1. Introduction
Highly purified colloidal silica is widely used in large-scale integration (LSI) manufacturing processes [
1,
2,
3]. Takahata et al. [
1] described a method for synthesizing colloidal silica from an active silicic acid solution (ASAS) derived from tetraethoxysilane (TEOS). Subsequent work [
2] has demonstrated that this process yields denser silica particles compared to those produced via the conventional Stöber method [
4] and proposed using
29Si CP/MAS NMR to evaluate the density of colloidal silica particles. Additionally, Higuchi et al. [
3] revealed that modifying the pH of the reaction mixture in a basic-acidic-basic sequence during the early stage of synthesis enables significant morphological control of the colloidal silica particles, which is an outcome that is not achievable with conventional methods [
1,
5,
6], which typically produce spherical or cocoon-like shapes.
Although ASAS allows for the synthesis of dense and morphologically modified colloidal silica particles, it offers limited control over the primary particle diameter (PPD), which can only be increased by adding additional ASAS. In contrast, the Stöber method enables the rapid synthesis of larger colloidal silica particles by adjusting the amounts of added ammonium and water [
4]. However, attempts to increase the particle size using a higher amount of ASAS face a significant challenge: as the particles grow, the silica deposition rate decreases exponentially, making it impractical to produce larger particles (e.g., >100 nm in diameter) within a reasonable time frame. This issue has been highlighted in paragraph (0005) of reference [
7].
Moreover, increasing the volume of the ASAS proportionally to the particle size risks overflowing the reaction vessel. This necessitates the division of the reaction liquid into multiple batches, making the process cumbersome and inefficient. Therefore, developing a method to control PPD independent of the increase in the amount of ASAS added is essential for establishing ASAS-based alternatives to the Stöber method.
During our study, we hypothesized that PPD could be governed by the silica deposition rate. During the initial stage of particle formation, seed particles form in response to the balance between the silica deposition rate and the surface area available for silica nucleation. Once a sufficient surface area is established, seed formation ceases and growth begins. Thus, controlling the silica deposition rate is key to manipulating PPD.
Aelion et al. [
8] suggest that increasing the acid concentration during ASAS preparation reduces the likelihood of reaction (2) in Equation (1) (which produces water), resulting in greater silanol retention. In other words, silanol was stabilized under acidic conditions. This understanding forms the basis of the present study.
As mentioned above, the conventional Stöber method allows particle size control through four primary parameters: the reaction temperature, water content, ammonium concentration, and feed rate of alkyl silicate. However, each parameter presents challenges. The hydrolysis of alkyl silicates is exothermic, requiring efficient heat exchange in order to maintain a stable reaction temperature. Over multiple batches, silica layers may form on the inner surface of the reaction vessels, reducing the heat transfer efficiency and causing property fluctuations in the final product. While Teflon® lining minimizes contamination and silica adhesion, it is less effective for heat exchange than glass linings, which carry a risk of metal contamination.
Controlling the water content and ammonium concentration by titration introduces variability owing to human error and sampling limitations. Similarly, mechanical issues with feed pumps compromise the control over the alkyl silicate feed rate. Additionally, the Stöber method often leaves unreacted alkyl silicate, owing to incomplete hydrolysis under basic conditions [
7,
9,
10]. To address this, the reaction pH must be lowered to 7, necessitating a time-consuming water exchange step.
A similar issue arises in the method described by Tasaki et al. [
11], which uses high-temperature water under basic conditions. This approach risks vapor phase reactions owing to the evaporation of alkyl silicate.
This study aims to resolve these limitations by developing an ASAS-based method that enables reliable control over the particle size and morphology without relying on the problematic variables inherent to the Stöber process.
2. Materials and Methods
- (1)
Preparation of ASAS
Distilled water (570 g) and a selected amount of 1N sulfuric acid were placed in a one-liter Erlenmeyer flask equipped with a magnetic stirrer. The flask was then immersed in a water bath at 30 °C and stirred for 30 min. Next, 68.4 g of tetramethoxysilane (TMOS; Colcoat, Tokyo, Japan) was placed in the same water bath to stabilize the temperature at 30 °C.
The temperature-regulated TMOS was then added simultaneously to the temperature-regulated water, followed by immediate cooling with crushed ice to keep the reaction temperature below 35 °C to avoid excessive condensation.
After confirming that the temperature had decreased, the preparation of the ASAS was complete. The obtained solution had a silica content of 6 wt% and was unstable as silanol condensation gradually progressed over time, causing the active silicic acid to polymerize. Therefore, the solution was stored in an ice bath (to keep the temperature below 10 °C) to prevent deterioration.
