Abstract
Langerhans cell sarcoma (LCS) is a rare and aggressive neoplasm characterized by a clonal proliferation of Langerhans cells (LCs), with multi-organ involvement and poor prognosis. Diagnostic challenges arise from its rarity and overlapping features with Langerhans cell histiocytosis (LCH), requiring immunophenotypic and histological analysis for differentiation. This case report discusses a 67-year-old male with multi-organ LCS involvement. Diagnosis was confirmed via liver biopsy and genetic analysis, revealing a MAP2K1 mutation. Treatment with subcutaneous cladribine and dexamethasone resulted in significant clinical and radiological improvement, despite hematological toxicity due to an underlying myelodysplastic neoplasm (MDS). This case proves the potential efficacy of cladribine for disseminated LCS and highlights the necessity for further research into optimal therapeutic approaches for this rare malignancy.
1. Introduction
Langerhans cell tumors are classified by the World Health Organization (WHO) into Langerhans cell sarcoma (LCS) and Langerhans cell histiocytosis (LCH) [1].
LCS is a rare clonal neoplastic proliferation of Langerhans cells. Information regarding this condition is limited and primarily based on case reports, making it difficult to understand its epidemiology, clinical features, and prognosis [2]. LCS is characterized by an aggressive clinical behavior, multi-organ involvement, and cytological features typical of malignant tumors [3], such as atypia, hyperchromatic nuclei, prominent nucleoli, and frequent mitotic figures, resulting in rapid growth, local invasion, and the ability to recur and metastasize. Flow cytometry analysis documented specific immunophenotypic markers, including CD1a, S100, langerin (CD207), and, less frequently, CD68 and weak lysozyme expression. The presence of Birbeck granules under electron microscopy is pathognomonic for Langerhans cell origin [4].
The major limitations of this pathology are its rarity, an unclear pathogenesis, and generally poor prognosis. An observational study based on SEER data from 2000 to 2019 in the United States, published in Medicine in 2024, showed that patients with LCS were mostly over 60 years old and predominantly Caucasian, with a nearly equal male-to-female ratio [2].
LCS is based on a combination of cell surface marker expression and characteristic histological features [5]. Langerhans cells can be distinguished by their peculiar morphology, characterized by characteristic longitudinally grooved nuclei, Birbeck granules, and immunohistochemical profiles [6]. Immunohistochemical staining positive for CD1a, CD207 (langerin), and S-100 protein confirms the LCS diagnosis [5]. Due to its rarity, there is no established evidence regarding the most appropriate treatment. Surgery, radiotherapy, and chemotherapy have been used with variable results and unclear responses [7]. Here, we present a case of Langerhans cell sarcoma with multi-organ involvement.
2. Case Report
A 67-year-old male patient with a past medical history of ectasia of the celiac tripod, focal dissection of the inferior mesenteric artery, and previous transurethral resection of the prostate due to a history of benign prostatic hyperplasia presented to the emergency department in July 2023 with abdominal pain, headache, and fever. He underwent CT scans showing an enlarged liver with multiple focal lesions (DM max 4 cm) in all segments, an enlarged spleen with an intraparenchymal lesion measuring 7 × 8 × 6 cm with central liquefactive phenomena, and numerous supra- and subdiaphragmatic lymphadenopathies. The patient was transferred to our center and repeated blood tests which showed Hemoglobin 11.6 g/dL, White Blood Cells 13,900/µL, Neutrophils 12,420/µL, Lymphocytes 630, Platelets 470,000/µL, coagulation within normal range, elevated liver function indices (grade 1), and negative virological screening. A new CT scan revealed further liver enlargement with architectural distortion due to multiple hypodense, confluent lesions with blurred margins distributed throughout both liver lobes, the largest measuring 60 mm in segment S5. Enlarged lymph nodes were noted at the hepatic hilum (largest measuring 38 × 21 mm), compressing the portal vein and inferior vena cava. Additional lymphadenopathies were observed along the gastric lesser curvature, splenic hilum (44 × 23 mm), and median supradiaphragmatic area (30 × 16 mm). The spleen was enlarged, with a 14 cm long axis containing a large hypodense lesion with a central necrotic core (80 × 69 mm), infiltrating the splenic vein (Figure 1).
