Abstract
Objective: The purpose of this scoping review was to identify gaps in the literature and summarize findings from studies examining the use of silicon-, silica-, and silicate-based toothpastes for the remineralization and repair of mineralized tooth tissues. Methods: A 10-year literature search was conducted using PubMed and Scopus, adhering to PRISMA 2020 guidelines. A total of 331 studies were initially identified, with 56 full-text review articles. After selecting the manuscripts, 27 studies were qualitatively analyzed by four reviewers, focusing on the results of both in vivo and in vitro methods. Results: The findings suggest that toothpastes containing silicon, silica, and silicate demonstrate promising results for remineralization and enamel repair, with evidence of mineral layer formation and/or deep enamel surface remineralization under various conditions. Additionally, the use of these toothpastes can lead to the obliteration of dentinal tubules within a few days. The results collectively support the efficacy of these toothpastes in enamel repair. Most of the clinical studies focused on dentine hypersensitivity, followed by white spot lesions. Conclusions: Silicon-, silica-, and silicate-based toothpastes (bioactive Si-toothpastes) can be considered effective based mostly on laboratory studies. There remains a need for more in vivo research studies on enamel and dentin mineral repair. Existing studies provide strong evidence that these technologies can reduce dentin hypersensitivity and promote enamel–dentin repair.
1. Introduction
The mechanisms underlying the remineralization of dental hard tissues are now increasingly well-understood. It is also important to recognize the importance of fluoride (F−) in this process, particularly when fluoride, in its ionic form, is available in toothpastes and other dental products [,]. Fluoride-containing toothpastes have been regarded as one of the most important strategies for dental caries prevention and for the caries decline in many countries. Therefore, fluoride-based strategies remain the standard for caries prevention [].
However, dental caries persists as a major public health problem in many parts of the world, and new approaches for the remineralization of dental hard tissues have been proposed in the last decade [,,,,,]. These technologies can be divided into two major groups: (a) the non-fluoride remineralization technologies (biomimetic enamel regenerative formulations) that claim to achieve a deeper remineralization of lesions; and (b) the group of products that enhance fluoride efficacy, known as F-boosters [].
A straightforward simple strategy to enhance fluoride efficacy and its bioavailability in the oral cavity would be to just add more fluoride into oral care products. However, this approach can raise concerns about the potential risk for dental fluorosis, as some children are exposed to multiple sources of fluoride. Moreover, a higher amount of fluoride in the mouth might not be efficacious for controlling erosive tooth wear and other oral complex conditions, such as MIH (molar incisor hypomineralization), associated with hypersensitivity [,,]. In this manner, non-fluoride remineralization technologies and F-boosters can be interesting approaches for consumers that avoid F-toothpastes and for those who prefer low F− in oral care products [,].
The incorporation of ions into the hydroxyapatite (HA) structure directly influences its physical, chemical, and physiological properties and, consequently, the tissue mineralization process. In general terms, demineralization is the removal of mineral ions from hydroxyapatite crystals of hard tissues (e.g.,: enamel, dentin, cementum, and bone). Remineralization is the opposite process and can be enhanced by substituting ionic species in the sites of the hydroxyapatite molecule []. The presence of substitute ions, either incorporated within the apatite lattice or only adsorbed on the surface, can modify the solubility of a dentin/enamel structure. These substitute ions could be anions such as fluoride and silicon.
The remineralization process can occur within the demineralized tooth structure as a natural repair event. Basically, remineralization is a net mineral gain since calcium (Ca2+) and phosphate (PO43−) ions are deposited into the crystal voids of the demineralized tooth structure [,].The incorporation of Ca2+ and PO43− ions into the crystal lattice can be enhanced by the presence of free fluoride (F−) ions in the oral environment. This process can result in an apatite that is significantly more resistant to a subsequent acid challenge [].
While studies on remineralization modulated by fluoride ions are already well studied, there are still gaps regarding the mechanisms of remineralization orchestrated by silicon. Each ionic grouping of apatite, namely calcium, phosphate, and hydroxyl, can be replaced by another ionic grouping of the same or different valence. In bones, the sites of hydroxyl (OH) and phosphate (PO4) of hydroxyapatite (HA) are noted as A and B, respectively. Hence, fluoride ions (F−) can substitute hydroxyls in site A to form Ca10(PO4)6(OH)2−2xF2x, whereas (SiO4)4− operates in site B. This substitution in site B will produce Ca10(PO4)6−x(SiO4)x(OH)2−x chemical structures [,].
Contrary to fluorine, silicon is a relatively inactive chemical element. However, these elements cannot be found in their isolated pure form in nature and both can form bioactive molecules. Silicon is a half-metallic element that reacts with oxygen to form silicon dioxide at high temperatures (SiO2). In this form, the (SiO4)4− anions are versatile tetrahedral molecules that can be connected in different ways and may become bioactive. This natural form of silicon dioxide is also known as “silica” and can be found in different crystalline or amorphous forms. Silicates occur when silicon dioxide is bound to other elements (e.g., calcium, aluminum, iron, and magnesium) and can be defined as salts []. All forms (silicon ion, silica, and silicate) can be of great importance in dental products.
Toothpastes are regarded as one of the most complex healthcare products to produce []. It is a mixture of abrasives suspended in an aqueous humectant phase to form a hydrocolloid material that active ingredients will be incorporated into. Furthermore, this material will form a slurry in the oral cavity, a mixture of saliva and denser solids from toothpastes. On one hand, saliva will facilitate the dispersion of ingredients in the oral cavity. On the other hand, saliva will dilute all toothpaste ingredients, including the bioactive ones. Finally, the bioactive ingredients must be stable in different pH environments.
The current knowledge in this field is certainly mostly based on laboratory experimental studies. Probably, there is a lack of data on treatment outcomes since few randomized clinical trials (efficacy studies) were carried out for F-boosters. Moreover, no insights coming from longitudinal effectiveness studies (real life studies) is expected.
The aims of this scoping revision were to identify gaps in the literature and summarize findings from several studies related to silicon-, silica-, and silicate- toothpastes that can be used for tooth remineralization and the repair of mineralized tissues, such as dental caries, molar incisor hypomineralization (MIH), and erosive tooth wear. It is also relevant to update information on this topic because there are many technologies claiming enamel–dentin repair. However, it appears that few clinical trials were carried out to provide robust evidence about the magnitude of the efficacy of these bioactive ingredients.