- (2)
Preparation of colloidal silica
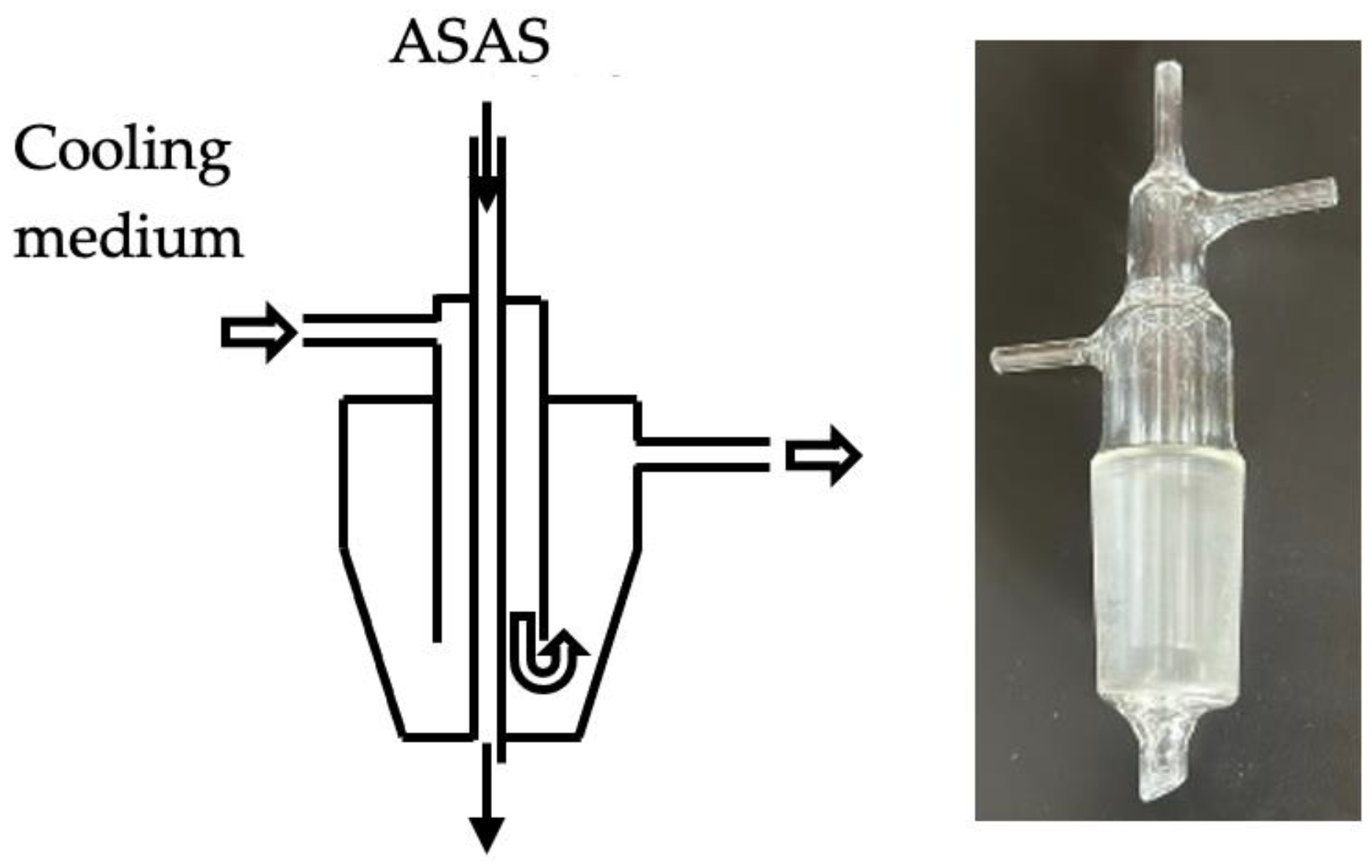
In a 2 L four-neck flask equipped with a Dean–Stark-type adaptor, a Dimroth cooling tube, a mechanical stirrer, and a specially prepared cooling feed adaptor (shown in
Figure 1) connected to a tube-type metering pump, 1 L of distilled water and 1 g of 1 N tetramethyl ammonium hydroxide (initial TMAH) were added. The flask was immersed in an oil bath maintained at approximately 150 °C to reflux the reaction mixture.
The ASAS prepared in
Section 1 was fed at a rate of 4.1 mL/min to complete the process in 3 h. This procedure was repeated six or ten times to obtain the desired colloidal silica, preventing ASAS degradation due to condensation during storage.
For the first addition of ASAS, the pH of the reaction mixture was monitored to control its acidity. To obtain morphologically modified particles, the pH of the reaction mixture should be lowered to a prescribed minimum value (the minimum pH) using the acidity of the ASAS under reflux. Under these acidic conditions, the seed particles agglomerate to form morphologically modified seed particles. This lowered pH of the reaction mixture must be maintained for 30 min before adding approximately 10 g of 1 N TMAH to raise the pH of the reaction mixture above 8, halting agglomeration before gelation occurs. Subsequently, the pH of the reaction mixture was kept above 8 by periodically adding 1 N TMAH, while some of the evaporated water (containing methanol from the hydrolysis of TMOS) was removed to prevent overflow through the Dean–Stark-type adaptor.
To obtain spherical particles, the pH of the reaction mixture was kept above 8 throughout the reaction period by periodically adding 1N TMAH. The removal of distillate began from the start to maintain the reaction volume. After the first addition of ASAS, distillate removal was continued to keep the reaction volume at approximately 1 L.
