Myeloid and Lymphoid Malignancies with Fusion Kinases Involving Spleen Tyrosine Kinase (SYK)—Emerging Rare Entities?
Abstract
1. Introduction
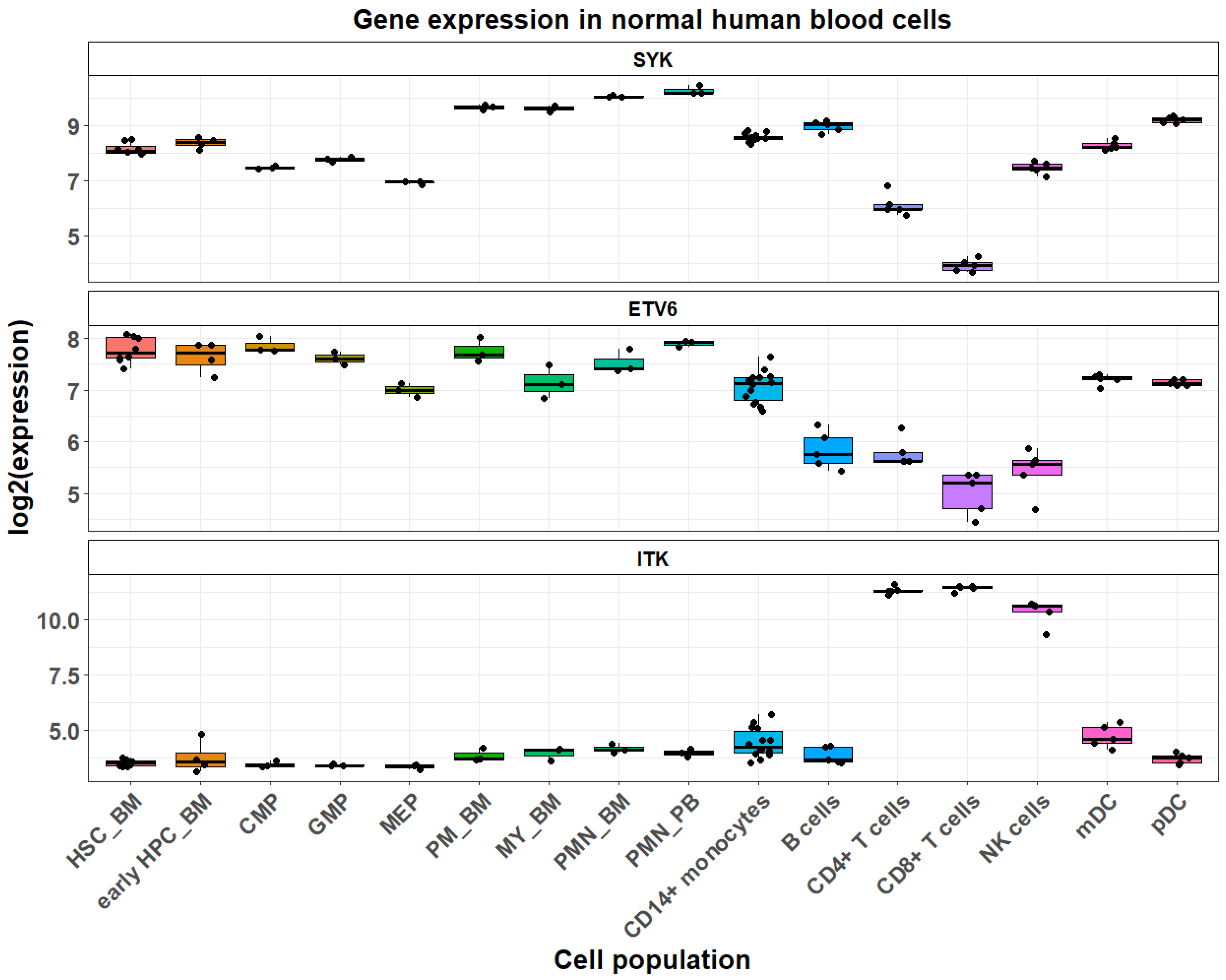
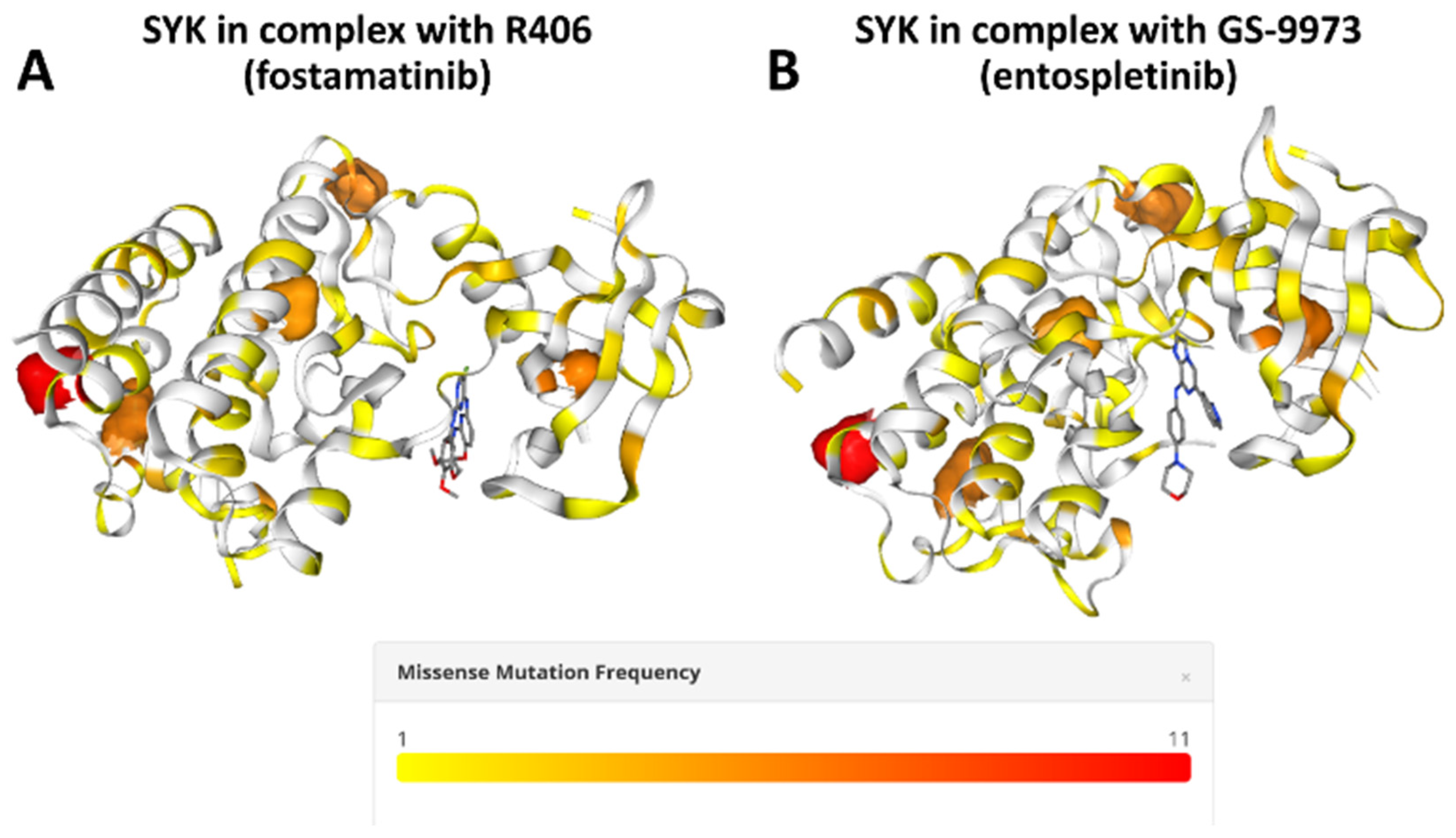
2. Spleen Tyrosine Kinase (SYK)
3. ETV6::SYK in Myeloid Neoplasms
4. ITK::SYK in Lymphoid Neoplasms
5. CTLC::SYK in Juvenile Xanthogranuloma
6. Other SYK Fusions Reported in Myeloid/Lymphoid Malignancies
7. Discussions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Mócsai, A.; Ruland, J.; Tybulewicz, V.L. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat. Rev. Immunol. 2010, 10, 387–402. [Google Scholar] [CrossRef]
- Hobbs, H.T.; Shah, N.H.; Badroos, J.M.; Gee, C.L.; Marqusee, S.; Kuriyan, J. Differences in the dynamics of the tandem-SH2 modules of the Syk and ZAP-70 tyrosine kinases. Protein Sci. 2021, 30, 2373–2384. [Google Scholar] [CrossRef]
- Bradshaw, J.M. The Src, Syk, and Tec family kinases: Distinct types of molecular switches. Cell. Signal. 2010, 22, 1175–1184. [Google Scholar] [CrossRef]
- Yanagi, S.; Inatome, R.; Takano, T.; Yamamura, H. Syk expression and novel function in a wide variety of tissues. Biochem. Biophys. Res. Commun. 2001, 288, 495–498. [Google Scholar] [CrossRef]
- Buchner, M.; Fuchs, S.; Prinz, G.; Pfeifer, D.; Bartholomé, K.; Burger, M.; Chevalier, N.; Vallat, L.; Timmer, J.; Gribben, J.G. Spleen tyrosine kinase is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukemia. Cancer Res. 2009, 69, 5424–5432. [Google Scholar] [CrossRef]
- Buchner, M.; Baer, C.; Prinz, G.; Dierks, C.; Burger, M.; Zenz, T.; Stilgenbauer, S.; Jumaa, H.; Veelken, H.; Zirlik, K. Spleen tyrosine kinase inhibition prevents chemokine-and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood 2010, 115, 4497–4506. [Google Scholar] [CrossRef]
- Feldman, A.; Sun, D.; Law, M.; Novak, A.; Attygalle, A.; Thorland, E.; Fink, S.; Vrana, J.; Caron, B.; Morice, W. Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia 2008, 22, 1139–1143. [Google Scholar] [CrossRef]