1.1. Enamel Regeneration or Repair: Background and Definitions
The term “regenerative dentistry” has several definitions that can overlap concepts and goals and raise some confusion. Regenerative dentistry and tissue engineering have been exploring at least three domains that aim to act as scaffolds to promote cell growth and differentiation for tissue regeneration/healing: stem cells, bioactive molecules, and biomaterials []. However, a direct approach to hard tissues brings into question whether this concept is suitable. Certainly, it is appropriate for bones and dentin, but for dental enamel, some additional thoughts are worth the effort.
Dental enamel is the only cell-free tissue in the human body, organized in a complex multilevel structure []. As a result, dental enamel cannot be biologically repaired or regenerated, and simply recovering the lost minerals may not be sufficient. The challenge lies in recreating the hierarchical structure on the surface of the damaged enamel, mimicking its original structure []. As for dentin, the concept of “healing” of the damaged tissue must include the organic portion. This challenging scenario is an open opportunity for a biomimetic approach within a broad perspective of the regenerative dentistry field.
Biomimetics in Dentistry was first proposed by Professor Stephen Mann in 1997: “…biomimetics concepts can be useful for the fabrication of biomaterial implants with controlled porosity and microstructure” []. The original idea was related to dental materials and was later expanded to other oral care products. Nevertheless, for both dental materials and toothpastes, the challenges continue: mature enamel, conversely, to dentin and bone, is acellular and does not resorb or remodel. Therefore, a true enamel regeneration cannot occur in vivo []; and considering how many toothpastes claim a full regeneration of enamel, the appropriate terminology for their beneficial effect is probably “enamel repair”. To justify the choice of the term “repair” instead of “regeneration”, one must bear in mind that these concepts are commonly used in soft tissue healing processes. A healing repair event takes place with a restored tissue with some failures or even scars from a previously damaged skin/mucosa. Whereas a regenerative healing process brings the idea of a complete restoration of the whole tissue architecture, function, and esthetics []. Transposing these definitions to hard tissue is of utmost importance to avoid some conflicting concepts.
It is relevant to point out that the concept of enamel regeneration is preserved when cell-based strategies, tissue engineering laboratory techniques, and similar methods are proposed. However, the focus of this review is the ion-based remineralization and repair of the enamel and dentin by the regular use of toothpastes.
There are more definitions that can raise confusion and need to be clarified, particularly for remineralization (e.g.,: functional remineralization, biomineralization, and biomimetic remineralization). Therefore, to clarify these terminologies and facilitate the rationale for using them, it is important to present a few definitions commonly used in these articles related to enamel/dentin mineral turnover.
Functional remineralization (FR) stands for remineralization that leads to improved tissue mechanics and functionality []. This is a close concept to enamel repair.
Biomineralization of hard tissues (enamel, dentin, and bone) involves the deposition of apatite mineral crystals within an organic matrix. Bone and teeth are regarded as biomaterials with unique biomechanical properties that are crucial to their function []. Hence, biomineralization is a complex series of events regulated by cells expressing matrix proteins that act as crystallization promoters or inhibitors [,]. As a result, the use of a bioactive agent for dental remineralization is not necessarily a biomineralization process.
Biomimetic mineralization (BIMIN) is another technique developed to stimulate the guided formation of a layer of fluorapatite that resembles enamel. This is conducted on a mineral substrate and has the potential to enhance the remineralization of superficial enamel and exposed demineralized dentin [].
Bioactive remineralization is a general term related to the net mineral gain of a demineralized dentin surface and the following clinical benefits promoted by bioactive agents: acid resistance (carious and erosive challenges), abrasion resistance (mechanical/masticatory wear), and surface impermeability (microleakage and hypersensitivity) [].
1.2. Silicon, Silica, and Silicate Toothpastes
In dentistry, silicon and silica are best known as hydrated silica SiO2·nH2O (silicon dioxide with a variable amount of water), which is used as the abrasive component of many brands of toothpastes. It is the abrasive of choice in clear gel formulations []. However, silicon, silica, and silicate can push forward and act as bioactive agents or compose a bioactive material for enamel/dentin remineralization or repair. Since the 1980s, a bioactive material has been explained as one that “elicits a specific biological response at the interface of the material that results in the formation of a bond between tissues and the material” [,,].
Bioactive glasses (BGs or BAG), also known as bioglasses, are a quaternary oxide system of SiO2–CaO–Na2O–P2O5. It is regarded as the first inorganic material with the ability to bond with living bone tissue and form a stable and tightly bound interface. In addition to bone regeneration, this material is highly versatile. It can deliver pharmaceutical compounds and be useful in many hard tissues engineering challenging conditions. Bioactive glass can mineralize dentine tubules to relieve tooth sensitivity. [,].
In the oral cavity, once interacting with saliva, bioglass particles trigger a complex phenomenon termed bioactivity. As a result, this ionic-rich bioglass environment fosters the creation of hydroxyapatite layers and calcium phosphate compounds (Ca3(PO4)2) within the tooth structure []. The formation of a crystalline hydroxyl carbonate apatite (HCA) layer is regarded as strong evidence of bioactivity in bones and other hard tissues []. Basically, a proposed mechanism of action of bioglass for remineralizing dental enamel relies on the following steps, as described by Dai et al. []: (a) the aqueous oral environment stimulates the exchange of sodium bioglass ions with hydrogen ions (H+); (b) calcium ions (Ca2+) in the particles as well as phosphate ions (PO43−) are released from the biomaterial; (c) a localized pH rise will allow the precipitates of calcium and phosphate ions, together with the ions from saliva, to form a calcium phosphate (Ca–P) layer on the hard tissue (lesion surface); (d) the silica network from the bioactive glass can react with the hydroxyl ions (OH−) from the aqueous solution and form soluble silanol compounds; (e) experimental observations indicate that the increase in Ca and P content would induce a decrease in the silicon content. Finally, the newly formed layer displays good resistance to abrasion and transforms into a hydroxyapatite layer, which is structurally like those of the original enamel and dentine [,,,]. It is important to keep in mind that this cycle has a continuum. Hence, it further increases calcium and phosphate ion concentrations and leads to the precipitation of calcium phosphate on the silica-rich layer [].
