Abstract
Background and Objectives: Stereolithography (SLA) enables the creation of physical replicas of digital models, offering surgeons a realistic representation of patient anatomy. This technology improves diagnostics and surgical planning, facilitates communication within the medical team, and enhances the doctor–patient relationship by promoting compliance. This study aims to place the intervention addressed in a broader context and highlight the utility of SLA models in complex surgical scenarios. Case Presentation: The study presents two cases of patients with severe maxillary atrophy: a 67-year-old healthy male (with well-controlled hypertension) and a 72-year-old female patient (with hypercholesterolemia). Intervention and Outcome: Zygomatic implant procedures with immediate loading were performed to resolve the bone atrophy in both cases. These procedures heavily leveraged preoperative planning using stereolithographic models. Both interventions resulted in a positive outcome, which was confirmed at the 6-month follow-up. Conclusions: The integration of stereolithographic models into the preoperative planning process improved the prognosis of these complex cases, confirming their value in managing severe maxillary atrophy.
1. Introduction
Oral and Maxillofacial Surgery, given its inherently complex and delicate nature, necessitates meticulous preoperative planning to minimize risks and maximize benefits for the patient. A fundamental advancement in this field was marked by the advent of 3D imaging and modelling technologies starting in the 1980s and 1990s [,]. Specifically, Computer-Aided Design/Computer-Aided Manufacturing (CAD/CAM) software enables the development of personalized treatment plans and the simulation of surgical procedures, thereby improving the visualization of anatomical structures and enhancing surgical precision [,].
The process begins with the acquisition of Computed Tomography (CT) data in DICOM (Digital Imaging and Communications in Medicine) format, from which CAD/CAM software generates high-definition three-dimensional digital models. Stereolithography (SLA), a key rapid prototyping technique, then allows these digital models to be transformed into tangible physical replicas []. Within the medical domain, this technology offers surgeons a realistic representation of the patient’s anatomy, which facilitates a deeper understanding of the craniofacial region and optimizes preoperative strategies [,]. As emphasized by Cutting, the integration of CAD/CAM design and stereolithography contributes significantly to improving both diagnostic accuracy and surgical precision [,].
The adoption of this technique has yielded numerous clinical advantages. Stereolithographic (SLA) models permit accurate visualization of the patient’s anatomy, which is crucial for developing a precise diagnosis and detailed surgical planning. Simulating the surgery on a physical model allows for the reduction in intraoperative complication risk and the anticipation of potential technical difficulties. Furthermore, SLA models are instrumental in creating custom surgical guides, accurately assessing bone defects for regenerative surgery, and designing custom prostheses and devices [,,]. The use of these models actively facilitates communication and collaboration among members of the medical team, thereby promoting a necessary multidisciplinary approach to treatment. They also represent a valuable communication tool for the patient, empowering them to better understand the proposed treatment plan and provide more informed consent [,].
This study aims to analyze the utility of SLA models in oral and maxillofacial surgery, specifically focusing on the importance of accurate planning for achieving a positive prognosis. Further objectives include promoting enhanced communication within the surgical team and strengthening the doctor–patient relationship.
2. Case Presentation
2.1. Fabrication of Stereolithographic Models
The fabrication of a SLA model for surgical planning commences with the acquisition of DICOM files derived from the patient’s Cone-Beam Computed Tomography (CBCT) scan using dedicated software. This data is subsequently processed using specialized software to create the digital SLA model by isolating and segmenting the specific area of interest. The finalized digital model is then transmitted to a 3D printer, where a photosensitive liquid resin is polymerized layer by layer according to the digital file’s specifications, thereby solidifying the resin into the desired anatomical shape. The model fabrication utilized a LightBuilder 4K 3D printer. The resin employed for the anatomical models was Pro Model, while Bio-Med Clear resin was used for the creation of the bone-supported surgical guides. Upon completion of the printing process, the physical model is carefully removed, cleaned, polished if required, and finally sterilized.
2.2. Clinical Application and Methodology
This two-case report presents the results of managing two patients afflicted with severe maxillary atrophy. Each patient underwent comprehensive clinical examinations and radiographic assessments, including both panoramic radiography and CBCT. The SLA models were then specifically created using Software Suite RealGUIDE™, RealGUIDE 5.4 version (https://www.realguide.com/it/home, accessed on 2 July 2025), which is designed to recognize CT scans in DICOM format with a layer thickness set at 0.5 mm. These scans were subsequently processed by a rapid prototyping program, which sectioned the digital model into successive layers, typically 0.5 mm thick. The 3D printing machine utilized this sliced data to construct the physical model layer by layer, ensuring each layer was perfectly bonded to the previous one.