Upon completion of the addition, the reaction mixture was refluxed for approximately 30 min to maturity, followed by concentration to achieve a silica content of approximately 20 wt.%. After cooling to below 40 °C, vacuum filtration using a 3-micron mixed cellulose membrane was performed to obtain the final colloidal silica product. If necessary, the acid can be removed from the colloidal silica via anion exchange.
The experimental conditions are presented in
Table 1.
The evaluation methods were as follows.
- 1
Specific surface area (SSA) (m2/g): BELSORP 28SA-85, Microtrac BEL Corp (Osaka, Japan).
- 2
PPD: calculated from S (SSA) (m2/g) measured using BELSORP, based on the relevant standard methodology and the following equation: S = (4π(d/2)2)/(4/3π(d/2)3ρ), which simplifies to d = 6/(ρS) (d: PPD (nm), ρ: specific gravity of silica). Therefore, d = 2727/S when ρ = 2.2 kg/L.
- 3
Secondary particle diameter (SPD) (nm) was measured using an ELSZ-2000S manufactured by Otsuka Electronics Co., Ltd. (Osaka, Japan), in accordance with the relevant standard.
- 4
Particle appearance: field emission scanning electron microscopy (FE-SEM) Gemini SEM 660 manufactured by Carl Zeiss Co., Ltd. (Tokyo, Japan).
- 5
Denseness: 29Si CP/MAS NMR Bruker Biospin GmbH (Berlin, Germany) AVANCE DSX300WB with 1wt. % of hexamethylcyclotrisiloxane as an internal standard.
3. Results and Discussion
- (1)
Influence of minimum pH
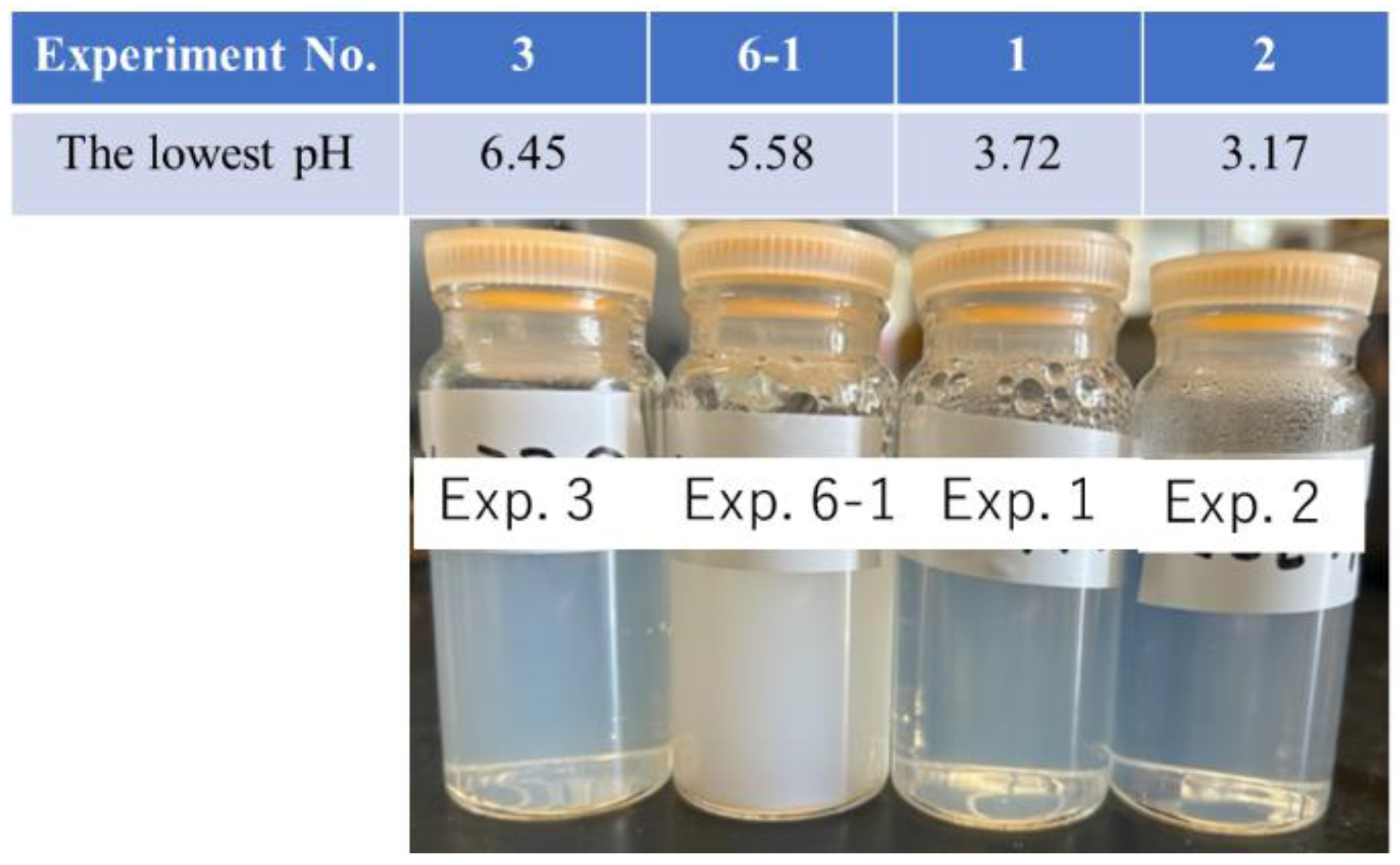
First, the influence of the minimum pH value was investigated in experiments numbered 1 to 3 and 6-1, which represents the sample obtained after the first addition of ASAS in Exp. 6. Their appearances are shown in
Figure 2.
