Abstract
Axiidea housed in the collection of the Museu de Oceanografia Prof. Petrônio Alves Coelho, Universidade Federal de Pernambuco (MOUFPE) were studied. This collection contains 66% of the total diversity of axiideans recorded from the continental shelf of the Brazilian coast. The species are listed by family and genus as follows: Axiidea: Axiidae (Axiopsis, Axiorygma, Calaxius, Coralaxius, Manaxius, Paraxiopsis), Callianassidae (Cheramoides), Callichiridae (Callichirus, Corallianassa, Lepidophthalmus, Neocallichirus), Ctenochelidae (Ctenocheles, Ctenocheloides, Dawsonius, Gourretia), Micheleidae (Marcusiaxius, Meticonaxius). Details on the biology and taxonomy of some of these species are given. In addition, certain poorly documented species are now better understood.
1. Introduction
The infraorder Axiidea de Saint Laurent, 1979 [1] encompasses the so-called ghost shrimps, mud lobsters or burrowing shrimps [2,3,4]. This infraorder represents one clade of marine decapods with a body form completely adapted for a fossorial lifestyle who are an important benthic component of sandy or muddy intertidal, shallow subtidal and deep-sea habitats worldwide [3,5,6].
Burrowing shrimps are known for constructing burrows of different shapes and depths [7] and for playing a significant role in shaping community structure [8]. Bioturbation produced by these species, i.e., the activity of water and sediment expulsion from its galleries, contributes to the suspension of organic matter and nitrogen fixation and increases food availability to other trophic levels [9,10].
Axiidean shrimps constitute an important component of the Brazilian benthic communities [11,12]. The axiidean fauna of Brazil has been summarized (as Thalassinidea) by Coelho [13], de Melo [14] and, recently (as Axiidea), by Hernáez et al. [12]. The latter work listed 35 species across seven families [12]. This study provides a comprehensive review of the Axiidea deposited in the zoological collection of the ‘Museu de Oceanografia Prof. Petrônio Alves Coelho (MOUFPE)’ of the northeastern region of Brazil. With more than 15,000 lots of crustaceans, this collection contains the largest holdings of Axiidea in their respective geographic area. The results of this revision are presented in this paper.
2. Materials and Methods
Material of Axiidea deposited in the MOUFPE collection primarily originates from a series of oceanographic expeditions conducted between 1965 and 1978 by Petrônio Alves Coelho. These collections were made on the continental shelf and slope off the coast of the north and northeastern regions of Brazil (e.g., [13,15,16]). Other lots deposited in the MOUFPE were collected by the author as part of ongoing studies on the systematics of burrowing shrimps of the infraorders Axiidea [1] and Gebiidea de Saint Laurent, 1979 [1] along the Brazilian coast.
Measurements (mm) were made under a stereomicroscope (Zeiss® Stemi® SV-6) equipped with a digital analysis image system (Zeiss® AxioCam® MRc5). Each image was digitized using an electronic tablet for graphic design (Wacom®).
Size (when given) is in total length (tl in mm) and carapace length (cl in mm) in the form (tl/cl).
Abbreviations include AK (station Akaroa), AS (Almirante Saldanha), CAN (Canopus Expedition), coll. (collector or collected by), GM (Geomar Expedition), REC (Recife Expedition), SUL (oceanographic campaign RV Almirante Saldanha).
This study follows the family and generic status as proposed by Poore et al. [4]. All species listed here are currently recognized as valid species by WoRMS (https://www.marinespecies.org; accessed on 10 April 2024).
3. Results
Taxonomy
Infraorder Axiidea de Saint Laurent, 1979 [1]
Family Axiidae Huxley, 1879 [17]
Genus Axiopsis Borradaile, 1903 [18]
Axiopsis brasiliensis Coelho and Ramos–Porto, 1991 [19]
Material—Brazil. Holotype, male, cl 16.1 mm, CAN # 98, 5.250° S, 34.983° W, 70 m, Touros, Rio Grande do Norte, 13 January 1966, MOUFPE 314; paratype, male, cl 12.2 mm, REC # IV, 8.165° S, 34.763° W, 27 m, Recife, Pernambuco, MOUFPE 311.
Type locality. Touros, Rio Grande do Norte, Brazil [19].
Remarks. The taxonomic validity of this species was questioned by Sakai [20], who considers A. brasiliensis to be a junior synonym of A. serratifrons. In Brazil, this species is distributed on calcareous bottoms over the continental shelf (30–80 m) between Ceará and Alagoas and has even been reported at the Atol das Rocas by Melo [14].
Genus Axiorygma Kensley and Simmons, 1988 [21]
Axiorygma nethertoni Kensley and Simmons, 1988 [21]
Material—Brazil. Male, cl 6.9 mm, GM # 206, 5.150° N, 50.700° W, 172 m, off Amapá, 1971, MOUFPE 1869.
Type locality. Key Largo National Marine Sanctuary, Florida, USA [21].
Remarks. The original description of A. nethertoni was based on several male and female specimens collected from the continental shelf off Florida at depths ranging between 30 and 59 m [21]. In Brazil, the only specimen identified by Coelho [13] as A. nethertoni was collected close to the continental shelf break at a depth of 172 m off the coast of Amapá, northern Brazil. This specimen, a male, was assigned by Coelho [13] to A. nethertoni without any morphological comparison with specimens from the coast of Florida, but based on the shape and ornamentation of carapace and chelipeds and the shape of uropods and telson, this specimen is assigned to this species and awaits further work.
Genus Calaxius Sakai and de Saint Laurent, 1989 [22]
Calaxius spinosus (Coelho, 1973) [23]
Material—Brazil. Paratype, female, cl 7.4 mm, damaged, GM # 194, 3.742° N, 50.125° W, 77 m, off Amapá, 1971, MOUFPE 1870; paratype, undetermined sex, damaged, AS # 1793B, 4.225° N, 50.433° W, 75 m, off Amapá, 18 November 1967, MOUFPE 8606.
Type locality. Amapá, Brazil [23].
Remarks. The holotype of C. spinosus is currently deposited in the collection of the Museu de Zoologia, Universidade de São Paulo (MZUSP), São Paulo, Brazil. Two specimens of C. spinosus are deposited in the MOUFPE collection. Both specimens correspond to paratypes. These specimens were collected off the coast of Amapá, northern Brazil, at a depth of between 75 and 77 m. There are no other reports of this species on the Brazilian coast.
Genus Coralaxius Kensley and Gore, 1981 [24]
Coralaxius nodulosus (Meinert, 1877) [25]
Material—Brazil. Undetermined sex, juvenile, cl 1.3 mm, Sergipe, 2001, MOUFPE 13711.
Type locality. North Sea [25].
Remarks. Axius nodulosus Meinert, 1877 [25] was described based on specimens from Nymindegab, North Sea, and transferred to Coralaxius Kensley and Gore, 1981 [24] by Sakai and de Saint Laurent, 1989 [22] in a major revision of Axiidae. Kensley and Gore [24] proposed the monotypic genus Coralaxius to accommodate a new species, C. abelei Kensley & Gore, 1981 [24], from the western Atlantic (Florida and Belize). Subsequently, Kensley (1994) [26] considered C. abelei to be synonymous with C. nodulosus from Nymindegab, North Sea. Currently, the geographic distribution of C. nodulosus along the western Atlantic includes the United States (South of Florida), Belize (Carrie Bow Cay, Curlew Cay), Jamaica, Costa Rica and Brazil (Sergipe; Rocas Atoll; Abrolhos Bank; Seamounts off coast of Bahia and Espírito Santo: Besnard, Eclaireur, Vitória, Montague, Jaseur, Davis, Almirante Saldanha; Martin Vaz Islands) (see [27]).