- Streubel, B.; Vinatzer, U.; Willheim, M.; Raderer, M.; Chott, A. Novel t (5; 9)(q33; q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia 2006, 20, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Kuno, Y.; Abe, A.; Emi, N.; Iida, M.; Yokozawa, T.; Towatari, M.; Tanimoto, M.; Saito, H. Constitutive kinase activation of the TEL-Syk fusion gene in myelodysplastic syndrome with t (9; 12)(q22; p12). Blood 2001, 97, 1050–1055. [Google Scholar] [CrossRef]
- Gíslason, M.H.; Demircan, G.S.; Prachar, M.; Furtwängler, B.; Schwaller, J.; Schoof, E.M.; Porse, B.T.; Rapin, N.; Bagger, F.O. BloodSpot 3.0: A database of gene and protein expression data in normal and malignant haematopoiesis. Nucleic Acids Res. 2024, 52, D1138–D1142. [Google Scholar]
- Bohlander, S.K. ETV6: A versatile player in leukemogenesis. Semin. Cancer Biol. 2005, 15, 162–174. [Google Scholar] [CrossRef] [PubMed]
- De Braekeleer, E.; Douet-Guilbert, N.; Morel, F.; Le Bris, M.-J.; Basinko, A.; De Braekeleer, M. ETV6 fusion genes in hematological malignancies: A review. Leuk. Res. 2012, 36, 945–961. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Thandla, S.P.; Ploski, J.E.; Raza-Egilmez, S.Z.; Chhalliyil, P.P.; Block, A.W.; De Jong, P.J.; Aplan, P.D. ETV6-AML1 translocation breakpoints cluster near a purine/pyrimidine repeat region in the ETV6 gene. Blood J. Am. Soc. Hematol. 1999, 93, 293–299. [Google Scholar]
- Kuno, Y.; Abe, A.; Emi, N.; Iida, M.; Yamamori, T.; Tanimoto, M.; Saito, H. An atypical myelodysplastic syndrome with t (9; 12)(q22; p12) and TEL gene rearrangement. Br. J. Haematol. 1999, 106, 570–571. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.; Swami, V.K.; Besa, E.C.; Punnett, H.H. A second case of myelodysplastic syndrome with t (9; 12)(q22; p12). Cancer Genet. Cytogenet. 2005, 157, 187–188. [Google Scholar] [CrossRef]
- Lierman, E.; Smits, S.; Debackere, K.; André, M.; Michaux, L.; Vandenberghe, P. t (9; 12)(q22; p13) ETV6:: SYK: A new recurrent cytogenetic aberration and tyrosine kinase gene fusion in myeloid or lymphoid neoplasms associated with eosinophilia. Br. J. Haematol. 2023, 200, 665–668. [Google Scholar] [CrossRef]