Based on the results shown in
Figure 2, it can be concluded that there is an optimal minimum pH for obtaining the largest colloidal silica particles. A strong white color was observed, indicating the scattering of visible light by the particles with a larger PPD. The optimal minimum pH range was between 4 and 6.
This phenomenon can be explained as follows: at a lower pH, ASAS stabilized in the liquid state, which prevented agglomeration. Upon the addition of an amine, the system shifted to a basic state, leading to the solidification of ASAS and the formation of spherical particles. In Exp. 3, the silica concentration was too low to induce particle agglomeration, as only a small amount of ASAS was added.
- (2)
Influence of acid concentration on PPD
Experiments were conducted to investigate the influence of acid concentration on PPD, specifically in Exp. 4 and 5, taking into account the results shown in
Figure 2. The results are summarized in
Table 2.
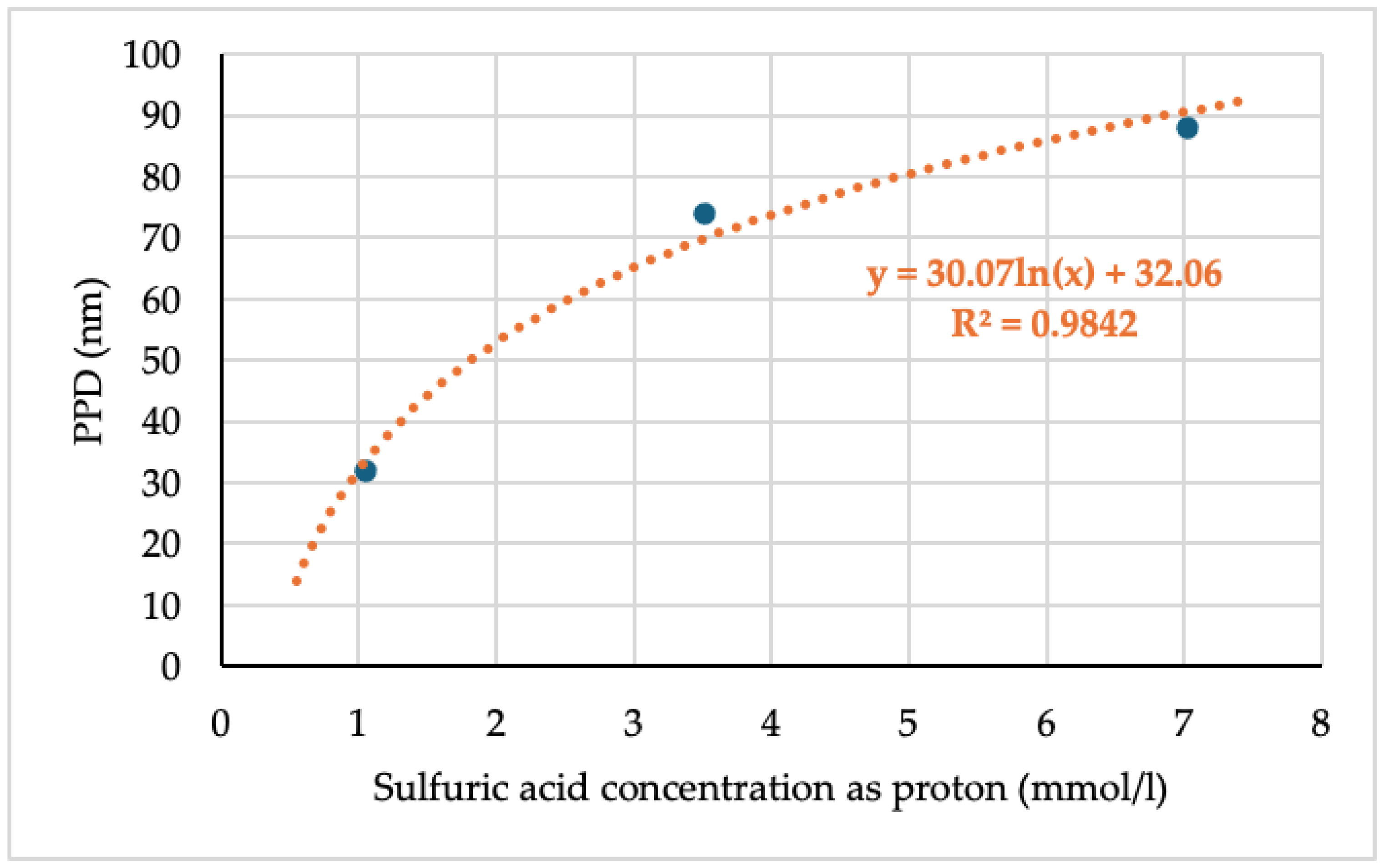
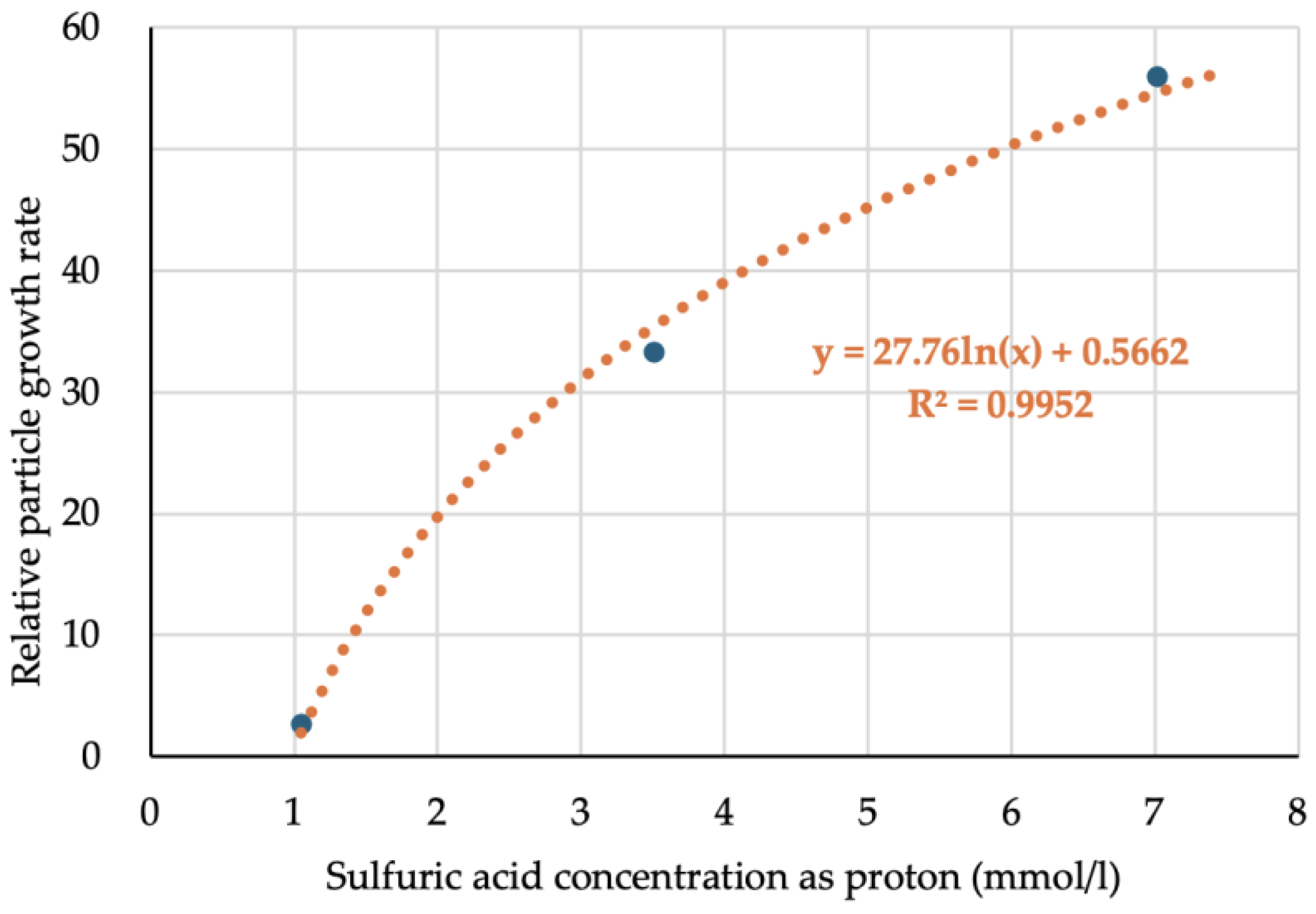
The relationship between PPD and acid concentration is shown in
Figure 3.
Acid concentration was determined based on data reported in the literature [
8]. As a result, a strong relationship between PPD and acid concentration was confirmed (
Figure 3), indicating that larger particles can be readily obtained in a short period of time by adding acid to hydrolyze TMOS for ASAS preparation.
In Exp. 8 (
Table 2), the effect of ASAS neutralization was tested. ASAS was prepared without acid addition and then neutralized with 0.1 N TMAH aq. solution, increasing the pH of ASAS from 4.04 to 6.69. While a smaller particle diameter was expected due to the reduction in silanol content, the result was the opposite, with the PPD increasing from 23 nm to 31 nm. As a result, the smallest particles were obtained from the ASAS without acid addition.