In Brazil, C. nodulosus has been reported as C. abelei from the continental shelf depths of between 40 and 270 m and from the Vitória-Trindade Seamount Chain at depths of between 48 and 81 m (see [27] and present study). The specimen of C. nodulosus from Brazil is characterized by having a rostrum short and triangular, not reaching the cornea; a cervical groove distinct; an antennular peduncle not overreaching antennal penultimate article; uropodal exopod with transverse suture and a telson subtriangular; and narrowing distally, among other characters. Several lots of C. abelei are registered in the database of MOUFPE collection (lots: 8612, 8613, 8614, 12602, 12829, 12940). Unfortunately, all of these batches were found to be missing at the time of this review.
Genus Manaxius Kensley, 2003 [28]
Manaxius angulatus (Coelho, 1973) [23]
Material—Brazil. Holotype, female, cl 4.8 mm, AS # 7077, 44 m, off Amapá, 9 June 1986, MOUFPE 306.
Type locality. Cabo Caciporé, Amapá, Brazil, 108–118 m [23].
Remarks. Manaxius angulatus is endemic to Brazil. This species was originally described as Calastacus angulatus by Coelho [23] and then transferred to Manaxius by Sakai [20]. Manaxius angulatus is characterized by having the fingers of the chelipeds shorter than the palm and the pleura 2–5 triangular ventrally (fig. 8b in [19]). In the MOUFPE collection, there is only one specimen (holotype) of this species deposited.
Genus Paraxiopsis de Man, 1905 [29]
Paraxiopsis defensus (Rathbun, 1901) [30]
Material—Brazil. Female, cl 7.9 mm, Tamandaré beach, Pernambuco, 1 July 1971, MOUFPE 1871; undetermined sex, juvenile, cl 3.4 mm, Bainema beach, Ilha de Boipeba Cairu, Bahia, A.O. Almeida, P.S. Santos and G.O. Soledade colls., 19 August 2012, MOUFPE 15545.
Type locality. Off Boca Prieta, Puerto Rico, 15.5 m, coral and sand [30].
Remarks. Paraxiopsis defensus (Rathbun, 1901 [30]) was described based on a female specimen from Boca Prieta, Puerto Rico. After its discovery, this species has been recorded in the Dominican Republic (Barahona Harbour [31]) and Brazil (Pernambuco and Bahia, northeastern region, present study). Morphology of P. defensus is characterized by having three to five pleura rounded ventrally [20]. Two specimens of this species are deposited in the MOUFPE collection. Both specimens examined in the MOUFPE are in good condition; both were collected in the northeastern region of Brazil.
Paraxiopsis vicina (Coelho and Ramos–Porto, 1991) [19]
Material—Brazil. Holotype, male, cl 7.9 mm, 2.225° S, 40.725° W, 53 m, continental shelf, Ceará, 29 October 1967, MOUFPE 1874.
Type locality. Off Ceará, 53 m, calcareous sediments [19].
Remarks. Paraxiopsis vicina is endemic to Brazil. This species is only known from the type locality (continental shelf of Ceara). Paraxiopsis vicina differs from other congeneric species in that in this species, the male P1 palm is distinctly tuberculate on the dorsal margin, bearing a tuberculate plate on the ventral margin [19,20,32].
Family Callianassidae Dana, 1852 [33]
Genus Cheramoides Sakai, 2011 [20]
Cheramoides aff. marginata (Rathbun, 1901) [30]
Material—Brazil. Female, major cheliped and abdomen missing, cl: 5.0 mm, station GM 211, 4.458° N, 50.0.25° W, 183–224 m, mud bottom, Amapá, 16 September 1969, MOUFPE 1878; 2 males, both damaged, cl: 3.0–3.7 mm, station GM 206, 5.150° N, 50.700° W, 172 m, mud bottom, Amapá, 1971, MOUFPE 8410; male, cl 3.2 mm, left second pereiopod missing, right cheliped detached, third to fifth pereiopods missing, station GM 08, 2.067° S, 42.717° W, 67 m, mud bottom, Maranhão, 2 June 1969, MOUFPE 8413; 5 males, cl: 2.7–3.1 mm, Maranhão, MOUFPE 21627; 6 females (3 ovigerous), cl: 2.0–3.6 mm, Maranhão, MOUFPE 21626; 2 males, cl: 4.5–4.6 mm, PAVASAS I, station DG 05, 2.258° S, 40.488° W, 45 m, mud bottom, Ceará, 20 July 1987, MOUFPE 8409; female, damaged, all pereiopods missing, cl: 3.8 mm, station AK 80, 10.097° S, 35.788° W, 290 m, mud bottom, Alagoas, 4 September 1965, MOUFPE 8417; 3 males, damaged, cl: 2.2–3.6 mm, 2 females, damaged, cl: 3.8–4.0, station AS 2167, 22.970° S, 41.933° W, 53 m, mud bottom, Rio de Janeiro, 16 September 1969, MOUFPE 8418.
Type locality. Puerto Rico, Mayagüez Harbour, 315 m [30].
Remarks. The record of Cheramoides marginata from Brazilian waters in the literature is highly questionable. While several studies previously reported the presence of this species in deeper areas on the continental shelf and slope between Amapá and Rio de Janeiro (see [13,14,15]), all of these records were undertaken without access to comparative materials from the Puerto Rican-type locality. Specimens of C. marginata herein examined disagree in some points from illustrations of C. marginata available in the literature (see [30,34,35]). In the specimens from Brazil, the rostrum exceeds eyestalks (Figure 1A), whereas in C. marginata from Puerto Rico, it is shorter than eyestalks (cf. fig. 16d in [34]). Also, the two populations can also be separated from each other by the different proportions in length of the antennular peduncle to the antennal peduncle, which are slightly shorter in Brazilian specimens (Figure 1A) but much shorter in C. marginata from Puerto Rico (cf. fig. 16d in [34]). These observations suggest the need for a future comparative study between the specimens identified as C. marginata from Brazil and the type material of this species from Puerto Rico.
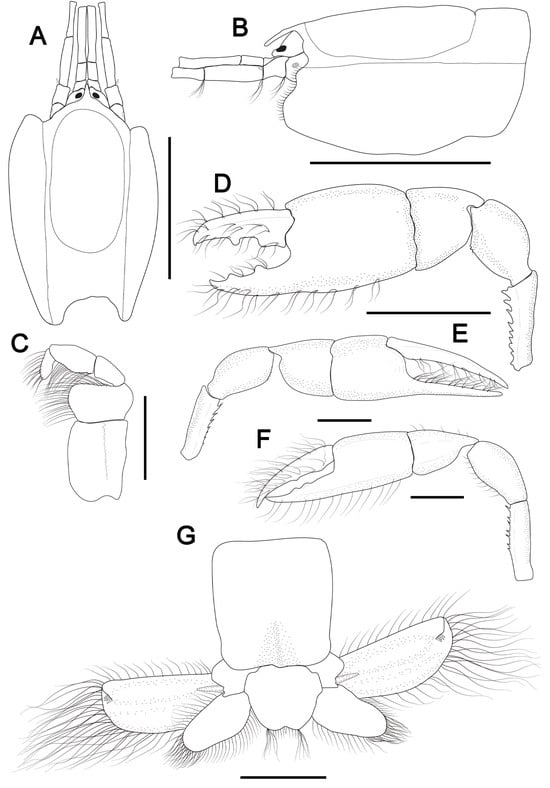
Figure 1.