Bioglass® 45S5 was the first commercial bioglass. Later, other formulations were developed: Class A and Class B, with and without sodium. NovaMin® is a calcium–sodium–phosphatesilicate glass also described as amorphous sodium calcium phosphosilicate (SCPS) that has the ability of a consistently release calcium ions. To increase remineralization performance, a bioglass combined with fluoride and high phosphate content was developed: BiominF® []. This product seems to form a more stable and resistant layer. Fluoride is particularly relevant in improving the bioactivity for enamel protection by the formation of the more acid-resistant fluorapatite, rather than hydroxyapatite []. In addition, fluoride-bioglass may enhance the remineralization of dentin and decrease the risk of dentin-matrix degradation. A further beneficial effect of bioglass is the antimicrobial activity of this material.
Conversely to other technologies that operate in an alkaline milieu, REFIX dental regeneration technology is an acidified bioactive complex produced mainly from compounds containing silica and phosphates [,]. Upon contact with the oral environment, they ionize, and these particles bind to the tooth structure, capturing calcium particles available in the oral environment. The interaction of these elements forms layers of hydroxyapatite enriched with silicon. REFIX technology has proven to be effective in tooth remineralization/regeneration, depositing minerals in areas deficient in mineral structures. This action reduces tooth sensitivity and prevents dental caries and erosion from tooth wear [].
The evidence supporting the efficacy of these technologies for enamel repair and reducing dentin hypersensitivity is increasing. For instance, a recent clinical study comparing NovaMin (Sensodyne Repair & Protect, GlaxoSmithKline, Philadelphia, PA, USA) and REFIX Technology (Regenerador Sensitive, Dentalclean, Londrina, PR, Brazil) showed that both products reduced the tooth hypersensitivity reported by the volunteers []. Similar results were also observed in a pilot study performed with participants that report dentin hypersensitivity (DH) caused by root surface exposure or periodontal treatment [].
NR5™ technology is known for its combination of two compounds—sodium phosphate and calcium silicate. When they encounter the tooth surface during brushing, the combination of these compounds fosters the formation of a new mineral layer of hydroxyapatite, depositing minerals in areas lacking mineral structures. This action reduces tooth sensitivity and prevents tooth decay and erosion []. The producers of the NR5™ technology claim that, when the REGENERATE Advanced Toothpaste is used in combination with the serum, it boosts the toothpaste’s effectiveness, enhancing the power of enamel regeneration [,].
Table 1 presents the main commercial brands in the market. Note that pH can differ from these technologies suggesting that bioactive silicon, silica, or silicates do not operate in the same manner.

Table 1.
Characteristics of toothpastes in the market that use bioactive Si-compounds.
1.3. Rationale for the Review—Guiding Question
Silica is an impressive, versatile compound that can be modified for many purposes in toothpastes. Recently, a modified silica surface produced a promising mucoadhesive compound that may increase the retention of toothpaste particles in the oral cavity [].
Until now, there have been few publications concerning the effect of bioactive silicon on tooth enamel. Silicon and silica, along with calcium, phosphates, and fluoride induce the remineralization of demineralized tooth enamel and dentine in vitro [,,,,,].
Therefore, this issue needs updated information regarding the positive points and potential limitations regarding the silicon-, silica-, and silicate-toothpastes aiming for dental remineralization and other therapeutic uses. Thus, the guiding question of the review can be summarized as: “Is there supporting evidence about silicon-, silica-, and silicate-toothpastes efficacy for remineralization and/or enamel repair of teeth and relieving dentin hypersensitivity (DH)?”
2. Material and Methods
This scoping review was performed following the 2020 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for the Scoping Reviews Checklist []. This review was registered with the OSF Registries (registration DOI: https://doi.org/10.17605/OSF.IO/U2B8D, accessed on 12 August 2024).
A literature research of papers published in the last 10 years was carried out on PubMed and Scopus (21 July 2024), using the following search strategy:
“silicon” OR “silica” OR “silicate”
AND
“toothpaste” OR “dental gel”
AND
“bioactive” OR “biomimetics”
AND
“teeth”.
It is important to point out that only articles shedding light on bioactive toothpastes/dentifrices/gels were included [,,,,,,,,]. Articles presenting results about hydroxyapatites in these products were excluded unless a combination with silicon, silica, and/or silicate was present. The primary target was enamel–dentin repair. Additionally, the relevant literature was hand-searched and included if the criteria for testing these silicon/silica/silicate technologies for enamel/dentin repair were tested for: “dental caries”; “molar incisor hypomineralization”; “dentine hypersensitivity”; “dental erosion”.
All types of studies were selected (including reviews, systematic reviews, and meta-analyses) but not included for the scoping review. The final reading focused on the therapeutic potential of these technologies in two major categories: A: “in vitro” or “laboratory studies” and B: “in vivo” studies, or general clinical studies, or clinical trials. Table 2 presents the inclusion and exclusion criteria.

Table 2.
Inclusion and exclusion criteria used in this scoping review.
A total of 331 studies were imported from screening (148 from PubMed and 183 from Scopus) by F.C.S. After this was the removal of articles related to dental materials and implants or other issues (169) and duplicates (106 studies). F.C.S. and F.V. screened 56 articles (title and abstract). The final number of studies retrieved from the query was 27 (Figure 1). Twelve articles had their full text read by N.L.S.F. and A.F.B.O. in group A (in vitro studies). A.C.C.G. and G.B.M. read 15 full-text articles in group B (in vivo, clinical trials).
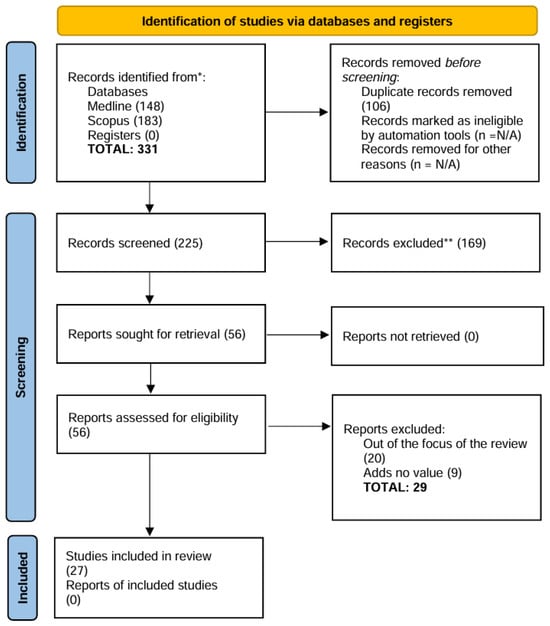
Figure 1.
