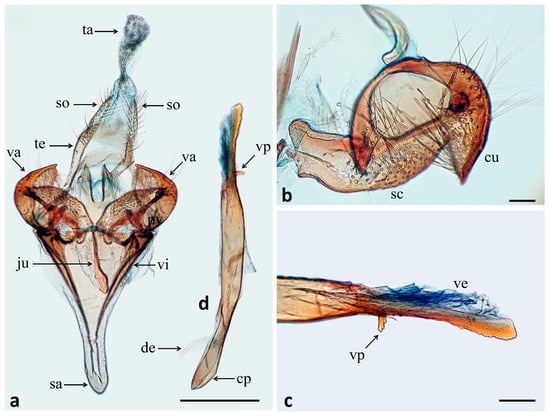
Figure 3.
Genital structures of Tebenna acanthophallus sp. nov. (a) Male genitalia (scale bar = 0.5 mm), (b) detail of valva, view of the internal surface (scale bar = 0.1 mm), (c) detail of aedeagus distal end (scale bar = 0.1 mm), (d) aedeagus. cp = coecum penis, cu = cucullus, dc = ductus ejaculatorius, ju = juxta, sa = saccus, sc = sacculus, so = socius, ta = tuba abdominalis, te = tegumen, va = valva, ve = vesica, vi = vinculum, vp = ventral process.
The abdomen was immersed in 10% KOH for 8 h at room temperature, cleaned in distilled water, stained in a 2% aqueous solution of Eosin “Y” for 4 h and cleaned in 20% ethanol. It was then stained in 1% Chlorazol black solution for 30 s and cleaned in 70% ethanol. Subsequently, the samples were dehydrated using 100% ethanol for 8 h. Permanent microscope slides were prepared with Euparal. All slides were observed using an Olympus SZ51 stereomicroscope. Photographs were obtained with a Cybershot DSC-W830 camera (Sony, Tokyo, Japan) using a Leitz Dialux 22 optical microscope (Leica Microsystems, Germany) and Nikon D7500 camera (Nikon, Japan). The material studied was deposited in the Entomological Collection of the Servicio Agrícola y Ganadero (CSAG), in the Insect Collection of the Instituto de Entomología, Universidad Metropolitana de las Ciencias de la Educación (IEUMCE) and in the “Colección de Tipos Entomológicos” of the Museo Nacional de Historia Natural (MNNC), Santiago, Chile.
One individual was selected for sequencing a fragment of approximately 650 bp of the mitochondrial DNA subunit I of the COI gene. DNA was extracted from the whole individual, and the COI gene was amplified using the primers LCO1490 (forward) and HCO2198 (reverse) [11]. DNA extraction and amplification were performed by the Australomic Laboratory of the Universidad Austral de Chile, Valdivia, Chile. Sequencing was performed at Macrogen (Seoul, South Korea) using the same universal primers. The obtained sequence was visualized and edited with the program Bioedit 7.2.5 [12], and aligned with the Clustal W algorithm including sequences from other 15 Tebenna species (Table 1), and 98 sequences from species in other genera of the family Choreutidae obtained from GenBank and BOLD, most of which were previously used in [13]. Only sequences with at least 300 bp were included in the final alignment, so Tebenna silphiella with 145 bp was excluded for the phylogenetic analyses. The complete list of sequence codes (BOLD Process ID) can be found at Figure 4, next to the species names in the tip labels, except for the species Tebenna micalis from Chile for which the GenBank accession codes are PQ452291 and PQ452291. The sequence from Tebenna acanthophallus sp. nov. is available from GenBank with accession number PX236114.

Table 1.
Data associated with the DNA sequences of the genus Tebenna obtained from BOLD and used in the Maximum Likelihood analysis, with addition of Tebenna acanthophallus sp. nov.
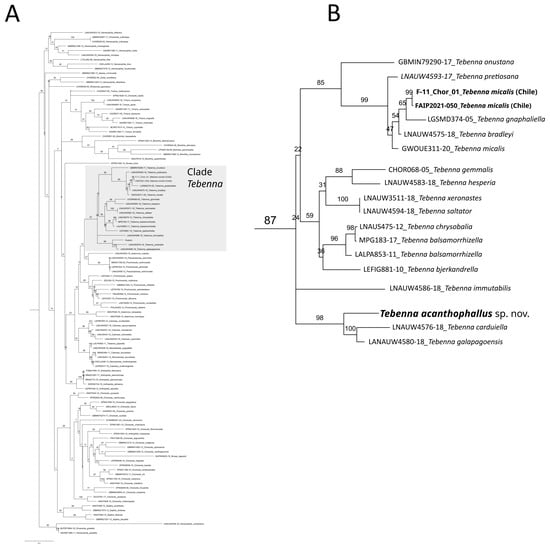
Figure 4.