Cheramoides aff. marginata [30]. (A–G), male, cl 3.2 mm, MOUFPE 8413; I, female (cl: 3.6 mm), MOUFPE 21626. (A), carapace front, eyestalk and antennular and antennal peduncles, dorsal view; (B), same, lateral view; (C), left maxilliped 3, internal surface; (D,E), male major and minor chelipeds, respectively, lateral view; (F), female minor cheliped, lateral view; (G), sixth pleomere, uropods and telson, dorsal view. Scale bars: (A,B,D) = 2 mm; (C,E–G) = 1.0 mm.
Family Callichiridae Manning and Felder, 1991 [35]
Genus Callichirus Stimpson, 1866 [36]
Callichirus corruptus Hernáez, Miranda, Rio and Pinheiro, 2022 [37]
Material—Brazil. Male, cl 11.8 mm, female, cl 12.3 mm, Praia do Pirangi, 5.974° S, 35.124° W, lower intertidal, Pirangi, Rio Grande do Norte, 10 January 1989, MOUFPE 8373; 5 males, cl: 7.9–15.9 mm, 3 females, cl: 7.8–15.3 mm, Praia Piedade, 8.167° S, 34.913° W, lower intertidal, Recife, Pernambuco, 31 July 1988, MOUFPE 8362; male, cl 9.6 mm, 3 females, cl: 12.9–17.7 mm, Praia do Tamandaré, lower intertidal, Tamandaré, Pernambuco, 5 February 1989, MOUFPE 8372; male, cl 10.9 mm, Praia do Gonzaga, 23.973° S, 46.334° W, lower intertidal, Santos, São Paulo, 20 January 1989, MOUFPE 8376.
Type locality. Praia do Gonzaga, intertidal, Santos, Brazil [37].
Remarks. Callichirus corruptus is one of the most common species of medium-grain sandy beaches along the Brazilian coast [37]. Until recently, Brazilian populations of Callichirus were assigned to C. major. Today, both species, geographically separated, are recognized as valid and distinct (see [37]). Callichirus corruptus is restricted to the Brazilian coast (between Pará and Santa Catarina), whereas C. major is from North Carolina down to Florida [37].
Genus Corallianassa Manning, 1987 [38]
Corallianassa hartmeyeri (Schmitt, 1935) [39]
Material—Brazil. Male, cl 6.4 mm, Station AS 6984B, 10.483° S, 36.267° W, Alagoas, 5 May 1986, MOUFPE 310.
Type locality. Kingston Harbor, Jamaica [39].
Remarks. The taxonomic history of C. hartmeyeri is complex. In 1924, the German zoologist Heinrich Balss identified a callianassid from Kingston (Jamaica), with Callianassa grandimana (Gibbes, 1850) (as Glypturus grandimanus) (see [40]). Subsequently, Schmitt [39], in his review of North American callianassids, recognized the existence of significant differences between Balss’s species and C. grandimana and proposed the name Callianassa hartmeyeri for Glypturus grandimanus sensu Balss, 1924 [40]. More than 50 years later, Manning [40] stated that the identity of C. hartmeyeri remains uncertain because there is no consensus among researchers regarding the identity of the material collected by Balss and assigned to Glypturus grandimanus. Despite the lack of consensus, C. hartmeyeri is considered a valid species, and together with Corallianassa longiventris A. Milne Edwards, 1870, constitute the two species of the genus recorded for the western Atlantic (see [41]).
In Brazil, C. hartmeyeri was recorded in Alagoas by Coelho [13]. The label on the only specimen (a male specimen) of C. hartmeyeri deposited in the MOUFPE collection provides no further information about the habitat of this species. The scarcity of Brazilian records of C. hartmeyeri suggests that the species may be less common in the southwestern Atlantic, at least along the continental coast of Brazil.
The comparison between the material identified as C. hartmeyeri from Brazil and the type material of C. hartemeyeri from Jamaica, available in Manning [40], must be conducted with caution as both specimens are of different sexes. The holotype of C. hartmeyeri is a female, whereas the specimen from Brazil is a male (see [40]). Therefore, certain sexually dimorphic appendages such as chelipeds and first pairs of pleopods should not be considered in any taxonomic comparison between specimens of opposite sexes in this and other families of Axiidea. Taking the above into consideration, the shape of the anterior region of carapace, uropodal endopod and telson as described by Manning (fig. 2a,b,h in [40]) for the reexamination of the holotype of C. hartmeyeri fit with the examined material of this species from Brazil (see also Figure 2A,E).
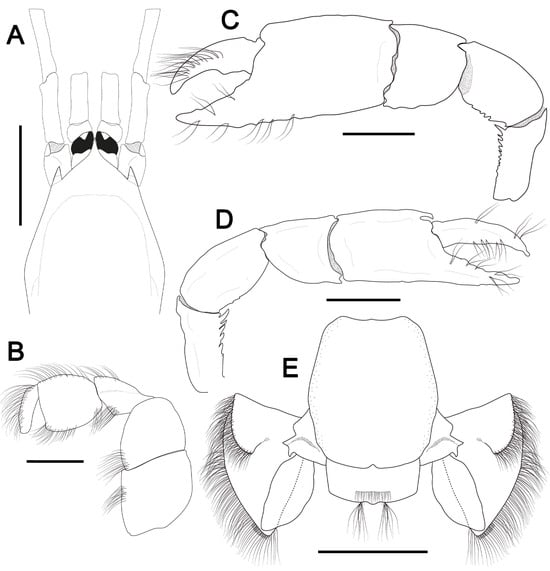
Figure 2.
Corallianassa hartmeyeri (Schmitt, 1935) [39]. (A–E), male, cl 6.4 mm, MOUFPE 310. (A), carapace front, eyestalk and antennular and antennal peduncles, dorsal view; (B), third maxilliped, external surface; (C,D), male major and minor cheliped, lateral view; (E), sixth abdominal somite, telson and uropods, dorsal view. Scale bars: (A,C–E) = 2 mm; (B) = 1 mm.
Corallianassa longiventris (A. Milne-Edwards, 1870) [42]
Material—Brazil. One specimen, destroyed, AS #1765A, 0.517° N, 47.817° W, 39 m, soft sediment, Pará, 12 November 1967, MOUFPE 1876; female, cl 9.1 mm, Station 331, Atol das Rochas, 22 m, Rio de Janeiro, 1 August 1992, MOUFPE 1880.
Type locality. Martinique [42].