- Debois, D.; Marot, L.; Andre, M.; Dachelet, C. Thalidomide as an effective treatment for adult multiple xanthogranuloma. JAAD Case Rep. 2018, 4, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Manuelyan, K.; Momcheva, I.; Angelova, S.; Nikolov, K.; Shivarov, V. Recurrent ETV6:: SYK rearrangement in myeloid malignancies confers partial susceptibility to MEK inhibition. Br. J. Haematol. 2024, 205, 382–386. [Google Scholar] [CrossRef]
- Risch, Z.; Kaffenberger, B.H.; Chung, C.G.; Samorodnitsky, E.; Hoskins, E.L.; Dao, T.; Smith, A.; Wall, S.A.; Brammer, J.; Reeser, J.W. Myeloid neoplasm with histiocytosis and spleen tyrosine kinase fusion responds to fostamatinib. Haematologica 2024, 109, 3816. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.-H.; Gao, L.; Jing, Y.; Xu, Y.-Y.; Ding, Y.; Wang, N.; Wang, W.; Li, M.-Y.; Han, X.-P.; Sun, J.-z. Detection of ETV6 gene rearrangements in adult acute lymphoblastic leukemia. Ann. Hematol. 2012, 91, 1235–1243. [Google Scholar] [CrossRef]
- Graham, M.T.; Abram, C.L.; Hu, Y.; Lowell, C.A. Expression of the TEL-Syk fusion protein in hematopoietic stem cells leads to rapidly fatal myelofibrosis in mice. PLoS ONE 2013, 8, e77542. [Google Scholar] [CrossRef] [PubMed]
- Sprissler, C.; Belenki, D.; Maurer, H.; Aumann, K.; Pfeifer, D.; Klein, C.; Müller, T.; Kissel, S.; Hülsdünker, J.; Alexandrovski, J. Depletion of STAT5 blocks TEL–SYK-induced APMF-type leukemia with myelofibrosis and myelodysplasia in mice. Blood Cancer J. 2014, 4, e240. [Google Scholar] [CrossRef]
- Villaseñor, A.G.; Kondru, R.; Ho, H.; Wang, S.; Papp, E.; Shaw, D.; Barnett, J.W.; Browner, M.F.; Kuglstatter, A. Structural insights for design of potent spleen tyrosine kinase inhibitors from crystallographic analysis of three inhibitor complexes. Chem. Biol. Drug Des. 2009, 73, 466–470. [Google Scholar] [CrossRef]
