Abstract
Lymphoproliferative neoplasms of uncertain biological significance are increasingly encountered due to widespread usage of immunophenotypic and molecular techniques. Considering that clearer biological criteria and patient management have been established for B-cell lymphoproliferative diseases of undetermined significance occurring in the peripheral blood, many issues are still obscure for early lesions detected in lymphoid tissues. Regardless that some categories of lymphoproliferative neoplasms of uncertain biological significance have been recognized by the 4th edition of the WHO, other anecdotal early lymphoproliferative lesions still remain fully undefined. Some early lesions frequently originate from the germinal center, including atypical germinal centers BCL2-negative, an early pattern of large B-cell lymphoma with IRF4 rearrangement, and “in situ” high-grade B lymphomas. Moreover, other early lymphoproliferative lesions arise outside the germinal center and include those developing within the setting of monocytoid B-cell hyperplasia, but they also can be directly or indirectly associated with chronic inflammations. This review aims to summarize the concepts discussed during the IV Workshop organized by the Italian Group of Hematopathology, focus on the state-of-the-art on B-cell lymphoproliferative neoplasms of uncertain biological significance, and offer operative insights to pathologists and clinicians in routine diagnostics.
Keywords:
lymphoproliferative neoplasms of uncertain biological significance; monoclonal B-cell lymphocytosis; “in situ” follicular neoplasia; “in situ” mantle cell neoplasia; atypical germinal centers; large B-cell lymphoma with IRF4 rearrangement; “in situ” high-grade B lymphomas; non-Hodgkin lymphoma; Hodgkin lymphoma 1. Introduction
The IV Workshop, organized by the Italian Group of Hematopathology, focused on lymphoproliferative neoplasms of uncertain biological significance. The long-standing concept of in situ/early neoplasia has been already established in epithelial tumors; the same approach is not so readily applicable to lymphoid proliferative disorders, due to the fact that lymphocytes are usually circulating and not sessile cells. Indeed, in this context, early lesions are often an incidental finding that partially/minimally involve an otherwise reactive-appearing lymph node and never progress in most instances. Along with the historical recognition of monoclonal immunoglobulins in the serum, namely monoclonal gammopathy of undetermined significance (MGUS), a clear conceptual definition of peripheral blood B-cell lymphoproliferative diseases of undetermined significance (monoclonal B-cell lymphocytosis) is well-established [1]. A series of early lesions histologically detected in lymphoid tissues, also in view of their uncertain risk of progression, are actually still seeking a biological determination. The early lesions within the lymphoid tissue accepted by the 4th edition of the World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues are “in situ” follicular neoplasia and “in situ” mantle cell neoplasia [2] that could actually be considered as the tissue counterpart of circulating elements with t(14;18) and t(11;14) rearrangements (Table 1). “In situ” follicular neoplasia (ISFN) is defined as partial or total colonization of germinal centers by clonal B cells carrying the BCL2 translocation characteristic of follicular lymphoma (FL) in a lymph node where the overall architecture is preserved [3,4]. ISFN is characterized by lack of polarization, closely packed centrocytes with very few, if present, centroblasts, although it is not commonly recognizable in routine H&E-stained sections [3]; this condition is frequently identified by an intense immunoreactivity for germinal center markers (e.g., CD10) and aberrant positivity for BCL2, along with a low proliferation index. ISFN must be distinguished from partial involvement by follicular lymphoma (pFL), where an architectural distortion is observed. The risk of progression of ISFN is very low (<5%) [5], and it does not seem to be influenced by the number of follicles involved within a single lymph node [5,6]. “In situ” mantle cell neoplasia (ISMCN) is defined as the occurrence of Cyclin D1-expressing B cells in nonexpanded mantle zones of otherwise morphologically reactive-appearing lymph nodes [4,7,8]. The clinical outcome has not been completely clarified; the progression into an overt mantle cell lymphoma (MCL) is uncommon [7], although ISMCN or minimal accumulations of Cyclin D1-positive B cells can sometimes be detected several years before developing an overt MCL [9]; additionally, it has been reported that ISMCN with expression of SOX11 has a higher chance to progress [7].