2.3. Case 1
A 67-year-old male, currently undergoing treatment for hypertension, presented for examination. Clinical and radiographic assessments confirmed severe maxillary atrophy, precluding the placement of implants using conventional techniques. Subsequent CT scans with 3D reconstruction corroborated the initial diagnosis and facilitated the creation of an SLA model.
This physical model was instrumental in accurately visualizing the patient’s intricate anatomical condition and establishing a definitive treatment plan. Furthermore, the SLA model was employed to clearly explain both the diagnosis and the rationale for the chosen treatment to the patient, thereby aiding the informed consent process.
The treatment involved an immediate loading Hybrid Zygoma procedure, performed under general anesthesia. The protocol included the placement of two zygomatic implants and two axial implants in the premaxilla. Crucially, the SLA model was utilized for a comprehensive pre-surgical simulation of the surgical site preparation phase immediately preceding the actual surgery on the patient. Following this simulation, a bone-supported surgical guide was employed. Once positioned in the surgical field, the guide accurately delineated the precise trajectory for both the osteotomy preparation and the subsequent implant insertion.
The patient experienced no immediate post-operative issues. The 6-month postoperative follow-up confirmed the stability of all implants, with no complications reported (Figure 1).
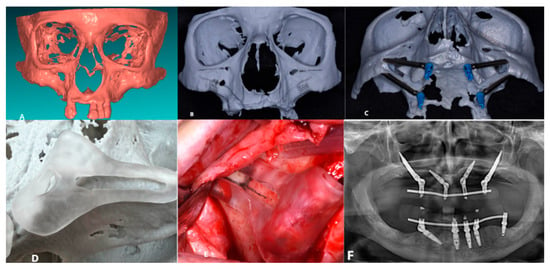
Figure 1.
(A) Creation of the SLA model; (B) Pre-operative study of the patient’s anatomy; (C) Pre-operative simulation of the implant preparation surgical procedure; (D) SLA and surgical guide; (E) Bone-supported surgical guide; (F) 6-Month Follow-Up.
2.4. Case 2
A 72-year-old female patient, who was being treated for hypercholesterolemia, presented for a comprehensive clinical and radiographic examination. Orthopantomography (OPT) and CBCT confirmed severe atrophy of the upper maxilla, a condition that rendered conventional implant placement unfeasible. Subsequent CT scans with 3D reconstruction corroborated the diagnosis and enabled the creation of an SLA model.
This physical replica was crucial for thoroughly visualizing and studying the patient’s anatomical condition, facilitating the establishment of the most suitable, problem-specific treatment plan. The model was also utilized to explain the detailed diagnosis and the proposed treatment plan to the patient.
Consequently, an immediate loading Quad Zygoma procedure was scheduled and performed under general anesthesia. The surgical protocol involved the insertion of four zygomatic implants and one axial implant in the premaxilla. The SLA model proved fundamental during the intraoperative phase; specifically, following the elevation of the full-thickness mucoperiosteal flap, the model was used to accurately identify and confirm the position of critical anatomical structures, such as the infraorbital nerve and the anterior wall of the maxilla. This preliminary study, along with the prior simulation of the surgical site preparation, was essential for the successful outcome of the surgery and the precise positioning of the implants as planned during the preoperative phase.
The patient reported no post-operative issues, which was substantiated by the findings at the 6-month follow-up (Figure 2).
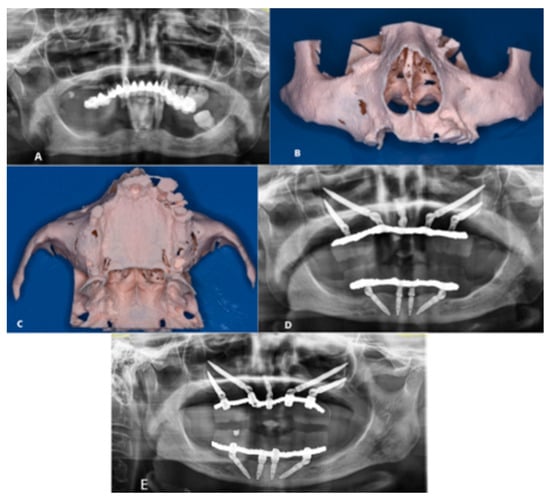
Figure 2.