- Braselmann, S.; Taylor, V.; Zhao, H.; Wang, S.; Sylvain, C.; Baluom, M.; Qu, K.; Herlaar, E.; Lau, A.; Young, C. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J. Pharmacol. Exp. Ther. 2006, 319, 998–1008. [Google Scholar] [CrossRef]
- Currie, K.S.; Kropf, J.E.; Lee, T.; Blomgren, P.; Xu, J.; Zhao, Z.; Gallion, S.; Whitney, J.A.; Maclin, D.; Lansdon, E.B. Discovery of GS-9973, a selective and orally efficacious inhibitor of spleen tyrosine kinase. J. Med. Chem. 2014, 57, 3856–3873. [Google Scholar] [CrossRef]
- Cremer, A.; Ellegast, J.M.; Alexe, G.; Frank, E.S.; Ross, L.; Chu, S.H.; Pikman, Y.; Robichaud, A.; Goodale, A.; Häupl, B. Resistance mechanisms to SYK inhibition in acute myeloid leukemia. Cancer Discov. 2020, 10, 214–231. [Google Scholar] [CrossRef]
- Jubb, H.C.; Saini, H.K.; Verdonk, M.L.; Forbes, S.A. COSMIC-3D provides structural perspectives on cancer genetics for drug discovery. Nat. Genet. 2018, 50, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Kannan, A.K.; August, A. Structure and function of Tec family kinase Itk. Biomol. Concepts 2011, 2, 223–232. [Google Scholar] [CrossRef][Green Version]
- Pechloff, K.; Holch, J.; Ferch, U.; Schweneker, M.; Brunner, K.; Kremer, M.; Sparwasser, T.; Quintanilla-Martinez, L.; Zimber-Strobl, U.; Streubel, B. The fusion kinase ITK-SYK mimics a T cell receptor signal and drives oncogenesis in conditional mouse models of peripheral T cell lymphoma. J. Exp. Med. 2010, 207, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Moreau, A.; Dupuis, J.; Streubel, B.; Petit, B.; Le Gouill, S.; Martin-Garcia, N.; Copie-Bergman, C.; Gaillard, F.; Qubaja, M. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am. J. Surg. Pathol. 2009, 33, 682–690. [Google Scholar] [CrossRef]
- Berg, L.J.; Finkelstein, L.D.; Lucas, J.A.; Schwartzberg, P.L. Tec family kinases in T lymphocyte development and function. Annu. Rev. Immunol. 2005, 23, 549–600. [Google Scholar] [CrossRef]
- Dierks, C.; Adrian, F.; Fisch, P.; Ma, H.; Maurer, H.; Herchenbach, D.; Forster, C.U.; Sprissler, C.; Liu, G.; Rottmann, S. The ITK-SYK fusion oncogene induces a T-cell lymphoproliferative disease in mice mimicking human disease. Cancer Res. 2010, 70, 6193–6204. [Google Scholar] [CrossRef] [PubMed]