Table 1.
Lymphoproliferative neoplasms of uncertain biological significance.
In cases of ISFN and ISMCN, it is necessary to proceed to a complete staging workup to exclude an overt lymphoma in another site. Additionally, a composite form, namely ISFN and ISMCN, can be associated with another overt lymphoma, as presented in the first three cases of the Workshop.
Other anecdotal early lymphoproliferative lesions with uncertain biological significance have been previously published and are not present in the 4th edition of the WHO Classification (Table 1). They mostly originate from the germinal centers and encompass atypical BCL2-negative germinal centers, an early pattern of large B-cell lymphoma (LBCL) with IRF4 rearrangement, and “in situ” high-grade B lymphomas. Nybakken et al. published a case series of atypical germinal centers in reactive/non-neoplastic lymph nodes characterized by isolated follicles with germinal centers substantially composed of aggregates of large centroblasts showing atypical mitoses without centrocytes [10]. These atypical follicles do not express BCL2 by immunohistochemistry and show the absence of BCL2/IGH translocation by FISH [10]: the differential diagnoses include a focal involvement by diffuse large B-cell lymphoma (DLBCL) or grade 3B, BCL2-negative FL, or a reactive follicular hyperplasia with unusual morphologic features.
Early LBL with IRF4 rearrangement has been reported in one case with predominant follicular hyperplasia that exhibited single atypical germinal centers enriched with centroblasts without accompanying tingible body macrophages displaying expression of CD20, CD10, BCL6, IRF4/MUM1, lambda immunoglobulin light chain restriction, and a gain and rearrangement of the IRF4 gene detected by FISH [11].
Two cases of so-called “in situ” high-grade B lymphomas with c-MYC rearrangement have been previously reported [12] as well. By definition, a very low tumor burden was noticed in contrast to other overt B-cell lymphomas with MYC gene rearrangements; this feature may suggest that these early “double hit” lesions may require additional genetic hits to progress [12,13]. From a practical perspective, these lymphoproliferations have to be assessed in the context of all clinical findings and accompanied by a strict-follow-up to rule out any possible evolution.
Some operative considerations arise from the previously reported situations. In routine practice, whenever we face potential atypical germinal centers, the immunohistochemical confirmation of the presence of follicular dendritic networks, the germinal center origin of the B cell population, and the occurrence of strong and diffuse immunoreactivity for IRF4, BCL2, and c-MYC must be assessed. In addition, FISH analysis for IRF4, BCL2, and c-MYC rearrangements is advisable. This approach will enable in most instances to rule out, focal area(s) of diffuse large B-cell lymphoma/high-grade B-cell lymphoma “double hit”, partial involvement by grade 3B BCL2-negative FL, and early LBCL with IRF4 rearrangement, respectively. Following the examples of ISFN and ISMCN, the exclusion of an overt disease with a complete staging workup is mandatory in all these cases.
Another niche where early lesions have been described beside the germinal center is monocytoid B-cell hyperplasia [14,15]. Monocytoid B cells (MBCs) were described for the first time by Karl Lennert as immature sinus histiocytosis, representing cells closely related to marginal zone hyperplasia [16]. Morphologically, MBCs measure one-and-a-half to three times the size of small lymphocytes, display round, oval, or indented nuclei with a slightly clumped to vesicular chromatin structure, small and inconspicuous (when present) nucleoli, and abundant pale cytoplasm. These cells can be arranged in perifollicular clusters within and around sinuses and are commonly admixed with scattered neutrophils. Unlike marginal zone B-lymphocytes, MBCs are negative for BCL2 [17]. Reactive MBCs are frequently associated with follicular hyperplasia and epithelioid macrophages [18] and have been recognized occurring in association with lymphoproliferative diseases, like classic Hodgkin lymphomas (cHL) [19,20]. More recently, clusters of Burkitt cells have been reported with MBC hyperplasia in both immunocompromised and immunocompetent patients [14,15]. In immunocompromised patients, it has been proven that these early lesions carry the risk of progression into an overt Burkitt lymphoma with a worse outcome [14,15].