(A) Pre-operative OPT; (B) Anterior view of the SLA model; (C) Inferior view of the SLA model; (D) Post-operative OPT; (E) 6-Month Follow-Up.
3. Intervention and Outcome
Technological advancements in imaging, notably the introduction of CBCT and 3D printing, have radically revolutionized the field of Oral and Maxillofacial Surgery. These innovations provide clinicians with highly effective tools for the diagnosis, planning, and precise execution of surgical procedures. Specifically, SLA models represent an invaluable resource during both the pre- and intra-operative phases, allowing for the accurate visualization of patient anatomy and directly contributing to an improved prognosis, encompassing both aesthetic and functional outcomes.
As highlighted extensively in the literature, SLA models find application across various domains of Oral and Maxillofacial Surgery, including the planning of complex procedures and the detailed visualization of anatomical structures adjacent to the surgical site []. SLA models have proven particularly effective in planning and executing corrective procedures for maxillofacial defects resulting from trauma or pathology. The direct visualization of anatomical structures facilitates surgical planning tasks such as defining incision sites, delineating resection margins, assessing bone defects, pre-adapting reconstruction plates, and fabricating custom prostheses. Notably, SLA models play a significant role in the rehabilitation of oncological patients, where they enable precise planning of reconstruction following tumor resection and the creation of custom prostheses, thereby improving the overall quality of life [,].
The usefulness of SLA models in preoperative planning is extensively documented, particularly concerning implant placement [,]. The ability to accurately assess patient anatomy, including critical relationships with structures such as the maxillary sinus and the inferior alveolar nerve, allows for the optimization of implant type, size, and angulation, which significantly reduces the risk of intraoperative complications [,]. The application of stereolithography, as supported by the present cases, is exceptionally beneficial in instances of severe maxillary atrophy. In the surgical procedures discussed in the previous section, the pre-operative study—comparing radiographs directly with the 3D model—allowed operators to accurately identify and visualize the anatomical portions of interest. Furthermore, the SLA model not only permits accurate structural study but also the simulation of the surgical procedure, which helps anticipate potential difficulties that might be encountered []. Accurate surgical planning, supported by SLA models, consequently minimizes both the duration of the surgery and the risk of complications, promoting a better prognosis []. Studies by Chiarelli and Chen support the effectiveness of SLA models in guided implantology, enabling greater precision in implant positioning, orientation, and depth through the use of bone-supported surgical guides created directly on the SLA model [,]. The surgical guide, as demonstrated in Case No. 1, proved essential in facilitating and respecting the implant trajectory during surgical site preparation. Similarly, the specific advantages offered by SLA models in pterygoid implantology transform the complexity of this procedure into a more controlled and predictable process, ensuring maximum precision in implant placement, allowing for the creation of custom guides, and improving patient communication [,].
Beyond the planning phase, the utility of SLA models extends to professional training and communication, both among team members and between patient and clinician. The patient’s ability to physically interact with a replica of their own anatomy facilitates their understanding of the chosen treatment plan, improving compliance and reducing preoperative anxiety [,]. For clinicians, SLA models offer a valuable opportunity to simulate procedures and gain experience in a controlled environment [].
Despite these numerous advantages, it is important to acknowledge the inherent limitations of stereolithography. The cost of the technology, although decreasing, can still pose an obstacle. The production of models requires time, and their accuracy is fundamentally dependent on the quality of the initial CBCT images and the precision of the printing process. Therefore, the use of high-quality CBCT data, reliable processing software, and biocompatible resins with high dimensional stability remains paramount [,,]. Furthermore, the effective use of SLA models and planning software necessitates a certain level of experience and an associated learning curve for both the surgeon and the technician [].