- Rigby, S.; Huang, Y.; Streubel, B.; Chott, A.; Du, M.-Q.; Turner, S.D.; Bacon, C.M. The lymphoma-associated fusion tyrosine kinase ITK-SYK requires pleckstrin homology domain-mediated membrane localization for activation and cellular transformation. J. Biol. Chem. 2009, 284, 26871–26881. [Google Scholar] [CrossRef]
- Kemps, P.G.; Baelde, H.J.; Vorderman, R.H.; Stelloo, E.; Swennenhuis, J.F.; Szuhai, K.; Lamers, M.H.; Kenkhuis, B.; Al-Hussaini, M.; Briaire-de Bruijn, I.H. Recurrent CLTC:: SYK fusions and CSF1R mutations in juvenile xanthogranuloma of soft tissue. Blood 2024, 144, 2439–2455. [Google Scholar] [CrossRef]
- Sugita, M.; Wilkes, D.C.; Bareja, R.; Eng, K.W.; Nataraj, S.; Jimenez-Flores, R.A.; Yan, L.; De Leon, J.P.; Croyle, J.A.; Kaner, J. Targeting the epichaperome as an effective precision medicine approach in a novel PML-SYK fusion acute myeloid leukemia. NPJ Precis. Oncol. 2021, 5, 44. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Feldman, T.A.; Ye, J.C.; Khodadoust, M.S.; Munoz, J.; Hamlin, P.A.; Kim, Y.H.; Wilcox, R.A.; Patel, M.R.; Coffey, G. Results from an open-label phase 2a study of cerdulatinib, a dual spleen tyrosine kinase/janus kinase inhibitor, in relapsed/refractory peripheral T-cell lymphoma. Leuk. Lymphoma 2025, 66, 1100–1110. [Google Scholar] [CrossRef]
| Case Number | Diagnosis | Age | Sex | Eosinophilia | Marrow Findings | Other Clinical Features | Treatment Given | Clinical Course | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1. | MDS | 36 | F | + | 20% blasts at diagnosis, myeloid hyperplasia, three-line dysplasia | Skin findings, Megakaryocytic differentiation of the blasts | Seven + three and conventional consolidation treatment | Obtained CR | [12,18] |
| 2. | CMML | 65 | M | - | 10% blasts, myeloid hyperplasia, three-line dysplasia, fibrosis, | Hydroxyurea and thalidomide without response, imatinib | Chronic course | [19] | |