Remarks. As with C. hartmeyeri, C. longiventris appears to be uncommon along the Brazilian coast. Coelho [13] recorded C. longiventris from the northern region of Brazil (#1876). Unfortunately, this specimen is destroyed, making it impossible to confirm the identification made by Coelho [13]. The other specimen deposited in the MOUFPE collection (#1880), a female collected in the southeastern region of Brazil, is in good condition. The examination of this specimen raises some doubts about its correct identification as C. longiventris. In C. longiventris from Martinique, the posterior margin of the telson is rounded with a median prominence, whereas in the specimen from Brazil, it is straight and has a small median depression (see fig. 6d in [38]; see also Figure 3E). The remaining characters (e.g., anterolateral spinous and articulated projections of carapace as well as ornamentation of chelipeds) seem to match the illustrations provided by Manning [38] for C. longiventris from Martinique. Further comparisons between Brazilian specimens assigned to C. hartmeyeri and C. longiventris and the type specimens of both species are necessary to ensure that these species are indeed present on the Brazilian coast.
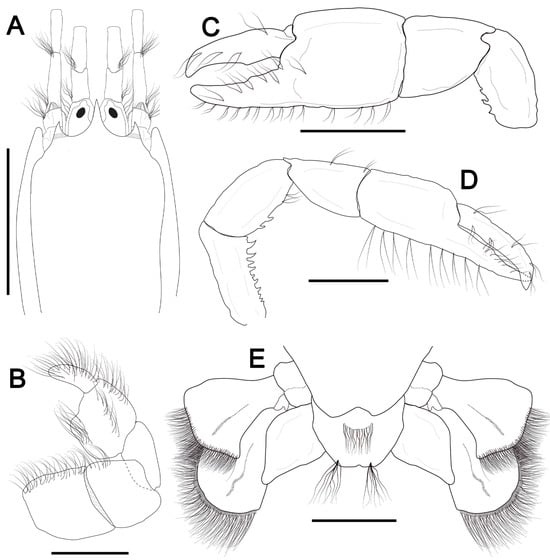
Figure 3.
Corallianassa longiventris (A. Milne-Edwards, 1870) [42]. (A–C), female, cl 9.1 mm, MOUFPE 1880. (A), carapace front, eyestalk and antennular and antennal peduncles, dorsal view; (B), third maxilliped, external surface; (C,D), female major and minor cheliped, lateral view; (E), sixth abdominal somite, telson and uropods, dorsal view. Scale bars: (A,C,D) = 4 mm; (B,E) = 2 mm.
Lepidophthalmus Holmes, 1904 [43]
Lepidophthalmus siriboia Felder and Rodrigues, 1993 [44]
Material—Brazil. 101 males, cl: 3.5–11.9 mm, 151 females, cl: 3.0–12.4 mm, mouth of Manguaba river, 9.161° S, 35.295° W, Porto de Pedras, intertidal, Alagoas, 27 June 2021, MOUFPE 20692.
Type locality. Mouth of Rio Anil, São Luís, Maranhão, Brazil [44].
Remarks. Lepidophthalmus siriboia is endemic to Brazil (from Pará to Bahia), occurring primarily in estuarine areas of the northeastern region [12,44]. This species is characterized by solitary habits [45]. Morphologically, L. siriboia shares with L. louisianensis and L. statoni the presence of ventral abdominal not sclerotized ([44], for the presence of ventral abdominal sclerotization, see [46]); however, this species can be distinguished from both L. louisianensis and L. statoni in having the terminal segments of the antennule and antenna with a parallel base (vs. non-parallel base of terminal segments of the antennule and antenna in L. louisianensis and L. statoni) (cf. fig. 8a in [12] and fig. 1a in [39]).
Genus Neocallichirus Sakai, 1988 [47]
Neocallichirus aff. grandimana (Gibbes, 1850) [48]
Material—Brazil. Male, cl 9.4 mm, Janga, intertidal, Paulista, Pernambuco, 17 August 1993, MOUFPE 8407; male, cl 10.3 mm, Candeais beach, intertidal, Recife, Pernambuco, 16 September 1989, MOUFPE 8404; 2 females, cl: 5.5–10.4 mm, Suape Port, intertidal, Pernambuco, 31 January 1964, MOUFPE 8405.
Type locality. Florida, Key West, USA [48].
Remarks. Neocallichirus grandimana is an emblematic species of the American coast, with records along both the western Atlantic and eastern Pacific coasts [14,20]. In Brazil, this species has been recorded along the northeastern region (Ceará and Pernambuco) by Pachelle et al. [49]. It is unclear whether the specimens from Brazil assigned to N. grandimana correspond to an undescribed species, as there has never been a thorough comparison between the Brazilian specimens and the type specimens of this species. In the MOUFPE collection, there are some specimens identified by Petrônio Coelho as N. grandimana that are in fair condition. This species can be easily distinguished from the other three congeners listed here, viz. N. guara Rodrigues, 1971 [50], N. guassutinga Rodrigues, 1971 [50], N. maryae Karasawa, 2004 [51], by a combination of characters, including frontal margin of the carapace having obtuse rounded anterolateral projections (vs. sharp anterolateral projections in N. maryae and N. guassutinga), and uropodal endopod widening distally (vs. narrowing distally in N. guara) (see [12]).
Neocallichirus guara (Rodrigues, 1971) [50]
Material—Brazil. Male, cl 10.3 mm, AS # 1992II, 1.617° N, 48.367° W, 32 m, Pará, 23 November 1968, MOUFPE 8398; female, cl 15.4 mm, GM # 47, 0.617° N, 57.850° W, 43 m, Pará, 9 June 1969, MOUFPE 8395.
Type locality. Santo Amaro Inlet, Guaruja, São Paulo, Brazil [50].
Remarks. Endemic to Brazil, N. guara has been recorded from Pará to São Paulo both in intertidal and shallow waters habitats. After its description, this species was never recorded again in the southeastern region of Brazil, being only later reported by Coelho [13] in the northeastern region of the country. Recently Pachelle et al. [49] and Hernáez et al. [12] reported N. guara from Ceará and Maranhão, respectively. Specimens of N. guara deposited in the MOUFPE collection are in very poor condition.
Neocallichirus guassutinga (Rodrigues, 1971) [50]
Material—Brazil. Female, cl 26.2 mm, Praia de Acaú, 7.546° S, 34.822° W, Paraíba, P. Coelho coll., 2 June 1996, MOUFPE 9767; male, cl 10.6 mm, Candeias, 8.193° S, 34.918° W, Recife, Pernambuco, P. Coelho coll., 8 April 1989, MOUFPE 8399.
Type locality. Araça beach, Sao Sebastião, São Paulo, Brazil [50].
Remarks. Neocallichirus guassutinga has been recorded along the western Atlantic from the coast of the USA (Florida, Louisiana, Texas), Mexico (Tamaulipas), Panama (Gulf of Panama) and Brazil (Paraiba, Pernambuco, São Paulo) (see [20], present study). This species closely resembles the congener species, N. maryae, but differs from it in that the uropodal exopod in N. guassutinga is much shorter than the inferior rami, whereas in N. maryae the two rami are similar in size [20]. Two specimens in good condition (one male and one female) are deposited in the MOUFPE collection.
Neocallichirus maryae Karasawa, 2004 [51]
Material—Brazil. Female, cl 10.8 mm, Tamandaré beach, 8.752° S, 35.091° W, Tamandaré, Pernambuco, P. Hernáez coll., 25 June 2021, MOUFPE 22045; male, cl 10.8 mm, Porto de Galinhas, 8.508° S, 35.000° W, Pernambuco, P. Hernáez coll., 25 June 2021, MOUFPE 22046.