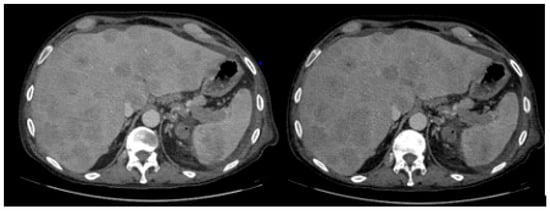
Figure 1.
Liver and spleen enlargement with architectural distortion due to multiple hypodense lesions.
The patient was admitted to the oncology ward and underwent a liver biopsy that revealed hepatic parenchyma almost entirely replaced by a malignant neoplasm with dendritic–histiocytic differentiation. The lesion consisted of pleomorphic giant cells with reniform nuclei, occasional grooves, prominent nucleoli, and the following immunophenotype: CD45+, CD68+, CD14+, CD4+, heterogeneous S100 (nuclear and cytoplasmic), CD1a+/−, Langerin+/−, Cyclin D1+/− (heterogeneous intensity). Numerous mitotic figures, including atypical ones, were present (Figure 2). The proliferative index (Ki67, clone MIB1) was 80%, and the BRAF gene mutation test was negative.
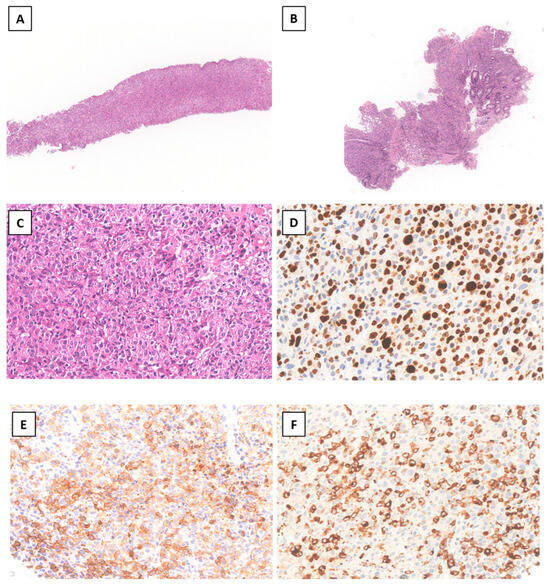
Figure 2.
Liver needle biopsy ((A) Hematoxylin–Eosin (H&E), original magnification (o.m.) × 50) and endoscopic colonic biopsy ((B) H&E o.m.× 50) showing diffuse infiltration by pleomorphic large tumor cells ((C), o.m. × 300) with high proliferation index ((D) o.m. × 400) and variable positivity for CD1a ((E) o.m. × 300) and langerin ((F) o.m. × 300).
Next-generation sequencing (NGS) of the liver biopsy identified a p.K57E MAP2K1 exon 2 mutation (VAF 38.35%). Colonoscopy with complete polyp excision was performed and revealed histiocytic/dendritic malignancy consistent with the liver biopsy lesions. Due to the histological findings, diagnosis of Langerhans cell sarcoma with hepatic, splenic, lymphatic, and intestinal involvement was made. Considering the presence of anemia and thrombocytopenia (grade 1), bone marrow aspiration and biopsy were performed. Cytology showed bilinear dysplasia with rare undifferentiated elements (<5%). Bone marrow biopsy excluded a histiocytic infiltration (CD1a negative) and confirmed the dysplasia seen at the aspiration. Cytogenetics revealed a normal karyotype, and NGS for myeloid neoplasm showed no high-risk alterations (only the DNMT3A mutation was detected, with a non-significant VAF). Thus, the patient was classified as low-risk myelodysplastic neoplasm (MDS) according to the Revised International Prognostic Scoring System (R-IPSS) and very low risk according to the Molecular International Prognostic Scoring System (M-IPSS).