| 3. | myelodysplastic/myeloproliferative neoplasm, unclassifiable with eosinophilia | 51 | M | + | Myeloid hyperplasia, marked megakaryocytic dysplasia | Itchy skin patches, histology suggestive of non-Langerhans histiocytosis | Thalidomide hydroxyurea and steroids, PEG-INF and photodynamic therapy, PEG-INF alone | Hematologic response, No skin response, Chronic course (OS > 5 years) | [20,21] |
| 4. | Myeloid/Lymphoid neoplasms with eosinophilia and tyrosine kinase fusions | 37 | F | + | Skin patches, Suggestive of non-Langerhans histiocytosis | adalimumab, cyclophosphamide upadacitinib cobematinib | [22] | ||
| 5. | Myeloid neoplasm with histiocytosis | 51 | F | + | Myeloid hyperplasia, no dysplasia described, eosinophilia | Prominent skin patches (60% BSA)—biopsy suggestive of non-Langerhans histiocytosis | Fostamatinib 100 mg BD, increased to 150 mg BD on progression, Allo-HSCT | Initial response for 6 months, Allo-HSCT at 18 months post diagnosis, died of GVHD 5 months post transplant | [23] |
| B-cell acute lymphoblastic leukemia (B-ALL) | 67 | M | Not reported | Not reported | Induction not followed by allo-HSCT | No CR after induction | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivarov, V.; Lozenov, S. Myeloid and Lymphoid Malignancies with Fusion Kinases Involving Spleen Tyrosine Kinase (SYK)—Emerging Rare Entities? Hemato 2025, 6, 17. https://doi.org/10.3390/hemato6020017
Shivarov V, Lozenov S. Myeloid and Lymphoid Malignancies with Fusion Kinases Involving Spleen Tyrosine Kinase (SYK)—Emerging Rare Entities? Hemato. 2025; 6(2):17. https://doi.org/10.3390/hemato6020017
Chicago/Turabian StyleShivarov, Velizar, and Stefan Lozenov. 2025. "Myeloid and Lymphoid Malignancies with Fusion Kinases Involving Spleen Tyrosine Kinase (SYK)—Emerging Rare Entities?" Hemato 6, no. 2: 17. https://doi.org/10.3390/hemato6020017
APA StyleShivarov, V., & Lozenov, S. (2025). Myeloid and Lymphoid Malignancies with Fusion Kinases Involving Spleen Tyrosine Kinase (SYK)—Emerging Rare Entities? Hemato, 6(2), 17. https://doi.org/10.3390/hemato6020017