- (3)
Evaluation of colloidal particle denseness
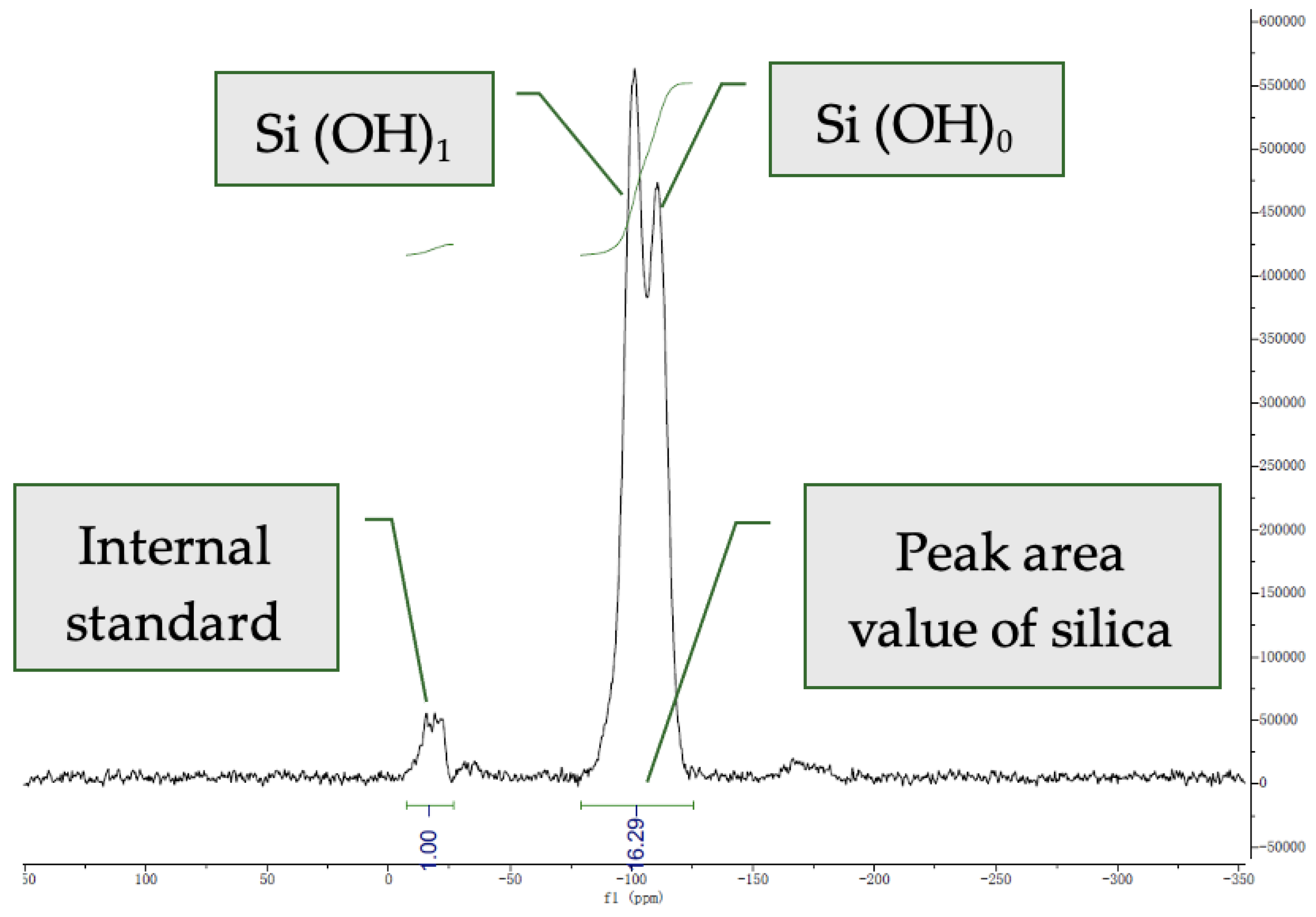
The denseness of the colloid particles was evaluated using 29Si CP/MAS NMR, which selectively detects Si-OH groups in proximity to protons. This technique enables the evaluation of silica particle denseness. The integration of the resulting peaks provides insight into the degree of densification.
To quantify the denseness, the peak area of the silica signal was normalized to that of an internal standard, following the method described in the literature [
2,
3]. An example of a
29Si CP/MAS NMR spectrum is shown in
Figure 4. The smaller the silica integration ratio relative to the internal standard, the denser the particle.
As the particle growth rate increased, the peak area of the silica also increased. However, as shown in
Table 2, a saturation tendency was observed. This can be explained by the increase in surface area along with particle growth, which slows the growth rate in the radial direction.
Exp. 7 was implemented as a standard without using acid. Its experimental conditions are the same as example 2 in the literature [
3]. As shown in
Table 2 and
Table 3, the
29Si CP/MAS NMR peak area for Exp. 7 was 8.2, while the peak area for example 2 in the literature [
3] was 11.1. This difference is likely due to the differences in measurement conditions. The NMR spectral analyzer used in [
2,
3] was an EX-270, manufactured by JEOL Ltd. (Tokyo, Japan), and the internal standard was polydimethylsilane, with a weight-average molecular weight of 2000.