Maximum Likelihood Tree based on COI DNA sequences of the family Choreutidae (A), with a zoom-in to the clade containing the species from genus Tebenna (B). Numbers above branches represent bootstrap values. Tip labels in bold represent sequences from Tebenna species present in Chile.
A maximum likelihood (ML) phylogenetic analysis including all aligned sequences was conducted in the program RaxMLGUI 2.0.10 [14]. GTR + G was selected as the best-fit evolutionary model for the phylogenetic analysis. A maximum of 1000 bootstrap replicates were run to estimate nodal support, followed by a search for the best tree. The tree was visualized and edited using the program FigTree v. 1.4.4. [15] and rooted with the mid-point method.
3. Results
3.1. Taxonomy
- Order: Lepidoptera
- Family: Choreutidae
- Genus: Tebenna Billberg, 1820
- Species: Tebenna acanthophallus sp. nov.
3.2. Diagnosis
In external morphology, Tebenna acanthophallus is similar to Tebenna micalis, but it can be distinguished from T. micalis by the absence of the elongated white spot near the anal margin on the hindwing. The male of Tebenna acanthophallus has a relatively longer valva than T. micalis, with a ventral edge with long, thick setae, aedeagus with perpendicular distal process and vesica without cornuti; in addition, on the eighth abdominal tergite, Tebenna acanthophallus has a sclerotized area shaped like a narrow transverse band. In Tebenna micalis, the hindwing has a white spot near the anal edge; in the male, the ventral edge of the valva is strongly convex and has short, thick setae, the aedeagus has no processes, and the vesica is armed with numerous spine-shaped cornuti; in the eighth abdominal tergite, the sclerotized area is subrectangular in shape.
3.3. Description
- Male. 9.0 to 11.0 mm wingspan (n = 10) (Figure 2a).
- Head. Vertex and frons dark brown, apex of scales lighter; antenna with scape dark brown, flagellum dark gray ringed with light gray; labial palpus brown at base and white toward apex, dotted with brown scales; haustellum covered with white scales.
- Thorax. Dark brown, with appressed scales, tegulae of the same color; forewing dark brown to reddish brown with two silvery gray bands antemedially and postmedially, ochre spot basally subcostal, black spots medially and subterminally enclosing clusters of metallic gray scales, fringe dark brown; hindwing dark brown with lighter terminal line, long fringe of the same color; M3 vein absent (Figure 2b); prothoracic leg light gray; meso- and metathoracic legs silvery gray, tibia and tarsus banded with dark gray.
- Abdomen. Dark brown with light gray lines, tergites and sternites sclerotized, first abdominal tergite with subrectangular sclerotized inner area, second abdominal sternite with broad subtriangular apodemes, eighth tergite with narrow transverse band-shaped sclerotized area (Figure 2c).
- Male genitalia (Figure 3). Tegumen narrow and band-shaped, widened medially; uncus slightly sclerotized and narrow, socii with long setae; vinculum-saccus broad basally, subtriangular, distal third narrow and slightly spatulate; juxta sclerotized rod-shaped (Figure 3a); valva longer than wide, ventral margin curved, covered by numerous long, thick setae; cucullus rounded with hooked ventral projection (Figure 3b); aedeagus 1.7 times vinculum length, substraight, straight spine-shaped ventral process located on distal fifth and protrusions toward apex (Figure 3c,d), coecum penis of similar thickness to rest of aedeagus, ductus ejaculatorius inserts dorsally; vesica not armed with cornuti.
- Female. Not studied.
- Material examined. Holotype (male): Chile, Región de Atacama, Huasco (28°28′21.7″ S 71°08′40.4″ W), altitude: 137 m.a.s.l., 2018, col. SAG. (CSAG). Paratypes: (3 males) with identical collection data as the holotype (Servicio Agrícola y Ganadero): (1 male) (CSAG), (1 male) (MNNC), (1 male) (IEUMCE). Additional material: CHILE—Atacama, Freirina, Trampa Opogona sach., 31 Mar. 2020, Leg. J. Torres (7 males), altitude: 207 m.a.s.l. CHILE—Atacama, Huasco, Trampa Opogona sach., 31 Mar. 2018, Leg. J. Torres (20 males), altitude: 137 m.a.s.l. All the additional material examined is deposited at the MNHN, Chile.