PRISMA 2020 flow diagram. * Databases and registers only, no websites; ** No automation tools were used in the process.
3. Results
Qualitative and quantitative analyses were carried out considering the therapeutic potential of silicon, silica, and silicates in toothpastes. Technical and methodological strengths and weaknesses were also explored. However, there are significant methodological differences that need to be considered. From 12 in vitro experiments, half of them used human enamel or dentin specimens, while the other half used bovine blocks for pH cycling and subsequently characterization by different techniques (e.g., scanning electron microscopy (SEM) imaging, EDX elemental analysis, surface microhardness and roughness). The technologies investigated also included non-silicon- and non-silica-toothpastes, such as arginine sodium monofluorophosphate and casein phosphopeptide-amorphous calcium phosphate (CCP-ACP).
Among the in vivo clinical studies (n = 15), 11 were randomized clinical trials (RCTs). Only two of these RCTs were testing the effect on white spot lesions, whereas the others aimed at the reduction of pain due to dentin hypersensitivity.
In general, we observed a substantial heterogeneity of pH cycling methods and techniques for measuring mineral loss/gain in laboratorial in vitro studies. Conversely, the randomized clinical trials were quite homogenous with short studies mainly carried out to evaluate the same outcome: pain relief.
This work retrieved in vitro studies from Brazil (n = 5), the United Arab Emirates (n = 2) and many other nationalities of Europe, Asia, and North America. For in vivo studies, more than half of the studies were concentrated in India, UK, China, and Egypt.
Table 3 and Table 4 present the overall characteristics and details about the in vitro and in vivo studies with these technologies, respectively.

Table 3.
Characteristics of the included articles (in vitro studies).

Table 4.
Characteristics of included articles (in vivo studies).
4. Discussion
The lack of consensus on terminologies related to these Si-based technologies for remineralizing dental hard tissues was a survey problem, since hydrated silica is a common component of many toothpastes. As a result, the search query used for this scoping review eventually selected studies testing abrasives. In addition, remineralization is a frequently surveyed issue of research related to bioactive materials for repairing or regenerating bones and teeth.
A similar commercial and academic terminology may be useful to encompass technologies using silicon (Si), silica (SiO2), and silicate (Si2O5)2n−n in toothpastes. This could facilitate communication and differentiate these products from regular hydrated silica. This approach is very important since the borderline between biomimetic products and F-boosters is increasingly blurred []. Accordingly, a parallel outcome of this scoping review is the proposal for using the term “bioactive Si-toothpaste” for biomimetic or F-boosters technologies for enamel repair that use silicon (Si), silica (SiO2), and silicate (Si2O5)2n−n in toothpastes.
As a result of this terminology issue, it was quite challenging to summarize the main findings and to identify a major pattern among the in vitro and in vivo studies (Table 3 and Table 4). Another limitation of this scoping review is the difficulty inaccessing the quality of the laboratory studies due to the high heterogeneity of pH cycling methods and techniques for measuring loss/gain mineral in laboratory studies.
The major technologies investigated in the 27 articles selected for this review were: NovaMin®, BiominF®, REFIX Technology, and NR5™technology (with and without serum booster).
In vitro study articles evaluating the NovaMin and REFIX technologies followed similar methodologies, involving the preparation of bovine enamel blocks, demineralization to create caries-like lesions, pH cycling with treatment based on different toothpastes, and subsequent analysis with surface microhardness, scanning electron microscopy, and EDS [].
However, if we take a closer look at the methodological aspects, some differences are worth highlighting. While some researchers [,] used human teeth, others [,,,] used bovine teeth. This may influence the comparability of the results due to differences in the composition and structure of human and bovine teeth. However, the polishing and sample preparation procedures are similar between the articles using bovine teeth, ensuring a uniform surface for the tests.
Most of the articles used demineralization methods with acetate solutions to simulate caries lesions and pH cycling, ensuring controlled conditions for evaluating the effects of the dentifrices tested. However, some studies used brushing methods associated with pH cycling, which varied between manual brushing [] and electric brushing [,], which may impact the effectiveness of the remineralization and protection effects observed.
For sample analysis and characterization, scanning electron microscopy (SEM) and EDS are the most common methods, allowing a detailed comparison of the morphology and chemical composition of the samples after treatment. However, quantitative methods such as surface microhardness and enamel surface roughness [,] are important and deserve to be highlighted, as they provide more robust evidence on the clinical applicability of these composites.
Methodological differences are crucial for interpreting the results of each study, as they directly influence conclusions about the effectiveness of the technologies evaluated and the comparison of data []. When it comes to methodological rigor, we must consider certain aspects, such as clarity and the detail of the methodology, control of variables, reproducibility, data analysis and interpretation, validity, and reliability of the methods used. Thus, the studies [,,,], demonstrate greater methodological rigor due to the following points: preparation and polishing of detailed samples, which ensures uniformity of the specimens; standardized procedures for demineralization and pH cycling, ensuring controlled conditions for the creation of caries lesions; use of electric brushing [,], as it reduces variability and improves the reproducibility of results; SEM and EDS analyses, offering a detailed characterization of the morphology and chemical composition of dental tissues after treatments [].
The main differences found in the results of the studies evaluated are related to the effectiveness of the different toothpastes in remineralizing enamel and forming new “layers” of minerals. Each study observed variations, depending on the compounds tested, in the presence of elements such as silicon and fluoride, as well as in the hardness and roughness properties of the enamel and dentin surfaces after treatment.
REFIX technology induces the formation of a mineralized layer on both enamel and dentin. On enamel, this layer is approximately 6 µm thick and forms on the surface after pH cycling. In dentin, after brushing with the same toothpaste, a mineralized layer is formed, only less thick, up to 3 µm. After 7 days of treatment, partial obliteration of the dentinal tubules is observed [].