Different non-Hodgkin lymphomas have been associated with chronic inflammation, either infectious or not. In cases of chronic infection due to non-lymphotropic agents, the pathogen’s role in establishing the proliferation and progression towards overt lymphoma radically differs from other lymphotropic agents. Moreover, IgG4-related disease (IgG4-RD) represents an important topic, as confirmed by the occurrence of a ISFN associated with this condition and presented during the Workshop. IgG4-RD is a fibroinflammatory disorder of uncertain etiology which affects almost any organ [21]. Although most patients with IgG4-RD do not present an association with lymphomas, some cases have been reported to develop concomitant or metachronous low- and high-grade lymphomas [22,23,24,25,26,27,28], suggesting that a potential pathogenetic link may lie in the context of a shared chronic antigenic stimulation [22,29,30]. This hypothesis is confirmed by cases #7 and #8 of the Workshop. On these grounds, a practical consideration is that monoclonal rearrangements can be demonstrated in a nonmalignant, antigen-driven proliferation of B cells, such as autoimmune disorders; accordingly, additional criteria of malignancy must be fulfilled to efficiently differentiate neoplastic proliferations from reactive conditions in this setting [31].
2. Submitted Cases of ISFN and ISMCN Associated with Overt Lymphoma
2.1. Case n. 1
Dr. Magnoli presented the previously published case [32] of a 75-year-old woman with multiple and bilateral lymphadenopathies that underwent an excisional biopsy of a right cervical lymph node. Morphological and immunohistochemical findings allowed to conclude for a diagnosis of a composite lymphoma encompassing FL, grade 1 to 2, “in situ” mantle cell neoplasia (ISMCN), and MCL with mantle zone growth pattern (MCLGP). A stage IVA was assessed, and the patient was treated with eight cycles of R-CVP (Rituximab plus a combination of Cyclophosphamide, Vincristine, and Prednisone) regimen, obtaining a complete remission. After two years, diffuse lymphadenopathy appeared, and the subsequent biopsy revealed a low-grade FL without any signs of a neoplastic mantle cell component, and after four years of “watch and wait”, a progression of the disease was documented; the patient died of lymphoma after being treated with a new line of chemotherapy with eight cycles of Chlorambucil chemotherapy, followed by a steroid-based support therapy. Karyotype and Spectral Karyotyping (SKY) FISH analyses on metaphases performed on the first biopsy tissue sample confirmed different cell clones. The neoplastic clone carrying the t(11;14) translocation involving CCND1 did not show additional cytogenetic abnormalities, whereas the neoplastic cell population with the t(14;18) translocation involving BCL2 was characterized by a triploid karyotype with several additional alterations, including also the rearrangement of BCL6. In this context, the observations led the authors to suggest that the progressively aggressive behavior of this B-cell lymphoma was determined by the complex karyotype of the overt FL [32]. On the contrary, the ISMCN did not harbor additional genetic abnormalities, nevertheless not affecting the clinical outcome [32]. Therefore, the following case confirms that the occurrence of additional genetic aberrations may drive progression and clinical outcomes.
2.2. Case n. 2
Dr. Granai discussed the case of a 45-year-old male who underwent the excisional biopsy of an enlarged axillary lymph node following the diagnosis of MCL of the appendix. The lymph node architecture was replaced by a diffuse proliferation of small monotonous lymphocytes with round nuclei and scant cytoplasm surrounding hyperplastic follicles with regularly structured germinal centers (Figure 1). Immunohistochemical investigations demonstrated the presence of two distinct lymphoid populations. The interfollicular cells were immunoreactive for CD20, CD5, CD23, and LEF1 (Figure 2). Cyclin D1 and SOX11 were expressed by reduced mantle zones surrounding reactive lymphoid follicles and absent in the interfollicular areas. Accordingly, a composite ISMCN and small lymphocytic lymphoma (SLL) diagnosis was made. IgH rearrangement confirmed the existence of two clonally unrelated B-cell neoplastic populations. The present case describes the well-known combination of ISMCN and SLL [33], exemplifies the concept that “in situ” lymphoma might be observed in association with overt MCL in distant sites, and highlights that the significance of the early lesion remains uncertain, thus suggesting that the clinical evaluation for evidence of overt lymphoma is highly recommended.