Despite his poor general condition (the patient required parenteral nutrition, oxygen therapy, and antibiotics), treatment was promptly initiated based on the International Collaborative Treatment Protocol for Children and Adolescents with Langerhans Cell Histiocytosis. Subcutaneous cladribine (5 mg/m2 daily) combined with dexamethasone (10 mg for 5 days) was administered. The patient completed six cycles, with progressive improvement. Due to grade 4 thrombocytopenia, cycle 3 and cycle 5 were postponed. Treatment resumed once hematological toxicity resolved to grade 3.
End-of-therapy imaging showed a significant reduction in the liver lesions (only one 2 × 1 cm lesion remaining) (Figure 3), a complete resolution of the previously described lymphadenopathies, and a reduction in the splenic lesion (30 × 20 mm vs. 55 × 40 mm). Liver function indices normalized. Hematological evaluation revealed persistent grade 2 anemia and thrombocytopenia, likely due to therapy and underlying MDS. The patient continued hematologic follow-up with periodic platelet transfusions. As of now, the patient is in good general condition, awaiting further evaluation of his Langerhans cell histiocytosis.
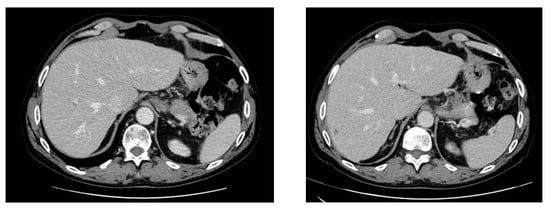
Figure 3.
Abdominal CT scan after the fifth and sixth cycles of subcutaneous cladribine treatment.
3. Discussion
LCS is a proliferative disorder of cells with morphological and phenotypic characteristics of Langerhans cells [8]. It may develop de novo or as progression from prior Langerhans cell histiocytosis [3]. LCS diagnosis and treatment remain challenging. Diagnostic criteria include cytological and histological features, such as nuclear atypia, hyperchromatic nuclei, prominent nucleoli, mitotic figures and expression of CD1a, S100 protein, and langerin (CD207) [9].
James E.F. Howard et al. described the complexity of this pathology, evaluating a case series of 66 patients with LCS diagnosed between 1966 and 2014. The most commonly affected sites were lymph nodes (74.2%), the skin (48.5%), lungs (28.8%), liver (16.7%), and spleen (15.2%). The most frequently used therapeutic approach was chemotherapy alone or in combination (71%), followed by surgery (47%) [10]. Localized disease showed a survival advantage. In according to the literature, our case documented an optimal response after complete excision of the intestinal lesion.
To date, no universally accepted international guidelines exist for LCS diagnosis and treatment due to the small number of cases [3]. Chemotherapy remains the best option for disseminated or metastatic disease. Studies have explored multiple options, including chemotherapeutic regimens such as cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone (CHOP); adriamycin, bleomycin, vincristine, and dacarbazine (ABVD); dexamethasone, high-dose cytarabine, and platinum (DHAP); etoposide, prednisone, oncovin, cyclophosphamide, and halotestin (EPOCH); ifosphamide, carboplatin, and etoposide (ICE); cisplatin/epirubicin, adriamycin, bleomycin, and oncovin (ABO); bleomycin, adriamycin, lomustine, and oncovin (BACO); plus L-asparaginase, vincristine, and daunorubicin, which have shown limited response rates [11]. A study published in 1999 in the journal Blood documented sustained complete responses with cladribine-based regimens, with a favorable toxicity profile, in adults with LCH [12].
LCS and LCH share immunohistochemical features, such as CD1a, Langerin, S100 positivity, and Birbeck granules, but differ in histological atypia and mitotic rates, with LCS having a worse prognosis [13]. MAPK pathway mutations, including BRAF, ARAF, MAP2K1, NRAS, and KRAS, occur in these histiocytic disorders, suggesting a common genetic pathway. MAP2K1 exon 2 – 3 mutations (as in our case) were documented in 33% of BRAF wild-type LCH lesions and somatic mutations of the MAPK pathway with ERK activation have been found in 75% of Langerhans cell histiocytosis (LCH) lesions.