In terms of erosion prevention, enamel samples treated with REFIX exhibit a smoother surface and resistance to subsequent erosive challenges. Elemental mapping analyses with EDS indicate that there are no significant changes in the chemical elements present on the surface after the erosive challenge, confirming the robustness of the layer formed [].
NovaMin technology, in turn, induces the formation of a mineralized layer both on the dentin surface and inside the dentin tubules. The mechanism of action suggested for this dentifrice is through the release of calcium and phosphate when in contact with saliva, promoting remineralization and obliteration of the dentinal tubules [].
However, the results presented in the studies show that NovaMin technology was less effective in forming a new mineral layer on the surface enamel compared to REFIX. In addition, there was a significant reduction in the percentage weight of calcium in NovaMin-treated samples after erosive challenges, indicating a lower resistance and stability of the mineral content.
In short, depending on what the study sets out to analyze, REFIX stands out in terms of enamel surface remineralization and erosion prevention. However, when applied to dentin, both technologies promote the obliteration of the dentinal tubules, which can help control tooth sensitivity. However, the mineral deposition provided by REFIX forms a thicker and more resistant layer over time.
All in vitro studies support that a net mineral gain is achieved and an enamel repair is also observed depending on the technique used. These in vitro investigations support the beneficial effect of these bioactive Si-toothpastes which are related to the potential control of dental caries lesions, erosive tooth wear [,,,,,,,,,,].
The clinical efficacy of various bioactive Si-toothpastes has been extensively studied, with a particular emphasis on formulations containing sodium calcium phosphosilicate (SCPS) and related technologies. Clinical studies have consistently demonstrated the ability of SCPS to occlude dentinal tubules, thereby alleviating pain associated with dentin hypersensitivity (DH). For instance, Chałas et al. (2015) [] and Zang et al. (2016) [] observed significant pain reduction with smaller SCPS particles, which exhibited greater efficiency in tubule occlusion. Similarly, Sufi et al. (2016) [] reported a marked reduction in DH with the use of toothpastes containing 5% SCPS. Hall et al. (2017) [] further confirmed the superiority of SCPS over arginine/calcium carbonate in long-term treatments, although a work performed later found no significant differences when comparing smaller SCPS particles (2.5%) to arginine/carbonate formulations [].
Studies by Patel et al. (2019) [] and Bhowmik et al. (2021) [] have also highlighted the efficacy of bioglass-containing toothpastes in reducing DH, particularly the remineralizing effect of BioMin-F compared to popular brands like Colgate Sensitive Pro-Relief and Sensodyne Rapid Action []. In a clinical comparison of four different interventions for DH reduction, Majjiand Murthy (2016) [] concluded that SCPS-based pastes were the most effective, a finding consistent with other investigation that demonstrated significant DH reduction using a toothpaste containing REFIX technology [].
Additionally, Seong et al. (2020) [] conducted a randomized controlled trial evaluating a toothpaste with calcium silicate and sodium phosphate, finding a significant pain reduction compared to a control group using only fluoridated toothpaste. Gallob et al. (2017) [] also supported the efficacy of experimental toothpastes containing silica, silicate, and silicon in reducing DH.
In the treatment of white spot lesions, few clinical studies explored bioglass-based technologies, showing that these formulations not only alleviate DH but also promote the remineralization of enamel, particularly in orthodontic patients [,]. A further study demonstrated that combining NovaMin® with a fluoridated toothpaste significantly improved the remineralization of white lesions, highlighting the potential of sodium monofluorophosphate (SMFP) to enhance fluoride therapy’s efficacy [].
Surprisingly, this review retrieved no clinical studies about these bioactive Si-toothpastes for DH associated with molar incisor hypomineralization (MIH). MIH is a developmental enamel defect primarily diagnosed in childhood and adolescence, which can lead to compromised function of the affected teeth []. Characterized by increased porosity and dentin tubule exposure [], this defect can result in post-eruptive fractures, dentin hypersensitivity, and pain. These conditions can significantly impact patients’ oral health-related quality of life (OHRQoL) [,], including difficulties with mastication, particularly when consuming hot or cold foods and beverages []. Moreover, MIH can lead to psychosocial challenges, such as decreased self-esteem, embarrassment when smiling, and difficulties with socialization [].
Given these challenges, silica-, silicate-, and silicon-based technologies have shown promise in dental remineralization and the reduction of dentin hypersensitivity through dentin tubule obliteration. Therefore, these materials can alleviate pain, reduce dentin hypersensitivity, and, in turn, reduce the negative impact on OHRQoL.
In vitro and in vivo studies are currently underway to evaluate the efficacy of these remineralizing agents in teeth with MIH, particularly in children. These studies aim to assess the potential of toothpastes containing these technologies as adjuncts in the treatment and management of dentin hypersensitivity and their impact on the oral health-related quality of life of these patients.
The authors acknowledge some strengths and limitations of the included studies. For instance, enamel/dentin repair and remineralization was surveyed by many different techniques. However, the heterogeneity of the laboratory studies in this field might not be interpreted as a limitation since all studies provided consistent evidence in terms of in vitro mineral gain when testing bioactive Si-toothpastes. The in vivo studies were of short duration (4 weeks) testing pain relief. Despite the homogeneity of these clinical trials, there still is a need for building up efficacy from longer randomized clinical trials and effectiveness evidence from “real life studies”. Future in vitro and in vivo researchers must also consider testing these products for other therapeutic purposes such as white spot lesions, MIH, dental fluorosis.
Finally, it must also be pointed out that the mechanisms of action of bioactive Si-toothpastes in the market may differ since there are products with different technologies and with acidic or alkaline formulations (Table 1). The combination of silicon and derivates with other strategies will probably be the next frontier for new bioactive Si-toothpastes on the market.
5. Conclusions
This scoping review retrieved in vitro (laboratory) and in vivo studies that support the evidence about the beneficial effects of using silicon-, silica-, and silicate-toothpastes for enamel repair and remineralization.
The clinical studies were mostly of short duration, but support the efficacy of SCPS, bioglass, and innovative technologies like REFIX in occluding dentinal tubules and remineralizing enamel, offering long-lasting relief from DH. The choice of treatment should be tailored to the patient’s specific needs and the properties of the desensitizing agent used.