Type locality. Bluefields, Jamaica [39].
Remarks. Neocallichirus maryae has been previously reported as Callianassa rathbunae by Schmitt [39]. The species is widely distributed along the western Atlantic coasts, including an isolated record in the Caribbean region (type locality, Jamaica) [39]. In Brazil, N. maryae has been recorded in the northeastern region (i.e., Maranhão, Piauí, Ceará, Pernambuco and Alagoas) by Botter-Carvalho et al. [52], Calado et al. [53], Pachelle et al. [11] and Hernáez et al. [12]. Neocallichirus maryae is characterized, among other traits, by the presence of an acute rostrum and by the relative size of the carpus and palm of the male major cheliped, which is about half as long as the palm in this species [12]. Several well-preserved specimens of N. maryae are deposited in the MOUFPE collection.
Family Ctenochelidae Manning and Felder, 1991 [35]
Genus Ctenocheles Kishinouye, 1926 [54]
Ctenocheles holthuisi Rodrigues, 1978 [55]
Material—Brazil. Female, cl 4.3 mm, Continental shelf of Salvador, 13.396° S, 38.852° W, 25–50 m, muddy bottom, Salvador, Bahia, A. Esteves coll., 1 Dezember 2011, MOUFPE 15060.
Type locality. Off the mouth of Rio São Francisco, Brazil [55].
Remarks. Ctenocheles holthuisi was described from an adult female specimen (cl 14 mm) collected on the continental shelf (75 m) of Bahia, Brazil [55]. In the original description of this species, there is no information about the catalog number of the holotype, which, according to Rodrigues [55], was deposited in the MOUFPE collection. After reviewing the collection material, it is possible to affirm that, unfortunately, the holotype of C. holthuisi is lost.
Genus Ctenocheloides Anker, 2010 [56]
Ctenocheloides almeidai Anker and Pachelle, 2013 [57]
Material—Brazil. Ovigerous female, cl 2.8 mm, Parque Municipal Marinho do Recife de Fora, 6.383° S, 38.983° W, 10–12 m, dead portions of coral head, Porto Seguro, Bahia, 13 April 2012, MOUFPE 15627; ovigerous female, cl 3.4 mm, same collection site, 5 May 2013, MOUFPE 15628.
Type locality. Off the mouth of Rio São Francisco, Brazil [57].
Remarks. Ctenocheloides almeidai was described by Anker and Pachelle [56] from a single female specimen collected in a coastal reef pool at Ponta Verde, Maceió. The second record of this species was presented three years later by Souza et al. [58], who reported three ovigerous females associated with dead coral at a depth of 10–12 m. According to the MOUFPE collection database, lots 15627, 15628 and 15629 contain, respectively, 1, 1 and 2 specimens of C. almeidae. Based on this review of the material, lot 15629 is missing.
Genus Dawsonius Manning and Felder, 1991 [35]
Dawsonius latispinus (Dawson, 1967) [59]
Material—Brazil. Female, cl 9.6 mm, incomplete specimen, only carapace preserved, AS#1793B station, 4.225° N, 50.433° W, sandy bottom, 75 m, Amapá, 18 November 1967, MOUFPE 8598; missing specimen, GM # 200 station, 3.667° N, 49.625° W, 92 m, Amapá, 1971, MOUFPE 8599; male, cl 5.0 mm, incomplete specimen, only carapace preserved, AS # 1784 station, 3.142° N, 48.117° W, sandy bottom, 85 m, Amapá, 16 November 1967, MOUFPE 1882; male, damage specimen, only major cheliped preserved, AS # 1906 station, 2.667° N, 49.000° W, sandy bottom, 78 m, Amapá, 4 May 1968, MOUFPE 8601; male, cl 2.0 mm, incomplete specimen, lacking legs and somites 4, 5, 6 and telson, AS # 1892 station, 1.750° N, 48.300° W, muddy bottom, 56 m, Pará, 1 May 1968, MOUFPE 309; missing specimen, AS#2476 station, 1.183° N, 45.925° W, 44 m, Pará, 1971, MOUFPE 8597.
Type locality. Off Grand Isle, Louisiana, 135 m [59].
Remarks. According to the original description, D. latispinus is distinguished from other burrowing shrimp species from the Gulf of Mexico by having a telson longer than wide and lacking spines or spinules; short uropods are distally rounded; the first abdominal segment is saddle-shaped; and there are dorso-antero-lateral projections of the sixth abdominal segment ([59]; see also Figure 4). In Brazil, this species has been collected in deep waters up to 80 m in the northern region of Brazil between Amapá and Pará. Unfortunately, most of the D. latispinus material deposited in the MOUFPE collection is in poor condition, with several lots where specimens have been definitively lost.
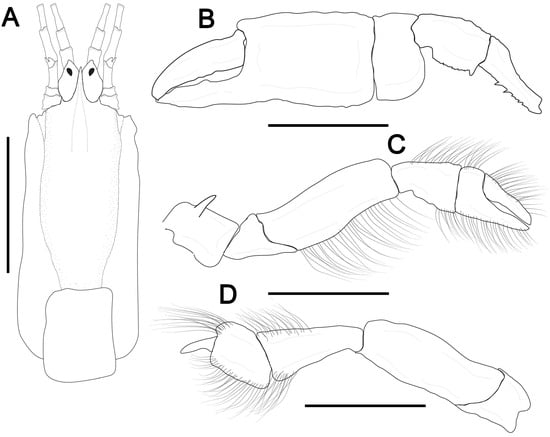
Figure 4.
Dawsonius latispinus (Dawson, 1967) [59]. (A–D), female, cl 9.6 mm, MOUFPE 8598. (A), carapace front, eyestalk, and antennular and antennal peduncles, dorsal view; (B), female major cheliped, lateral view; (C,D), second and third pereopods, lateral view. Scale bars: (A) = 5 mm; (B–D) = 2 mm.
Genus Gourretia de Saint Laurent, 1973 [60]
Gourretia laresi Blanco Rambla and Liñero Arana, 1994 [61]
Material—Brazil. Female, cl 4 mm, Continental shelf of Salvador, 13.489° S, 38.831° W, 25–50 m, muddy bottom, Salvador, Bahia, A. Esteves coll., 10 October 2011, MOUFPE 15059.
Type locality. Northwest Chimana Islands, 71 m on the clay bottom, Venezuela [61].
Remarks. Pachelle et al. [62] first reported G. laresi on the Brazilian coast. They examined a female specimen collected from the continental shelf (20–50 m) off Bahia. This single specimen, in excellent condition, is deposited in the MOUFPE collection.
Family Micheleidae Sakai, 1992 [63]
Genus Marcusiaxius de Carvalho and Rodrigues, 1972 [64]
Marcusiaxius lemoscastroi de Carvalho and Rodrigues, 1972 [64]
Material—Brazil. Male, cl 9.1 mm, GM # 194, 3.742° N, 50.125° W, 77 m, continental shelf, Amapá, 1971, MOUFPE 8474.
Type locality. Amapá, north coast of Brazil, littoral [64].