The impact of different MAPK mutations remains uncertain; however, the frequency of MAPK pathway mutations in LCH suggests its functional role in the pathological activation of ERK, which is physiologically involved in myeloid cell differentiation and maturation [14].
Another critical finding is the discovery of the myeloid origin of Langerhans cells (LCs) in LCH, confirmed by multiple studies. Gene expression analysis of cells within ICL lesions revealed that the transcriptional profile of CD207+ CL present in these lesions closely resembles that of myeloid-derived dendritic cells rather than CD207+ epidermal CL [15]. This distinction is fundamental for guiding therapeutic decisions.
Cladribine, an antimetabolite, selectively destroys quiescent lymphocytes and exhibits high toxicity toward monocytes. This property supports its potential application in treating monocyte-derived neoplasms and chronic inflammatory conditions. Tumors originating from Langerhans cells (LCH), as previously mentioned, result from the accumulation of tissue histiocytes derived from the same progenitor cells as monocytes.
Based on these considerations, the patient underwent excision of the intestinal lesion followed by systemic chemotherapy. We opted to treat our patient with subcutaneous injections of Cladribine, administered over five consecutive days for a total of six cycles, achieving an optimal response. Although the therapy was generally well-tolerated, the patient experienced significant hematologic toxicity, particularly severe thrombocytopenia (grade 4), likely exacerbated by a concomitant diagnosis of MDS.
This case underscores the severity of this rare condition, which carries a high mortality rate. Reported overall disease-specific survival (DSS) at one year is 58%, dropping to 28% at five years for all patients with LCS. The five-year disease-free survival (DFS) rate is 25%, with no disseminated disease patients surviving beyond five years (10). In our case, the patient remains in overall good general condition 1.5 years post-diagnosis.
Our findings align with the literature, highlighting that DSS and DFS significantly deteriorate as the disease progresses from local to loco-regional and finally to disseminated stages (Figure 4) [10].
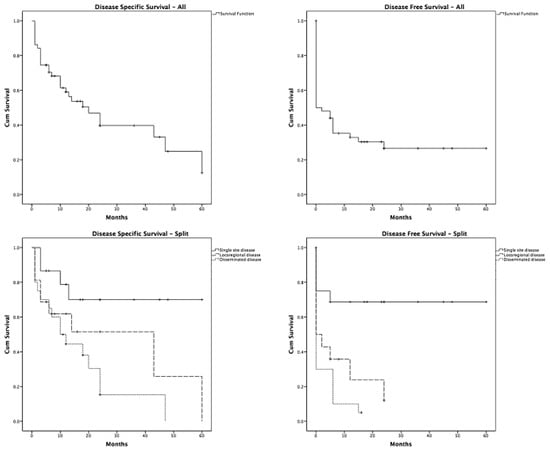
Figure 4.
Disease-specific survival (DSS) and disease-free survival (DFS) in patients with LCS.
4. Conclusions
LCS is a rare disease with a poor prognosis. While understanding its pathogenesis remains scarce, MAPK pathway mutations suggest a common genetic basis for these disorders [9], and greater understanding could guide the choice of the most appropriate treatment for patients affected by these rare entities.
Localized disease often has an excellent prognosis, typically managed with surgical excision [16]. Systemic therapy is required for multifocal and disseminated disease [5]. The main problem remains the selection of the most effective regimen for this condition.
Our case demonstrates the efficacy and tolerability of cladribine for disseminated LCS, suggesting its use as a viable and effective treatment option in patients with LCH and LCS.
Author Contributions
G.P. (Giulia Pileggi) conceptualized and wrote the manuscript; S.M., M.P., A.d.N., E.P., E.C., V.D.S., F.L.L., E.R., C.T. (Chiara Togni) and C.T. (Caterina Tatarelli)researched the data; G.M. edited and reviewed the manuscript; S.M., G.P. (Giovanna Palumbo) and A.T. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study and written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Due to patient privacy, all data pertaining to the patient in this case report cannot be shared or distributed.