- Geographic distribution. Tebenna acanthophallus is known in Huasco (28°28′21.7″ S 71°08′40.4″ W) and Freirina (28°29′46.3″ S 71°04′21.9″ W), both in the Huasco Province, Atacama Region. According to the biogeographic classification proposed by [16], these localities belong to the Coquimbo Province, which is located between 26° and 33° south latitude of the Central Chilean Subregion, in the Andean Region (Figure 1).
- Etymology: The name acanthophallus refers to the particular spine present in the aedeagus of this species.
3.4. Phylogenetic Relationships and Genetic Distance to Closely Related Species
The maximum likelihood phylogenetic analysis, which includes a large number of species and genera of the Choreutidae family, resulted in a largely resolved tree, as depicted in Figure 4A. This tree places Tebenna acanthophallus within a partially resolved clade containing all other Tebenna species, confirming its correct placement within the genus. Within this clade, Tebenna acanthophallus sp. nov. appears as the sister species to T. galapagoensis and T. corduiella in a highly supported subclade (bootstrap support = 98). Tebenna micalis, the only other Tebenna species with presence in Chile, clusters within another well supported clade (bootstrap support = 85) along with T. onustana, T. pretiosana, T. bradleyi, and T. gnaphaliella.
Regarding genetic distance (K2P distance) against its closest relatives, Tebenna acanthophallus presented a distance of 0.05 and 0.054 against T. galapagoensis and T. carduiella, respectively. Genetic distance between Tebenna acanthophallus and T. micalis from Chile was 0.095.
4. Discussion
We described a new species of Lepidoptera in the genus Tebenna, family Choreutidae, and estimated a phylogeny containing 112 species within the family to better understand its phylogenetic placement. The phylogenetic analysis supports the placement of Tebenna acanthophallus sp. nov. within the clade containing all other Tebenna species, being close to species T. galapagoensis and T. carduiella, but in a separate subclade from the other Tebenna species present in Chile which were previously identified as T. micalis [5] (Figure 4B). The phylogenetic placement of the Chilean Tebenna species in different clades indicates that Chilean species are not monophyletic. This suggest that the presence of Tebenna in the country could be explained by independent colonization events from different Tebenna lineages.
The external morphology of T. acanthophallus share certain similarities with T. micalis from Central Chile in both the general coloration of the wings and the rest of the body. However, some elements of the coloration pattern and the genital morphology of males allow the distinction of both species (see diagnosis).
A puzzling result of our study is the relatively distant relationship between the sequence of Tebenna micalis from Chile and the sequence of Tebenna micalis from Italy, used in our study. These sequences do not cluster together in a sister-group relationship, but present paraphyletic relationships within a clade that also included sequences from T. gnaphaliella and T. bradleyi. The species T. bradleyi is currently considered a synonym of T. micalis, but this seems not to be the case for T. gnaphaliella, which seems to be a valid species [17]. The distribution of T. micalis is described as cosmopolitan, but a global genetic analysis is required to evaluate its genetic structure and whether it may or may not comprise a group of cryptic species. Previous examination of the genitalia of T. micalis from Chile revealed no differences from the original description of T. micalis [5].
Our phylogenetic tree allows us to pinpoint several additional taxonomic considerations. For example, Figure 4A shows a sequence assigned to Tebenna piperella (Bold code: LALPA905-11), outside the clade containing all other Tebenna species and closer to the genus Caloreas and Neocaloreas. This is most likely due to a misidentification problem in the BOLD data base, although we cannot, with the data at hand, rule out the possibility that this species actually belongs to a group not related with Tebenna. Another issue revealed by the phylogenetic tree is the extreme similarity between species T. saltator and T. xeronastes (Figure 4B). The sequences of these two species show no genetic differences, suggesting they are probably from a single species. All these examples indicate that a thorough revision of the genus Tebenna is required to better understand the actual dimension of the diversity of this genus. These examples also highlight the extreme gap in knowledge we still have within microlepidoptera relative to other better known lepidoptera lineages such as Papilionoidea and certain families of Heterocera [18].
This new finding rises to 32 the number of species of the genus Tebenna Billberg, 1820 worldwide and represents the second Tebenna species in Chile, after T. micalis [5]. In addition, the existence of a new species of Tebenna native to Chile resolves the question about whether the family Choreutidae was natively distributed in Chile.