Future prospects for stereolithography in the medical field are highly promising. Increased use of biocompatible materials for printing definitive prostheses and the development of dynamic models that simulate tissue movement are anticipated. The integration of Augmented Reality (AR) could allow surgeons to visualize SLA models superimposed on the patient’s anatomy during surgery, further enhancing accuracy and safety. Additionally, Artificial Intelligence (AI) is expected to automate several steps in the design and production workflow, ultimately making the technology more accessible and efficient. Current research is also focused on developing new resins with improved mechanical and biological properties []. Furthermore, the integration of advanced materials and therapeutic nanotechnology is a growing frontier, particularly in oral oncology. For instance, research is progressing on the application of metal-based inorganic nanocrystals for biological Sonodynamic Therapy (SDT) []. SDT is a non-invasive treatment that utilizes ultrasound (which possesses high tissue penetration) to activate sonosensitizers (like these nanocrystals) to produce cytotoxic Reactive Oxygen Species (ROS) to eradicate tumor and bacterial cells []. The development of such precise, deep-penetrating therapies could one day complement the highly accurate surgical planning achieved with SLA models, particularly in the management of complex maxillofacial tumors. Advances in Augmented Reality (AR) are, in parallel, transforming craniofacial reconstruction by further improving accuracy, planning, and prognosis; AR integrates three-dimensional imaging with real-time overlays, allowing surgeons to visualize and interact with the patient’s specific anatomy throughout each stage of the surgical process. Preoperative planning benefits significantly from AR’s ability to generate dynamic simulations of procedures, enabling surgeons to anticipate challenges and refine techniques [].
4. Conclusions
This case report firmly confirms the value of SLA models in oral surgery, particularly for resolving complex implant planning stages and facilitating intraoperative procedures. Furthermore, these models serve an essential role in patient education, enhancing the patient’s comprehension and awareness of the proposed treatment, which ultimately improves compliance. As demonstrated, the use of SLA models also significantly facilitates communication and collaboration among colleagues, allowing the surgical team to simulate procedures, thereby increasing the precision and accuracy of the intervention and simultaneously reducing operating times. Despite the known limitations pertaining to the initial cost and the necessity of a learning curve, SLA models, when produced from high-quality CBCT images and a precise printing process, represent a revolutionary and sound investment for the future of oral and maxillofacial surgery.
Author Contributions
Conceptualization, A.G. and G.C. (Giulia Caporro); methodology, A.G.; investigation, S.R.; resources, G.C. (Giulia Caporro); data curation, A.G. and S.R.; writing—original draft preparation, A.G., S.R. and G.C. (Giulia Ciciarelli); visualization, A.C. and M.A.; supervision, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it is a retrospective case report that does not involve any intervention or prospective data collection, in accordance with the local regulations (GDPR—EU Regulation 679/2016 and the Code regarding the protection of personal data).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abe, M.; Tabuchi, K.; Goto, M.; Uchino, A. Model-based surgical planning and simulation of cranial base surgery. Neurol. Med.-Chir. 1998, 38, 746–750; discussion 750–751. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.; Brown, J.M.; Connell, M.; Craven, C.M.; Efford, N.D.; Radjenovic, A.; Smith, M.A. Preliminary experience with medical applications of rapid prototyping by selective laser sintering. Med. Eng. Phys. 1997, 19, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Nayar, S.; Bhuminathan, S.; Bhat, W.M. Rapid prototyping and stereolithography in dentistry. J. Pharm. Bioallied Sci. 2015, 7 (Suppl. S1), S216–S219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehra, P.; Miner, J.; D’Innocenzo, R.; Nadershah, M. Use of 3-d stereolithographic models in oral and maxillofacial surgery. J. Maxillofac. Oral Surg. 2011, 10, 6–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cutting, C.B.; Bookstein, F.L.; Grayson, B.; Fellingham, L.; McCarthy, J.G. Three-dimensional computer-aided design of craniofacial surgical procedures: Optimization and interaction with cephalometric and CT-based models. Plast. Reconstr. Surg. 1986, 77, 877–885. [Google Scholar] [CrossRef]