Acknowledgments
The authors would like to thank all those who contributed to the treatment of the patient. We would also like to thank the patient for giving us consent to write this case report.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pathology & Genetics: Tumours of Haematopoietic and Lymphoid Tissues; Jaffe, E.S., Harris, N.L., Stein, H., Vardiman, J.W., Eds.; IARC Press: Lyon, France, 2001; p. 283. [Google Scholar]
- Mali, B.; Mali, A.; Mali, A.; Abdulrazzak, M.; Jobran, A.W. The epidemiological and clinical characteristics of Langerhans cell sarcoma in the United States: A population study based on SEER data from 2000 to 2019. Medicine 2024, 103, e39315. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.T.; Friesenbichler, J.; Holzer, L.A.; Liegl-Atzwanger, B.; Beham-Schmid, C.; Leithner, A. Langerhans cell sarcoma: A case report and review of the literature. Pol. J. Pathol. 2016, 67, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Valladeau, J.; Clair-Moninot, V.; Dezutter-Dambuyant, C.; Pin, J.-J.; Kissenpfennig, A.; Mattéi, M.-G.; Ait-Yahia, S.; Bates, E.E.M.; Malissen, B.; Koch, F.; et al. Identification of mouse Langerin/CD207 in Langerhans cells and some dendritic cells of lymphoid tissues. J. Immunol. 2002, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Ferringer, T.; Banks, P.M.; Metcalf, J.S. Langerhans cell sarcoma. Am. J. Dermatopathol. 2006, 28, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Pileri, S.A.; Grogan, T.M.; Harris, N.L.; Banks, P.; Campo, E.; Chan, J.K.C.; Favera, R.D.; Delsol, G.; De Wolf-Peeters, C.; Falini, B.; et al. Tumours of histiocytes and accessory dendritic cells: An immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology 2002, 41, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Charfi, M.; Lajnaf, M.; Kallel, F.; Saguem, I.; Kassar, O.; Elloumi, M. Complexity of diagnosing and treating langerhans cell sarcoma: A case report. Tunis Med. 2023, 101, 651–653. [Google Scholar] [PubMed] [PubMed Central]
- Nakamine, H.; Yamakawa, M.; Yoshino, T.; Fukumoto, T.; Enomoto, Y.; Matsumura, I. Langerhans Cell Histiocytosis and Langerhans Cell Sarcoma: Current Understanding and Differential Diagnosis. J. Clin. Exp. Hematop. 2016, 56, 109–118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yi, W.; Chen, W.Y.; Yang, T.X.; Lan, J.P.; Liang, W.N. Langerhans cell sarcoma arising from antecedent langerhans cell histiocytosis: A case report. Medicine 2019, 98, e14531. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.E.; Dwivedi, R.C.; Masterson, L.; Jani, P. Langerhans cell sarcoma: A systematic review. Cancer Treat. Rev. 2015, 41, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Valentín-Nogueras, S.M.; Seijo-Montes, R.; Montalván-Miró, E.; Sánchez, J.L. Langerhans cell sarcoma: A case report. J. Cutan. Pathol. 2013, 40, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Saven, A.; Burian, C. Cladribine activity in adult langerhans-cell histiocytosis. Blood 1999, 93, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Takahashi, K.; Hori, M.; Okumura, T.; Saito, M.; Yamakawa, M.; Tabuchi, K.; Hara, A. Langerhans cell sarcoma of the cervical lymph node: A case report and literature review. Auris Nasus Larynx 2010, 37, 7503. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Hampton, O.A.; Shen, X.; Simko, S.J.; Shih, A.; Abhyankar, H.; Lim, K.P.H.; Covington, K.R.; Trevino, L.; Dewal, N.; et al. Mutually exclusive recurrent somatic mutations in MAP2K1 and BRAF support a central role for ERK activation in LCH pathogenesis. Blood 2014, 124, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.E.; Li, L.; Peters, T.L.; Leung, H.C.; Yu, A.; Man, T.K.; Gurusiddappa, S.; Phillips, M.T.; Hicks, M.J.; Gaikwad, A.; et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J. Immunol. 2010, 184, 4557–4567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Zhou, X.G.; Wang, Z. Langerhans cell sarcoma in the cervical lymph node: A case report and literature review. Acta Haematol. 2013, 129, 114–120. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).