Author Contributions
Conceptualization, G.V., F.U. and C.P.M.-R.; methodology, F.U., C.P.M.-R. and G.V.; formal analysis, C.P.M.-R. and G.V.; investigation, G.V.; resources, F.U., S.R. and C.P.M.-R.; data curation, G.V., F.U. and S.R.; writing—original draft preparation, G.V.; writing—review and editing, G.V., F.U. and C.P.M.-R.; visualization, G.V., F.U. and C.P.M.-R.; supervision, F.U. and C.P.M.-R.; funding acquisition, F.U. and C.P.M.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by DIUMCE 08-2025-ID (to C.P.M.-R.), FONDECYT 11220703 (to C.P.M.-R.), and Fondo de Apoyo a la Investigación Patrimonial, SERPAT, project FAIP N-40-INV, 2021 (to F.U.).
Data Availability Statement
Type material can be found at collections of Servicio Agrícola y Ganadero, Chile (CSAG), Museo Nacional de Historia Natural de Chile (MNNC), and Instituto de Entomología, Universidad Metropolitana de Ciencias de la Educación, Chile (IEUMCE). The DNA sequence used for the phylogenetic analysis can be found at GenBank with accession number PX236114.
Acknowledgments
The authors thank Rodrigo Soto, from the Servicio Agrícola y Ganadero (SAG), Chile, for providing valuable samples for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dugdale, J.S. A new generic name for the New Zealand species previously assigned to Simaethis auctorum (Lepidoptera: Choreutidae), with description of a new species. N. Zeal. J. Zool. 1979, 6, 461–466. [Google Scholar] [CrossRef][Green Version]
- Heppner, J.B. Nearctic metalmark moths, 7. Genus Tebenna and new genus Pseudotebenna (Lepidoptera: Choreutidae: Choreutinae). Lepid. Novae 2023, 15, 121–172. [Google Scholar][Green Version]
- Heppner, J.B. A new genus and new assignments in the American Choreutidae (Lepidoptera Sesioidea). Proc. Entomol. Soc. Wash. 1977, 79, 631–636. [Google Scholar][Green Version]
- Clarke, J.F. The Lepidoptera of Rapa Island. Smithson. Contrib. Zool. 1971, 56, 166–167. [Google Scholar] [CrossRef]
- Valenzuela, G.; Urra, F.; Muñoz-Ramírez, C. Evidencia morfológica y molecular revela primer registro de Tebenna micalis (Lepidoptera) y la familia Choreutidae en Chile. Gayana 2025, 89, 81–88. [Google Scholar]
- Muñoz-Ramírez, C.P. The Phylogenetic position of Ceroglossus ochsenii Germain and Ceroglossus guerini Germain (Coleoptera: Carabidae), two endemic ground beetles from the Valdivian forest of Chile. Rev. Chil. Entomol. 2015, 40, 14–21. [Google Scholar]
- Lee, S.; Brown, R.L. A New Method for Preparing Slide Mounts of Whole Bodies of Microlepidoptera. J. Asia-Pac. Entomol. 2006, 9, 249–253. [Google Scholar] [CrossRef]
- Robinson, G.S. The preparation of slides of Lepidoptera genitalia with special reference to microlepidoptera. Entomol. Gaz. 1976, 27, 127–132. [Google Scholar]
- Klots, A.B. Lepidoptera. In Taxonomist’s Glossary of Genitalia in Insects, 2nd ed.; Tuxen, S.L., Ed.; Munksgaard: Copenhagen, Dinamarca, 1970; Volume 25, pp. 115–130. [Google Scholar]
- Rota, J. A new genus and new species of metalmark moths (Lepidoptera: Choreutidae) from Costa Rica. Zootaxa 2008, 1993, 12–18. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Mayer, C.; Dietz, L.; Call, E.; Kukowka, S.; Martin, S.; Espeland, M. Adding leaves to the Lepidoptera tree: Capturing hundreds of nuclear genes from old museum specimens. Syst. Entomol. 2021, 46, 649–671. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Rambaut, A. Figtree Software, v 1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 30 November 2024).
- Morrone, J.J. Biogeographical regionalisation of the Andean region. Zootaxa 2015, 3936, 207–236. [Google Scholar] [CrossRef]
- Beccaloni, G.; Scoble, M.; Kitching, I.; Simonsen, T.; Robinson, G.; Pitkin, B.; Hine, A.; Lyal, C.; Ollerenshaw, J.; Wing, P.; et al. Global Lepidoptera Index (version 1.1.24.256). In Catalogue of Life (Version 2024-09-25); Bánki, O., Roskov, Y., Döring, M., Ower, G., Hernández Robles, D.R., Plata Corredor, C.A., Stjernegaard Jeppesen, T., Örn, A., Vandepitte, L., Pape, T., et al., Eds.; Catalogue of Life: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Scoble, M.J. The Lepidoptera. Form, Function and Diversity; Natural History Museum Publications (RU); Oxford University Press: Oxford, UK, 1992; pp. 1–404. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).