Author Contributions
Conceptualization, F.V.V. and F.C.S.; methodology, F.V.V., F.C.S., A.F.B.d.O., N.L.S.F., A.C.C.G. and G.B.M.; data curation, A.F.B.d.O., N.L.S.F., A.C.C.G. and G.B.M.; writing—original draft, F.V.V., F.C.S., A.F.B.d.O., N.L.S.F., A.C.C.G. and G.B.M.; review and editing, M.J.S.B., M.A.B.P. and P.H.P.D.; supervision, F.V.V. and F.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
All authors acknowledge the support of the Post graduation Program in Dentistry at the Federal University of Paraíba (UFPB) and the FORP/USP Graduate Program in Pediatric Dentistry for the academic support for N.L.S.F. (UFPB) and A.C.C.G. and G.B.M. (USP).
Conflicts of Interest
Author Fabiano Vieira Vilhena has been involved as a consultant for Dentalclean. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M. Enhancing fluoride: Clinical human studies of alternatives or boosters for caries management. Caries Res. 2016, 50 (Suppl. S1), 22–37. [Google Scholar] [CrossRef]
- Sampaio, F.C.; Bonecker, M.; Paiva, S.M.; Martignon, S.; Ricomini Filho, A.P.; Pozos-Guillen, A.; Oliveira, B.H.; Bullen, M.; Naidu, R.; Guarnizo-Herreno, C.; et al. Dental caries prevalence, prospects, and challenges for Latin America and Caribbean countries: A summary and final recommendations from a Regional Consensus. Braz. Oral Res. 2021, 35, e056. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.L.S.; Silva, J.; de Sousa, E.B.G.; D’Alpino, P.H.P.; de Oliveira, A.F.B.; de Jong, E.J.; Sampaio, F.C. Effectiveness of fluoride-containing toothpastes associated with different technologies to remineralize enamel after pH cycling: An in vitro study. BMC Oral Health 2022, 22, 489. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.S.; Patel, A.N.; Al Botros, R.; Snowden, M.E.; McKelvey, K.; Unwin, P.R.; Ashcroft, A.T.; Carvell, M.; Joiner, A.; Peruffo, M. Measurement of the efficacy of calcium silicate for the protection and repair of dental enamel. J. Dent. 2014, 42 (Suppl. S1), S21–S29. [Google Scholar] [CrossRef] [PubMed]
- Joiner, A.; Schäfer, F.; Naeeni, M.M.; Gupta, A.K.; Zero, D.T. Remineralisation effect of a dual-phase calcium silicate/phosphate gel combined with calcium silicate/phosphate toothpaste on acid-challenged enamel in situ. J. Dent. 2014, 42, S53–S59. [Google Scholar] [CrossRef] [PubMed]
- Vilhena, F.V.; Grecco, S.D.S.; Gonzalez, A.H.M.; D’Alpino, P.H.P. Regenerative and protective effects on dental tissues of a fluoride-silicon-rich toothpaste associated with a calcium booster: An in vitro study. Dent. J. 2023, 11, 153. [Google Scholar] [CrossRef]
- Pandya, M.; Diekwisch, T.G.H. Enamel biomimetics-fiction or future of dentistry. Int. J. Oral Sci. 2019, 11, 8. [Google Scholar] [CrossRef]
- Philip, N. State of the art enamel remineralization systems: The next frontier in caries management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef]
- Seong, J.; Newcombe, R.G.; Matheson, J.R.; Weddell, L.; Edwards, M.; West, N.X. A randomised controlled trial investigating efficacy of a novel toothpaste containing calcium silicate and sodium phosphate in dentine hypersensitivity pain reduction compared to a fluoride control toothpaste. J. Dent. 2020, 98, 103320. [Google Scholar] [CrossRef]
- Meyer, F.; Amaechi, B.T.; Fabritius, H.O.; Enax, J. Overview of calcium phosphates used in biomimetic oral care. Open Dent. J. 2018, 12, 406–423. [Google Scholar] [CrossRef] [PubMed]
- Vilhena, F.V.; de Oliveira, S.M.L.; Matochek, M.H.M.; Tomaz, P.L.S.; Oliveira, T.S.; D’Alpino, P.H.P. Biomimetic Mechanism of Action of Fluoridated Toothpaste Containing Proprietary REFIX Technology on the Remineralization and Repair of Demineralized Dental Tissues: An In Vitro Study. Eur. J. Dent. 2021, 15, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Arcos, D. Silicon substituted hydroxyapatites. A method to upgrade calcium phosphate based implants. J. Mater. Chem. 2005, 15, 1509–1516. [Google Scholar] [CrossRef]
- Ten Cate, J.M.; Buzalaf, M.A.R. Fluoride Mode of Action: Once There Was an Observant Dentist. J. Dent. Res. 2019, 98, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.R.; Huang, J.; Best, S.M.; Bonfield, W. Enhanced in vitro cell activity and surface apatite layer formation on novel silicon-substituted hydroxyapatites. In Proceedings of the 12th International Symposium on Ceramics in Medicine, Nam, Japan, 8–11 October 1999. [Google Scholar]
- Patel, N.; Best, S.M.; Bonfield, W.; Gibson, I.R.; Hing, K.A.; Damien, E.; Revell, P.A. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J. Mater. Sci. Mater. Med. 2002, 13, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Luhrs, A.K.; Geurtsen, W. The application of silicon and silicates in dentistry: A review. Prog. Mol. Subcell. Biol. 2009, 47, 359–380. [Google Scholar] [CrossRef]
- Lippert, F. An introduction to toothpaste—Its purpose, history and ingredients. Monogr. Oral Sci. 2013, 23, 1–14. [Google Scholar] [CrossRef]
- Thalakiriyawa, D.S.; Dissanayaka, W.L. Advances in Regenerative Dentistry Approaches: An Update. Int. Dent. J. 2024, 74, 25–34. [Google Scholar] [CrossRef]
- Luo, X.; Niu, J.; Su, G.; Zhou, L.; Zhang, X.; Liu, Y.; Wang, Q.; Sun, N. Research progress of biomimetic materials in oral medicine. J. Biol. Eng. 2023, 17, 72. [Google Scholar] [CrossRef]