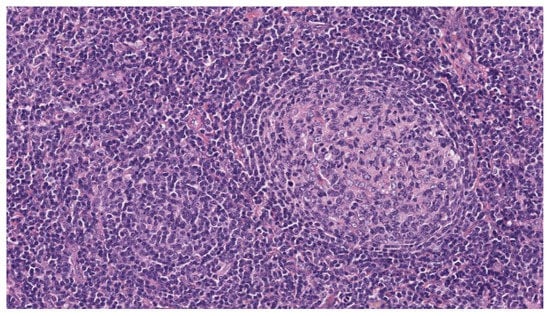
Figure 1.
H&E original magnification 20×. The lymph node architecture is replaced by a diffuse, interfollicular monotonous proliferation of small lymphocytes with round nuclei and scant cytoplasm surrounding hyperplastic follicles with regularly structured germinal centers.
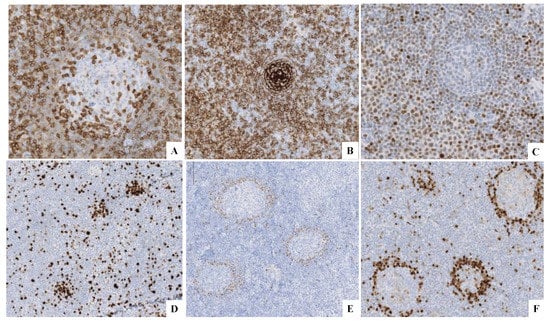
Figure 2.
(A) CD5+ in both components. (B) CD23+ in the SLL component. (C) LEF1+ in the SLL component. (D) Ki-67 highlights the proliferation centers. (E) Cyclin D1+ in the ISMCN. (F) SOX11+ in the ISMCN.
2.3. Case n. 3
Dr. Di Stefano submitted the case of a 45-year-old male patient that received a diagnosis of chronic lymphocytic leukemia (CLL) with atypical phenotype according to a bone marrow biopsy and the flow cytometric analysis results. The bone marrow biopsy showed a diffuse interstitial infiltrate of small B lymphocytes with a round nucleus and scant cytoplasm, immunoreactive for CD20, BCL2, and CD5 and negative for CD23, Cyclin D1, SOX11, CD10, and BCL6. A few weeks later, the patient underwent an excisional biopsy of three small cervical lymph nodes. The lymph node architecture was effaced by a small-to-medium-sized population with an immunophenotypic profile consistent with CLL with an atypical phenotype [34,35]. Two residual follicular structures were present, and their germinal centers showed a strong immunoreactivity for CD10 and BCL2 with a low proliferation index. The final diagnosis was CLL with an atypical phenotype and ISFN. In the present case, it is also confirmed that ISFN can be associated with other forms of overt lymphoma such as CLL. In such an event, it is necessary to always report the early lesion to guarantee appropriate clinical management.
3. Submitted Cases of Early Lymphoproliferative Lesions Originating from the Germinal Center Other Than ISFN and ISMCN
3.1. Case n. 4
Dr. Lazzi submitted the case of a 77-year-old male patient that underwent radical prostatectomy with bilateral lymphadenectomy for prostatic adenocarcinoma. Within one of the right iliac-obturator lymph nodes, two isolated follicles (Figure 3A–C) displayed germinal centers with atypical features such as the absence of polarization and enrichment of centroblasts. The atypical follicles were positive for B-cell and germinal center-related markers but negative for BCL2 by immunohistochemistry and for BCL2 translocation by FISH analysis (Figure 3D–F). An overt FL grade 3A was found in a contralateral lymph node (Figure 3G,H), which was negative for BCL2, as well both by immunohistochemistry and FISH; therefore, the final diagnosis was of FL, grade 3A with focal localization on the contralateral lymph node. The histologic interpretation of isolated atypical germinal centers can be challenging, but an overt FL helped achieve the correct diagnosis. A Next-Generation Sequencing (NGS) analysis was performed using an Illumina custom 80-gene-targeted NGS panel for B-cell lymphomas and revealed genetic variants in TNFAIP3 and NOTCH2.