Remarks. The whereabouts of the M. lemoscastroi holotype is the ‘Instituto de Biologia da Universidade Federal, Bahia’. In addition to Brazil, this species has been recorded along the Caribbean coast of Central and South America [20]. In the MOUFPE collection, a single specimen of this species is deposited.
Marcusiaxius minutus (Coelho, 1973) [65]
Material—Brazil. Female, cl 12.3 mm, GM # 199, 3.792° N, 49.700° W, 91 m, continental shelf, Amapá, 1971, MOUFPE 1872.
Type locality. Off Amapá, Brazil [65].
Remarks. The original description of M. minutus is incomplete [65]. Marcusiaxius minutus is endemic to Brazil. In the MOUFPE collection, a female specimen of this species is deposited.
Genus Meticonaxius de Man, 1905 [29]
Meticonaxius capricorni Coelho, 1987 [66]
Material—Brazil. Paratype, female, cl. 14.6 mm, incomplete, SUL II # 10, Guanabara Bay, 23.867° S, 43.183° W, 165 m, continental shelf, Rio de Janeiro, 27 March 1972, MOUFPE 15327; paratype, male, cl. 17.4 mm, SUL II # 6, off São Paulo, 24.133° S, 44.550° W, 139 m, continental shelf, São Paulo, 26 March 1972, MOUFPE 15325; paratype, female, cl 20.3 mm, SUL II # 3, off São Paulo, 24.550° S, 44.733° W, 148 m, continental shelf, São Paulo, 26 March 1972, MOUFPE 15326.
Type locality. Rio de Janeiro, 156 m, Brazil [66].
Remarks. Endemic to Brazil, this species has been recorded exclusively in the continental shelf of Rio de Janeiro [12]. Two lots (MOUFPE: 15326, 15327), each containing one specimen, collected at two sites along the coast of São Paulo, extend the distribution of this species 800 km southward.
4. Discussion
Holdings of Axiidea deposited in the MOUFPE collection are summarized according to habitat in Table 1. This collection contains specimens of 23 out of the 35 Axiidea ghost shrimp species recorded on the Brazilian coast. Many more comparative studies between Axiidea material from the Brazilian coast and the respective types of each of these species are necessary to establish the taxonomic validity of Brazilian records, especially in the case of those species originally described in the northern hemisphere and reported for the Brazilian coast without any comparative work. From here, certainly, additional species of Axiidea have to be expected.

Table 1.
Burrowing shrimp species of the infraorder Axiidea deposited in the Museu de Oceanografia Prof. Petrônio Alves Coelho, Universidade Federal de Pernambuco (MOUFPE). The habitat of each species, intertidal or subtidal, is represented by ‘I’ and ‘S’, respectively. The reference for a Brazilian record is provided here.
Author Contributions
Conceptualization, P.H.; methodology, P.H. and J.F.S.-F.; formal analysis, P.H.; investigation, P.H. and J.F.S.-F.; resources, P.H. and J.F.S.-F.; data curation, P.H. and J.F.S.-F.; writing—original draft preparation, P.H.; writing—review and editing, P.H.; supervision, J.F.S.-F.; funding acquisition, P.H. and J.F.S.-F. All authors have read and agreed to the published version of the manuscript.
Funding
P.H. is grateful to Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) for financial aid through a Researcher Fixation Scholarship (process BFP-0196-1.08/20).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We would like to express our sincere gratitude to Jessica Frias and Fabiola Couto, technical staff of the Museu de Oceanografia Prof. Petrônio Alves Coelho, for providing access to the material of Axiidea from the zoological collection. Both authors also thanks to their respective families for the con-tinuing support. This paper has greatly benefited from the helpful comments and suggestions of three anonymous reviewers.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- de Saint Laurent, M. Vers une nouvelle classification des Crustacés Décapodes Reptantia. Bull. Off. Natl. Pêche Tunis. 1979, 3, 15–31. [Google Scholar]
- Dworschak, P.C.; Felder, D.L.; Tudge, C.C. Infraorders Axiidea de Saint Laurent, 1979 and Gebiidea de Saint Laurent, 1979 (formerly known collectively as Thalassinidea). In Treatise on Zoology–Anatomy, Taxonomy, Biology; The Crustacea. Complementary to the Volumes Translated from the French of the Traité de Zoologie [Founded by P.-P. Grassé], Volume 9 Part B. Eucarida: Decapoda: Astacidea p.p. (Enoplometopoidea, Nephropoidea), Glypheidea, Axiidea, Gebiidea, and Anomura; Schram, F.R., von Vaupel Klein, J.C., Forest, J., Charmantier-Daures, M., Eds.; Brill: Leiden, The Netherlands, 2012; pp. 109–219. [Google Scholar]
- Hernáez, P. An update on reproduction in ghost shrimps (Decapoda: Axiidea) and mud lobsters (Decapoda: Gebiidea). In Marine Ecology; Chapter 11; Türkoğlu, M., Önal, U., Ismen, A., Eds.; IntechOpen: London, UK, 2018; pp. 231–253. [Google Scholar]
- Poore, G.C.B.; Dworschak, P.C.; Robles, R.; Mantelatto, F.L.; Felder, D.L. A new classification of Callianassidae and related families (Crustacea: Decapoda: Axiidea) derived from a molecular phylogeny with morphological support. Mem. Mus. Vic. 2019, 78, 73–146. [Google Scholar] [CrossRef]
- Dworschak, P.C. Global diversity in the Thalassinidea (Decapoda): An update (1998–2004). Nauplius 2005, 13, 57–63. [Google Scholar]
- Hernáez, P.; Fenberg, P.B.; Rivadeneira, M.M. Departing from an ideal: An asymmetric, bimodal and non-Equatorial latitudinal gradient of marine diversity in Western Atlantic burrowing shrimps (Decapoda: Axiidea and Gebiidea). J. Biogeogr. 2021, 48, 650–661. [Google Scholar] [CrossRef]
- Griffis, R.B.; Suchanek, T.H. A model of burrow architecture and trophic modes in thalassinidean shrimp (Decapoda: Thalassinidea). Mar. Ecol. Prog. Ser. 1991, 79, 171–183. [Google Scholar] [CrossRef]
- Pillay, D. Ecosystem engineering by Thalassinidean crustaceans: Response variability, contextual dependencies and perspectives on future research. Diversity 2019, 11, 64. [Google Scholar] [CrossRef]
- Ziebis, W.; Forster, S.; Huettel, M.; Jørgensen, B. Complex burrows of the mud shrimp Callianassa truncata and their geochemical impact in the sea bed. Nature 1996, 382, 619–622. [Google Scholar] [CrossRef]
- Bertics, V.J.; Sohm, J.A.; Treude, T.; Ch-ET, C.; Capone, D.G.; Fuhrman, J.A.; Ziebis, W. Burrowing deeper into benthic nitrogen cycling: The impact of bioturbation on nitrogen fixation coupled to sulfate reduction. Mar. Ecol. Prog. Ser. 2010, 409, 1–15. [Google Scholar] [CrossRef]