- Volponi, A.A.; Zaugg, L.K.; Neves, V.; Liu, Y.; Sharpe, P.T. Tooth repair and regeneration. Curr. Oral Health Rep. 2018, 5, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Mann, S. The biomimetics of enamel: A paradigm for organized biomaterials synthesis. Ciba Found. Symp. 1997, 205, 261–269; discussion 269–274. [Google Scholar] [PubMed]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yoon, M.; Choi, K.Y. Approaches for Regenerative Healing of Cutaneous Wound with an Emphasis on Strategies Activating the Wnt/beta-Catenin Pathway. Adv. Wound Care 2022, 11, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Habelitz, S. Current developments on enamel and dentin remineralization. Curr. Oral Health Rep. 2019, 6, 257–263. [Google Scholar] [CrossRef]
- Moradian-Oldak, J.; George, A. Biomineralization of Enamel and Dentin Mediated by Matrix Proteins. J. Dent. Res. 2021, 100, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef]
- Moradian-Oldak, J. Protein-mediated enamel mineralization. Front. Biosci. 2012, 17, 1996–2023. [Google Scholar] [CrossRef]
- Singer, L.; Fouda, A.; Bourauel, C. Biomimetic approaches and materials in restorative and regenerative dentistry: Review article. BMC Oral Health 2023, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Devadiga, D.; Shetty, P.; Hegde, M.N.; Reddy, U. Bioactive remineralization of dentin surface with calcium phosphate-based agents: An in vitro analysis. J. Conserv. Dent. JCD 2022, 25, 93–97. [Google Scholar] [CrossRef]
- Darvell, B.W.; Smith, A.J. Inert to bioactive—A multidimensional spectrum. Dent. Mater. 2022, 38, 2–6. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Hench, L.L.; Forbers, D.; Bowman, L.S. History of bioceramics. Ceram. Int. 1982, 8, 131–140. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Al Berakdar, G.; Mkawi, E.M.; et al. Silica-Based Bioactive Glasses and Their Applications in Hard Tissue Regeneration: A Review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef]
- Prasad, P.S.; Pasha, M.B.; Rao, R.N.; Rao, P.V.; Madaboosi, N.; Ozcan, M. A Review on Enhancing the Life of Teeth by Toothpaste Containing Bioactive Glass Particles. Curr. Oral Health Rep. 2024, 11, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, D. Bioglass at 50—A look at Larry Hench’s legacy and bioactive materials. Biomed. Glas. 2019, 5, 178–184. [Google Scholar] [CrossRef]
- Dai, L.L.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. Mechanisms of Bioactive Glass on Caries Management: A Review. Materials 2019, 12, 4183. [Google Scholar] [CrossRef] [PubMed]
- El-Wassefy, N.A. Remineralizing effect of cold plasma and/or bioglass on demineralized enamel. Dent. Mater. J. 2017, 36, 157–167. [Google Scholar] [CrossRef]
- Ramadoss, R.; Padmanaban, R.; Subramanian, B. Role of bioglass in enamel remineralization: Existing strategies and future prospects—A narrative review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [PubMed]
- Vilhena, F.V.; Lonni, A.A.S.G.; D’Alpino, P.H.P. Silicon-enriched hydroxyapatite formed induced by REFIX-based toothpaste on the enamel surface. Braz. Dent. Sci. 2021, 24, 1–7. [Google Scholar] [CrossRef]
- Alonso, R.C.B.; Oliveira, L.d.; Silva, J.A.B.; Santos, W.B.B.d.; Ferreira, L.R.d.S.L.; Guiraldo, R.D.; Vilhena, F.V.; D’Alpino, P.H.P. Effectiveness of Bioactive Toothpastes against Dentin Hypersensitivity Using Evaporative and Tactile Analyses: A Randomized Clinical Trial. Oral 2024, 4, 36–49. [Google Scholar] [CrossRef]
- Zangrando, M.S.R.; Silva, G.F.F.; Bigotto, M.L.B.; Cintra, F.M.R.N.; Damante, C.A.; Sant’Ana, A.C.P.; Vilhena, F.V. Blocking tubules technologies for dentin hypersensitivity in periodontal patients—Pilot study. Res. Soc. Dev. 2021, 10, e35101320398. [Google Scholar] [CrossRef]
- Moras, C.G.; Acharya, S.R.; Adarsh, U.K.; Unnikrishnan, V.K. Regenerative biomineralization potential of commercially available remineralizing agents as a preventive treatment approach for tooth erosion—An in vitro laser-induced breakdown spectroscopy analysis. J. Conserv. Dent. JCD 2023, 26, 165–169. [Google Scholar] [CrossRef]
- Rahman, B.; El-Damanhoury, H.M.; Sheela, S.; Ngo, H.C. Effect Of Calcium Silicate, Sodium Phosphate, and Fluoride on Dentinal Tubule Occlusion and Permeability in Comparison to Desensitizing Toothpaste: An In Vitro Study. Oper. Dent. 2021, 46, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Mc Gowan, J.; Straus, S.; Moher, D.; Langlois, E.V.; O’Brien, K.K.; Horsley, T.; Aldcroft, A.; Zarin, W.; Garitty, C.M.; Hempel, S.; et al. Reporting scoping reviews-PRISMA ScR extension. J. Clin. Epidemiol. 2020, 123, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadou, D.; Eymael, D.; Hajhamid, B.; Carneiro, K.M.M.; Prakki, A. Chemical and Ultrastructural Characterization of Dentin Treated with Remineralizing Dentifrices. J. Funct. Biomater. 2024, 15, 25. [Google Scholar] [CrossRef]
- Tomaz, P.L.S.; Sousa, L.A.; Aguiar, K.F.; Oliveira, T.S.; Matochek, M.H.M.; Polassi, M.R.; D’Alpino, P.H.P. Effects of 1450-ppm fluoride-containing toothpastes associated with boosters on the enamel remineralization and surface roughness after cariogenic challenge. Eur. J. Dent. 2020, 14, 161–170. [Google Scholar] [CrossRef]
- Fernandes, N.L.S.; Juliellen, L.D.C.; Andressa, F.B.O.; D’Alpino, H.P.P.; Sampaio, C.F. Resistance against erosive challenge of dental enamel treated with 1,450-PPM fluoride toothpastes containing different biomimetic compounds. Eur. J. Dent. 2021, 15, 433–439. [Google Scholar] [CrossRef]