Table 3 presents a comparison of the
29Si CP/MAS NMR peak areas. The largest particles in Exp. 6, with a PPD of 88 nm, have a peak area value of 16.3, which is significantly smaller than the value of 35.3 obtained by the Stöber method sample, which has a PPD of about 20 nm. This indicates that even larger particles processed using this method maintain sufficient denseness. The value of 8.2 from Exp. 7 is in reasonable agreement with the value of 9.2 from Exp. 4.
- (4)
Influence of acid concentration on relative particle growth rates
The relative particle growth rates are presented in
Table 4 and
Figure 5. Each relative particle growth rate was calculated using Equation (2).
Figure 5 shows a graph of the relationship between relative particle growth rate and acid concentration. The particle growth rate was expected to be proportional to the silica deposition rate. We defined the relative silica deposition rate as the rate at which ASAS reacts with seed particles, expressed in relation to a standard. We estimate that this rate is directly influenced by the concentration of silanol groups in the ASAS. Specifically, higher silanol content correlates with a faster deposition rate. The particle growth rate refers to the rate at which the volume of the particle increases. When the surface area of the existing particles is sufficient to accommodate incoming silica, the particle growth rate becomes equivalent to the silica deposition rate. However, if the available area is insufficient, additional nucleation occurs to match the silica deposition rate.
As shown in
Figure 5, a clear correlation between the relative particle growth rate and acid concentration was established.
- (5)
Study of colloidal particle shapes
The colloidal particle shapes primarily depend on the acid concentration and other factors, as summarized in
Table 5.
In the case of spherical particles, the amount of TMAH initially added affects the PPD, as shown in
Table 6.
For spherical particles, the addition of acid during the preparation of ASAS was also effective in obtaining colloidal silica with a larger PPD. However, as shown in
Table 7, its effect on the formation of spherical particles appears to be less pronounced, despite the addition of a similar amount of acid.
This difference can be attributed to the reduction in surface area that accompanies particle agglomeration in an acidic environment, leading to morphological modifications. Such surface area reduction does not occur during the formation of spherical particles, resulting in a smaller PPD for spherical particles compared to that of morphologically modified particles.
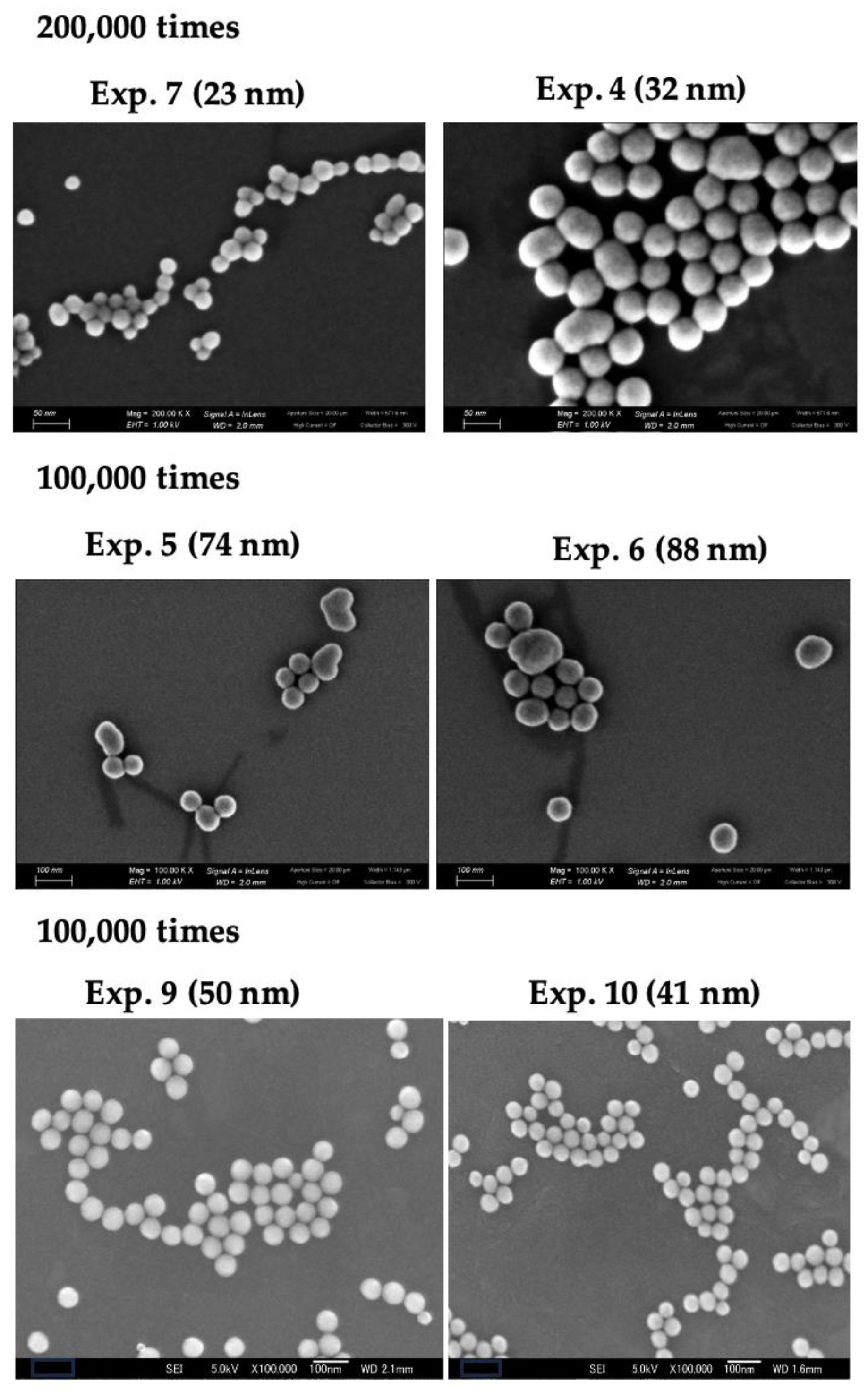
The appearance of the particles obtained in this study was confirmed by FE-SEM. The FE-SEM images are shown in
Figure 6. The particles obtained in this study mainly have a round shape, whereas the images of Exp. 4 to 6 include some nodular-type particles.