- Pachelle, P.P.G.; Anker, A.; Mendes, C.B.; Bezerra, L.E.A. Decapod crustaceans from the state of Ceará, northeastern Brazil: An updated checklist of marine and estuarine species, with 23 new records. Zootaxa 2016, 4131, 1–63. [Google Scholar] [CrossRef]
- Hernáez, P.; Pinheiro, M.A.A.; Alves-Junior, F.A.; Santana, W. Intertidal burrowing shrimps (Axiidea: Callianassidae, Callichiridae; Gebiidea: Axiannassidae, Upogebiidae) collected along the Brazilian coast. Mar. Biol. Res. 2022, 18, 380–408. [Google Scholar] [CrossRef]
- Coelho, P.A. Revisão das espécies de Thalassinidea encontradas em Pernambuco, Brasil (Crustacea, Decapoda). Trop. Oceanogr. 1997, 25, 137–161. [Google Scholar] [CrossRef]
- Melo, G.A.S. Manual de Identificação dos Crustacea Decapoda do Litoral Brasileiro: Anomura, Thalassinidea, Palinuridea, Astacidea; Editora Plêiade/FAPESP: São Paulo, Brazil, 1999; 551p. [Google Scholar]
- Coelho, P.A.; Ramos-Porto, M. A constituição e a distribuição da fauna de decápodos do litoral Leste da América do Sul entre as latitudes de 5°N e 39°S. Trop. Oceanogr. 1972, 13, 133–236. [Google Scholar] [CrossRef]
- Coelho, P.A.; Coelho-Santos, M.A. A família Callianassidae no litoral do Estado de Pernambuco (Crustacea, Decapoda, Thalassinidea). Trop. Oceanogr. 1993, 22, 243–257. [Google Scholar] [CrossRef]
- Huxley, T.H. On the classification and the distribution of the crayfishes. Proc. Sci. Meet. Zool. Soc. Lond. 1879, 1878, 752–788. [Google Scholar] [CrossRef]
- Borradaile, L.A. On the classification of the Thalassinidea. Ann. Mag. Nat. Hist. 1903, 12, 534–551. [Google Scholar] [CrossRef]
- Coelho, P.A.; Ramos-Porto, M. Sinopse dos crustaceos decapodos brasilieros (familias Scyllaridae, Palinuridae, Nephropidae, Parastacidae e Axiidae). An. Univ. Fed. Pernamb. Ciência Biol. 1991, 8/10, 47–88. [Google Scholar]
- Sakai, K. Axioidea of the World and a Reconsideration of the Callianassoidea (Decapoda, Thalassinidea, Callianassida); Brill: Leiden, The Netherlands, 2011; Volume 13, pp. 1–616. [Google Scholar]
- Kensley, B.; Simmons, G.M. Axiorygma nethertoni, a new genus and species of thalassinidean shrimp from Florida (Decapoda: Axiidae). J. Crust. Biol. 1988, 8, 657–667. [Google Scholar] [CrossRef]
- Sakai, K.; de Saint Laurent, M. A check list of Axiidae (Decapoda, Crustacea, Thalassinidea, Anomula), with remarks and in addition descriptions of one new subfamily, eleven new genera and two new species. Nat. Publ. Tokushima Biol. Lab. Shikoku Univ. 1989, 3, 1–104. [Google Scholar]
- Coelho, P.A. Descricao preliminar de Calastacus angulatus, n. sp., e de C. spinosus, n. sp., do Norte do Brasil (Crust., Decapoda, Axiidae). Ciência Cult. São Paulo 1973, 25, 344–345. [Google Scholar]
- Kensley, B.; Gore, R.H. Coralaxius abelei, new genus and new species (Crustacea: Decapoda: Thalassinidea: Axiidae): A coral-inhabiting shrimp from the Florida Keys and the western Caribbean Sea. Proc. Biol. Soc. Wash. 1981, 93, 1277–1294. [Google Scholar]
- Meinert, F. Crustacea Isopoda, Amphipoda et Decapoda Daniae: Forteguelse over Danmarks isopode, amphipode og decapode krebsdyr. Naturhist Tidsskr. 1877, 3, 57–248. [Google Scholar]
- Kensley, B. The genus Coralaxius redefined, with descriptions of two new species (Crustacea: Decapoda: Axiidae). J. Nat. Hist. 1994, 28, 813–828. [Google Scholar] [CrossRef]
- Pachelle, P.P.G.; Tavares, M. Axiidean ghost shrimps (Decapoda: Axiidae, Callianassidae, Callichiridae, Micheleidae) of the Trindade and Martin Vaz Archipelago, Vitória-Trindade Seamounts Chain and Abrolhos, off southeastern Brazil. Zootaxa 2020, 4758, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Kensley, B. Axioid shrimps from Guam (Crustacea, Decapoda, Thalassinidea). Micronesica 2003, 35–36, 359–384. [Google Scholar]
- de Man, J.G. Diagnoses of new species of macrurous decapod Crustacea from the “Siboga-Expedition”. Tijdschr. Ned. Dierkd. Ver. 1905, 9, 587–614. [Google Scholar]
- Rathbun, M.J. Investigations of the Aquatic Resources and Fisheries of Porto Rico by the United States Fish Commission Steamer Fish Hawk in 1899. The Brachyura and Macrura of Porto Rico. Bull. US Fish. Comm. 1901, 20, 1–127. [Google Scholar]
- Kensley, B. The genus Paraxiopsis De Man, with descriptions of new species from the western Atlantic (Crustacea; Decapoda; Axiidae). Bull. Mar. Sci. 1996, 58, 709–729. [Google Scholar]
- Kensley, B. A new species of the axiid shrimp genus Acanthaxius from the Caribbean (Crustacea: Decapoda: Thalassinidea). Proc. Biol. Soc. Wash. 1996, 109, 70–74. [Google Scholar]
- Dana, J.D. Conspectus Crustaceorum, etc. conspectus of the Crustacea of the exploring expedition under Capt. Wilkes, U.S.N., including the Crustacea Cancroidea Corystoidea. Proc. Acad. Nat. Sci. Phil. 1852, 6, 73–86. [Google Scholar]
- Biffar, T.A. New species of Callianassa (Decapoda, Thalassinidea) from the western Atlantic. Crustaceana 1971, 21, 225–236. [Google Scholar] [CrossRef]
- Manning, R.B.; Felder, D.L. Revision of the American Callianassidae (Crustacea: Decapoda: Thalassinidea). Proc. Biol. Soc. Wash. 1991, 104, 764–792. [Google Scholar]
- Stimpson, W. Descriptions of new genera and species of Macrurous Crustacea from the coasts of North America. Proc. Chic. Acad. Sci. 1866, 1, 46–48. [Google Scholar]
- Hernáez, P.; Miranda, M.S.; Rio, J.P.; Pinheiro, M.A. A new Callichirus ghost shrimp species from the south-western Atlantic, long confounded with C. major (Say, 1818) (Decapoda: Axiidea: Callichiridae). J. Nat. Hist. 2022, 56, 533–563. [Google Scholar] [CrossRef]
- Manning, R.B. Notes on western Atlantic Callianassidae (Crustacea: Decapoda: Thalassinidea). Proc. Biol. Soc. Wash. 1987, 100, 386–401. [Google Scholar]
- Schmitt, W. Mud shrimps of the Atlantic coast of North America. Smithson. Misc. Collect. 1935, 95, 1–5; pls. 1–5. [Google Scholar]
- Manning, R.B. The status of Callianassa hartmeyeri Schmitt, 1935, with the description of Corallianassa xutha from the west coast of America (Crustacea, Decapoda, Thalassinidea). Proc. Biol. Soc. Wash. 1988, 101, 883–889. [Google Scholar]
- DecaNet eds. DecaNet. Corallianassa Manning. Accessed through World Register of Marine Species. 1987. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=246268 (accessed on 10 April 2024).