- Cutting, C.; Grayson, B.; Bookstein, F.; Fellingham, L.; McCarthy, J.G. Computer-aided planning and evaluation of facial and orthognathic surgery. Clin. Plast. Surg. 1986, 13, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; ArunVignesh, K.R.; Krishnakumar, R.; Ananthanarayanan, V.; Muthalagappan, P.L. Preoperative Assessment for Surgical Accuracy in Maxillofacial Surgery Cases Using Stereolithographic Models. Cureus 2024, 16, e60233. [Google Scholar] [CrossRef]
- Mukhtarkhanov, M.; Perveen, A.; Talamona, D. Application of Stereolithography Based 3D Printing Technology in Investment Casting. Micromachines 2020, 11, 946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, C.C.; Ishikawa, M.; Maida, T.; Cheng, H.C.; Ou, K.L.; Nezu, T.; Endo, K. Stereolithographic Surgical Guide with a Combination of Tooth and Bone Support: Accuracy of Guided Implant Surgery in Distal Extension Situation. J. Clin. Med. 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Urso, P.S.; Barker, T.M.; Earwaker, W.J.; Bruce, L.J.; Atkinson, R.L.; Lanigan, M.W.; Arvier, J.F.; Effeney, D.J. Stereolithographic biomodelling in cranio-maxillofacial surgery: A prospective trial. J. Cranio-Maxillofac. Surg. 1999, 27, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Al-Momani, Z.; Hodson, N.; Nixon, P.; Mitchell, D. Computerized tomography, stereolithography and dental implants in the rehabilitation of oral cancer patients. Dent. Update 2013, 40, 564–566, 569–570, 573–574 passim. [Google Scholar] [CrossRef] [PubMed]
- Sinn, D.P.; Cillo, J.E., Jr.; Miles, B.A. Stereolithography for craniofacial surgery. J. Craniofacial Surg. 2006, 17, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Matsuda, S.; Ohba, S.; Minegishi, Y.; Nakai, K.; Fujieda, S.; Sano, K. Stereolithographic model-assisted reconstruction of the mandibular condyle with a vascularized fibular flap following hemimandibulectomy: Evaluation of morphological and functional outcomes. Oncol. Lett. 2017, 14, 5471–5483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.; Yuan, J.; Wang, C.; Huang, Y.; Kang, L. Modular preoperative planning software for computer-aided oral implantology and the application of a novel stereolithographic template: A pilot study. Clin. Implant. Dent. Relat. Res. 2010, 12, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K. Zygomatic implant analogue simulation surgery and stereolithographic 3D models. Br. J. Oral Maxillofac. Surg. 2023, 61, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Sykes, L.M.; Parrott, A.M.; Owen, C.P.; Snaddon, D.R. Applications of rapid prototyping technology in maxillofacial prosthetics. Int. J. Prosthodont. 2004, 17, 454–459. [Google Scholar] [PubMed]
- Chiarelli, T.; Franchini, F.; Lamma, A.; Lamma, E.; Sansoni, T. From implant planning to surgical execution: An integrated approach for surgery in oral implantology. Int. J. Med. Robot. 2012, 8, 57–66. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, M.; Orsijena, A.; Franco, R.; Chiacchia, M.; Jahjah, A.; Capogreco, M. Clinical achievements of implantology in the pterygoid region: A systematic review and meta-analysis of the literature. J. Stomatol. Oral Maxillofac. Surg. 2024, 125 (Suppl. S2), 101951. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, S.; Capogreco, M.; D’Amario, M.; Falisi, G.; Severino, M.; Iacomino, E. Pterygoid implants: A viable alternative for the rehabilitation of the posterior sectors of the atrophic maxilla. Oral Implantol. 2024, 16, 38–43. [Google Scholar] [CrossRef]
- Tavassol, F.; Gellrich, N.C. Kompetenz und Kommunikation bei der Umsetzung computergestützter chirurgischer Planung [Competence and communication in the implementation of computer-assisted surgical planning]. De Chir. 2021, 92, 194–199. (In German) [Google Scholar] [CrossRef] [PubMed]
- Sidhom, M.; Zaghloul, H.; Mosleh, I.E.; Eldwakhly, E. Effect of Different CAD/CAM Milling and 3D Printing Digital Fabrication Techniques on the Accuracy of PMMA Working Models and Vertical Marginal Fit of PMMA Provisional Dental Prosthesis: An In Vitro Study. Polymers 2022, 14, 1285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Y.K.; Yau, H.T.; Wang, I.C.; Zheng, C.; Chung, K.H. A novel dental implant guided surgery based on integration of surgical template and augmented reality. Clin. Implant. Dent. Relat. Res. 2015, 17, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, J. Metal-based inorganic nanocrystals for biological sonodynamic therapy applications: Recent progress and perspectives. Rare Met. 2023, 43, 413–430. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).