We would like to mention the types of particle growth. There are two types of particle growth, “inward” and “outward”. For example, propylene polymerization using Mg (OEt)2 as a catalyst carrier is the first type of particle growth. In this process, polymerization occurs exclusively on the surface of the catalyst carrier, on which a metallic catalyst exists, and proceeds to make the polymer particles grow inward, resulting in a shape similar to that of the catalyst carrier particles.
In contrast, the growth of colloidal silica particles occurs in the latter. Colloidal silica particles grow by covering the surfaces of seed particles with newly added silica, similar to layers of snow accumulation. As a result, the colloidal silica particles tend toward a spherical shape as they increase in size.
Based on the above discussion, it may be difficult for larger particles, such as those observed in Exp. 5 and 6, to become highly morphologically modified silica particles like those with bent and/or branched structures described in the literature [
3].
This research focused on controlling not SPD, but PPD. Further research is necessary if more morphologically modified large particles are required. For example, adding a strong acid can reduce the amount of ASAS, thereby lowering the pH of the reaction mixture. At a sufficiently low pH of the reaction mixture, the concentration of silica would be insufficient to promote particle agglomeration. Therefore, the initial amount of water used as a solvent might need to be reduced, and/or the holding period in the acidic state used to promote agglomeration should be extended.
Finally, we considered the optimal timing of acid addition to facilitate particle growth while preventing the formation of new seed particles. Because acid produces a salt that destabilizes the colloidal silica, excessive acid addition is not advisable.
As mentioned above, the growth rate in the radial direction decreased as the surface area increased, which allowed the silica deposition rate to decrease. When the silica deposition rate and the silica accepting rate corresponding to the particle growth rate of the particle reached equilibrium, the formation of the seed particles was complete. The surface area of the seed particles must be sufficient to accept the added ASAS. However, because the surface area of each seed particle is unknown, we need to assume the PPD of the seed particle. If the PPD of the seed particle and the current PPD are assumed to be 2 and 18 nm, respectively, the corresponding surface areas can be calculated, as shown in
Table 8.
Thus, starting from the current particle, the silica deposition rate, which is thought to be proportional to the particle growth rate, could potentially be reduced by a factor of 1/64. To confirm this assumption, in Exp. 9 and 10, acid was not added from the second preparation of ASAS onward, as noted in the footnote of
Table 6. The acid concentration for the first preparation was 3.20 mmol/l, leading to a calculated relative particle growth rate of 33, as shown in the equation in
Figure 5. Therefore, the silica deposition rate should have decreased by a factor of 1/33. As a result, even without acid, no small particles were observed in the FE-SEM images in
Figure 6, supporting the validity of the assumption.
If small spherical particles are observed, it would suggest that the assumed diameter of the seed particles is too small.
Therefore, the addition of acid to control PPD is only necessary for the first preparation of ASAS. From the second preparation of ASAS onward, the addition of acid is not always required, and its amount can be drastically reduced to avoid the accumulation of undesired salts. However, as mentioned earlier, acid plays a role in stabilizing ASAS, so the addition of a small amount of acid is recommended. The addition of acid can also improve particle monodispersity due to the increased uniformity of ASAS.
Conversely, if the acid concentration is low, rather than reducing the acid amount, the addition rate of ASAS can be accelerated, although this is restricted by the ability to remove water through distillation.
In this research sulfuric acid was used as the acid. Other acids, including organic ones, can also be used. However, volatile acids such as hydrochloric acid are not preferable due to the possibility of unpredictable vapor phase reactions, corrosion, and other issues.