- Milne-Edwards, A. Révision du genre Callianassa (Leach) et description de plusieurs espèces nouvelles de ce groupe faisant partie de la collection du Muséum. Nouv. Arch. Mus. Hist. Nat. Paris 1870, 6, 75–102; pls. I–II. [Google Scholar]
- Holmes, S.J. On some new or imperfectly known species of west American Crustacea. Proc. Calif. Acad. Sci. 1904, 3, 307–322; pls. 35–37. [Google Scholar]
- Felder, D.L.; Rodrigues, S.d.A. Reexamination of the ghost shrimp Lepidophthalmus louisianensis (Schmitt, 1935) from the northern Gulf of Mexico and comparison to L. siriboia, new species, from Brazil (Decapoda: Thalassinidea: Callianassidae). J. Crust. Biol. 1993, 13, 357–376. [Google Scholar] [CrossRef]
- Hernáez, P.; Forbes, A.; de Souza, P.R.C.M.; Souza-Filho, J.F. Unraveling the mating system of the burrowing shrimp Lepidophthalmus siriboia (Decapoda Callichiridae) based on life history traits. Ethol. Ecol. Evol. 2024, 36, 150–174. [Google Scholar] [CrossRef]
- Felder, D.L. Ventrally sclerotized members of Lepidophthalmus (Crustacea: Decapoda: Callianassidae) from the Eastern Pacific. Ann. Naturhist Mus. Wien 2003, 104, 429–442. [Google Scholar]
- Sakai, K. A new genus and five new species of Callianassidae (Crustacea: Decapoda: Thalassinidea) from northern Australia. Beagle Occas. Pap. North. Territ. Mus. Arts Sci. 1988, 5, 51–69. [Google Scholar] [CrossRef]
- Gibbes, L.R. On the carcinological collections of the United States, and an enumeration of species contained in them, with notes on the most remarkable, and descriptions of new species. Proc. Am. Assoc. Adv. Sci. 1850, 3, 165–201. [Google Scholar]
- Pachelle, P.P.G.; Anker, A.; Bezerra, L.E.A. Re-identification of the material of Neocallichirus maryae Karasawa, 2004 from Ceará, northeastern Brazil, with the first record of N. cacahuate Felder and Manning, 1995 in the southwestern Atlantic. Zootaxa 2017, 4276, 346–356. [Google Scholar] [CrossRef]
- Rodrigues, S.A. Mud shrimps of the genus Callianassa Leach from the Brazilian coast (Crustacea, Decapoda). Arq. Zool. 1971, 20, 191–223. [Google Scholar] [CrossRef]
- Karasawa, H. Neocallichirus maryae, a replacement name for Neocallichirus rathbunae (Schmitt, 1935) (Crustacea: Decapoda: Thalassinidea). Paleontol. Res. 2004, 8, 87. [Google Scholar] [CrossRef]
- Botter-Carvalho, M.L.; Carvalho, P.V.V.D.B.C.; Ramos-Porto, M. Registro de Neocallichirus rathbunae (Schmitt, 1935) em águas do litoral brasileiro (Decapoda: Callianassidae). Trop. Oceanogr. 1995, 23, 199–202. [Google Scholar] [CrossRef][Green Version]
- Calado, T.C.S.; Silva, M.P.; Aires, A.F. Crustáceos decápodos e estomatópodos de praias arenosas do litoral norte e centro do estado de Alagoas. Trab. Oceanog. Univ. Fed. Pernamb. 1998, 26, 107–115. [Google Scholar] [CrossRef][Green Version]
- Kishinouye, K. Two rare and remarkable forms of macrurous Crustacea from Japan. Annot. Zool. Jpn. 1926, 11, 63–70. [Google Scholar]
- Rodrigues, S.d.A. Ctenocheles holthuisi (Decapoda, Thalassinidea), a new remarkable mud shrimp from the Atlantic Ocean. Crustaceana 1978, 34, 113–120. [Google Scholar] [CrossRef]
- Anker, A. Ctenocheloides attenboroughi n. gen., n. sp. (Crustacea: Decapoda: Axiidea: Ctenochelidae), a new ghost shrimp with pectinate claw fingers from Madagascar. J. Nat. Hist. 2010, 44, 1789–1805. [Google Scholar] [CrossRef]
- Anker, A.; Pachelle, P.P.G. Ctenocheloides almeidai sp. nov., a new ghost shrimp from Brazil (Decapoda, Ctenochelidae). Zootaxa 2013, 3613, 482–492. [Google Scholar] [CrossRef]
- Souza-Santos, P.; Soledade, G.O.; Almeida, A.O. Second finding of the south western Atlantic ghost shrimp Ctenocheloides almeidai Anker and Pachelle, 2013 (Crustacea: Axiidea). Nauplius 2016, 24, e2016005. [Google Scholar]
- Dawson, C.E. Callianassa latispina (Decapoda, Thalassinidea), a new mud shrimp from the Northern Gulf of Mexico. Crustaceana 1967, 13, 190–196. [Google Scholar] [CrossRef]
- de Saint Laurent, M. Sur la systématique et la phylogénie des Thalassinidea: Définition des familles des Callianassidae et des Upogebiidae et diagnose de cinq genres nouveaux. C. R. Hebd. Séances Acad. Sci. Paris 1973, 277, 513–516. [Google Scholar]
- Blanco Rambla, J.P.; Liñero Arana, I. New records and new species of ghost shrimps (Crustacea: Thalassinidea) from Venezuela. Bull. Mar. Sci. 1994, 55, 16–29. [Google Scholar]
- Pachelle, P.P.G.; Bezerra, L.E.A.; Anker, A. Second findings of the ghost shrimps Gourretia laresi Blanco Rambla and Liñero Arana, 1994 and Ctenocheles holthuisi Rodrigues, 1978 (Decapoda, Axiidea, Ctenochelidae). Crustaceana 2013, 86, 110–120. [Google Scholar] [CrossRef]
- Sakai, K. The families Callianideidae and Thalassinidae, with the description of two new subfamilies, one new genus and two new species (Decapoda, Thalassinidea). Nat. Publ. Tokushima Biol. Lab. Shikoku Univ. 1992, 4, 1–33. [Google Scholar]
- Rodrigues, S.d.A.; de Carvalho, H.A. Marcusiaxius lemoscastroi, g. n., sp. n., primeira occurrência da família Axiidae (Crustacea, Decàpoda, Thalassinidea) no Brasil. Ciência Cult. São Paulo 1972, 24, 357. [Google Scholar]
- Coelho, P.A. Descriçao preliminar de Meticonaxius minutus, sp. n., do Norte de Brasil (Crustacea, Decapoda, Axiidae). Ciência Cult. São Paulo 1973, 25, 345. [Google Scholar]
- Coelho, P.A. Uma espécie nova de Meticonaxius do Brasil (Crustácea, Decapoda, Callianideidae). Rev. Bras. Zool. 1987, 4, 63–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).