- Poggio, C.; Gulino, C.; Mirando, M.; Colombo, M.; Pietrocola, G. Preventive effects of different protective agents on dentin erosion: An in vitro investigation. J. Clin. Exp. Dent. 2017, 9, e7–e12. [Google Scholar] [CrossRef]
- Altan, H.; Goztas, Z.; Kahraman, K.; Kus, M.; Tosun, G. Inhibition Effects of Different Toothpastes on Demineralisation of Incipient Enamel Lesions. Oral Health Prev. Dent. 2019, 17, 179–185. [Google Scholar] [CrossRef]
- El-Damanhoury, H.M.; Elsahn, N.A.; Sheela, S.; Bastaty, T. In Vitro Enamel Remineralization Efficacy of Calcium Silicate-Sodium Phosphate-Fluoride Salts versus NovaMin Bioactive Glass, Following Tooth Whitening. Eur. J. Dent. 2021, 15, 515–522. [Google Scholar] [CrossRef]
- Chalas, R.; Wojcik-Checinska, I.; Zamoscinska, J.; Bachanek, T. Assessment of Pain Intensity in Patients with Dentin Hypersensitivity After Application of Prophylaxis Paste Based on Calcium Sodium Phosphosilicate Formula. Med. Sci. Monit. 2015, 21, 2950–2955. [Google Scholar] [CrossRef] [PubMed]
- Zang, P.; Parkinson, C.; Hall, C.; Wang, N.; Jiang, H.; Zhang, J.; Du, M. A Randomized Clinical Trial Investigating the Effect of Particle Size of Calcium Sodium Phosphosilicate (CSPS) on the Efficacy of CSPS-containing Dentifrices for the Relief of Dentin Hypersensitivity. J. Clin. Dent. 2016, 27, 54–60. [Google Scholar] [PubMed]
- Sufi, F.; Hall, C.; Mason, S.; Shaw, D.; Milleman, J.; Milleman, K. Efficacy of an experimental toothpaste containing 5% calcium sodium phosphosilicate in the relief of dentin hypersensitivity: An 8-week randomized study (Study 2). Am. J. Dent. 2016, 29, 101–109. [Google Scholar]
- Hall, C.; Mason, S.; Cooke, J. Exploratory randomised controlled clinical study to evaluate the comparative efficacy of two occluding toothpastes—A 5% calcium sodium phosphosilicate toothpaste and an 8% arginine/calcium carbonate toothpaste—For the longer-term relief of dentine hypersensitivity. J. Dent. 2017, 60, 36–43. [Google Scholar] [CrossRef]
- Fu, Y.; Sufi, F.; Wang, N.; Young, S.; Feng, X. An Exploratory Randomised Study to Evaluate the Efficacy of an Experimental Occlusion-based Dentifrice in the Relief of Dentin Hypersensitivity. Oral Health Prev. Dent. 2019, 17, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, E.; Pawar Chandrashekhar, D.; Sharma Hareesha, M. Comparative evaluation of fluorinol and calcium sodium phosphosilicate-containing toothpastes in the treatment of dentin hypersensitivity. Int. J. Dent. Hyg. 2021, 19, 421–428. [Google Scholar] [CrossRef]
- Arshad, S.; Zaidi, S.J.A.; Farooqui, W.A. Comparative efficacy of BioMin-F, Colgate Sensitive Pro-relief and Sensodyne Rapid Action in relieving dentin hypersensitivity: A randomized controlled trial. BMC Oral Health 2021, 21, 498. [Google Scholar] [CrossRef] [PubMed]
- Majji, P.; Murthy, K.R. Clinical efficacy of four interventions in the reduction of dentinal hypersensitivity: A 2-month study. Indian J. Dent. Res. 2016, 27, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Gallob, J.; Sufi, F.; Amini, P.; Siddiqi, M.; Mason, S. A randomised exploratory clinical evaluation of dentifrices used as controls in dentinal hypersensitivity studies. J. Dent. 2017, 64, 80–87. [Google Scholar] [CrossRef]
- Salah, R.; Afifi, R.R.; Kehela, H.A.; Aly, N.M.; Rashwan, M.; Hill, R.G. Efficacy of novel bioactive glass in the treatment of enamel white spot lesions: A randomized controlled trial. J. Evid. Based Dent. Pract. 2022, 22, 101725. [Google Scholar] [CrossRef]
- Hamdi, K.; Hamama, H.H.; Motawea, A.; Fawzy, A.; Mahmoud, S.H. Long-term evaluation of early-enamel lesions treated with novel experimental tricalcium silicate paste: A 2-year randomized clinical trial. J. Esthet. Restor. Dent. 2022, 34, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Mollabashi, V.; Heydarpour, M.; Farhadifard, H.; Alafchi, B. DIAGNOdent pen quantification of the synergy of NovaMin(R) in fluoride toothpaste to remineralize white spot lesions in patients with fixed orthodontic appliances: A double-blind, randomized, controlled clinical trial. Int. Orthod. 2022, 20, 100632. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, L.; Ebel, M.; Bekes, K.; Klode, C.; Hirsch, C. Influence of caries and molar incisor hypomineralization on oral health-related quality of life in children. Clin. Oral Investig. 2021, 25, 5205–5216. [Google Scholar] [CrossRef] [PubMed]
- Weerheijm, K.L. Molar incisor hypomineralisation (MIH). Eur. J. Paediatr. Dent. 2003, 4, 114–120. [Google Scholar] [PubMed]
- Jalevik, B.; Klingberg, G. Treatment outcomes and dental anxiety in 18-year-olds with MIH, comparisons with healthy controls—A longitudinal study. Int. J. Paediatr. Dent. 2012, 22, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Jälevik, B.; Sabel, N.; Robertson, A. Can molar incisor hypomineralization cause dental fear and anxiety or influence the oral health-related quality of life in children and adolescents?—A systematic review. Eur. Arch. Paediatr. Dent. 2022, 23, 65–78. [Google Scholar] [CrossRef]
- Raposo, F.; de Carvalho Rodrigues, A.C.; Lia, É.N.; Leal, S.C. Prevalence of Hypersensitivity in Teeth Affected by Molar-Incisor Hypomineralization (MIH). Caries Res. 2019, 53, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Rodd, H.D.; Graham, A.; Tajmehr, N.; Timms, L.; Hasmun, N. Molar Incisor Hypomineralisation: Current Knowledge and Practice. Int. Dent. J. 2021, 71, 285–291. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).