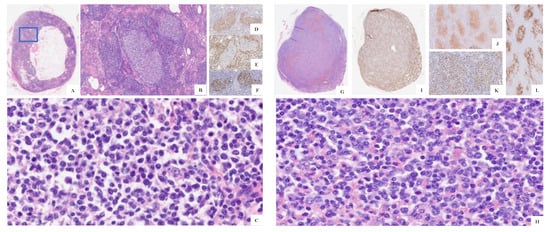
Figure 3.
(A) H&E original magnification 2× of the right iliac-obturator lymph node displaying two atypical follicles highlighted in the blue rectangle. (B) H&E original magnification 10× of the atypical follicles. (C) H&E original magnification 40× of the atypical germinal centers with the absence of polarization and enrichment in centroblasts. (D) CD10+, (E) BCL2-, and (F) Ki67 immunostains. (G) H&E original magnification ×4 of the left iliac-obturator lymph node. (H) H&E original magnification 40× of the overt FL on the left iliac-obturator lymph node. (I) BCL2-. (J) CD10+. (K) Ki67. (L) CD23 immunostains.
3.2. Case n. 5
Dr. Granai submitted and recently published [36] the case of a 72-year-old woman without significant medical history that underwent colonoscopy for enduring complaints and constipation; a 5-mm sessile polypoid lesion in the sigmoid colon was noted. Microscopically, the polypoid lesion displayed a submucosal lymphoid infiltrate with a follicular growth pattern and predominant centroblasts, along with very few centrocytes. The lymphoid population showed immunoreactivity for CD20, CD10, BCL6, BCL2, and MYC, in the context of expanded and disrupted follicular dendritic meshworks. FISH studies identified both IGH/BCL2 and IGH/MYC gene rearrangements, allowing the diagnosis of an extranodal FL grade 3A, with BCL2 and MYC rearrangements (DH-FL). Staging did not show additional sites of disease, and the patient is currently in good health and has never progressed. This case raises several questions related to patient’s management, since it is not clear whether to consider DH-FL an indolent lymphoma based on its morphologic aspects or an aggressive lymphoma given its cytogenetic features [37,38,39,40,41]. This dilemma related to the outcome is further enhanced when DH-FL displays partial involvement or is limited to a single site. More studies are needed to clarify this issue.
4. Submitted Cases of Early Lymphoproliferative Lesions Arising in the Setting of Monocytoid Hyperplasia
Case n. 6
Dr. Di Stefano presented the case of a 61-year-old woman with a prior history of invasive breast carcinoma treated with surgical resection, chemotherapy, and radiotherapy, who during follow up developed left supraclavicular lymphadenopathy. The excised lymph node showed follicular hyperplasia with polarized germinal centers; in the perifollicular areas, aggregates of MBC were admixed with rare, scattered large mononucleated or binucleated cells equipped with eosinophilic nucleolus and basophilic cytoplasm (Figure 4). The large cells were immunoreactive for CD30, CD15, PAX5 weak (Figure 5), and MUM1 and negative for CD20, CD3, and CD45. EBV was negative by EBER in situ hybridization. The molecular analysis performed on microdissected atypical, perifollicular areas detected a polyclonal IGH rearrangement, supporting the diagnosis of partial involvement by cHL. Since the radiologic and clinical evidence showed a localized disease, a “watch and wait” approach was chosen, and in the following years, subsequent nodal biopsies of the supraclavicular lymph nodes displayed the same morphological picture. Five years after the first diagnosis, the patient exhibited left inguinal lymphadenopathy and underwent an incisional biopsy. The morphologic examination revealed a subverted architecture by small T lymphocytes, eosinophils, and histiocytes intermingled with classical Reed–Sternberg (RS) and Hodgkin cells (Figure 6). The morpho–phenotypical profile was consistent with cHL, mixed cellularity. After the diagnosis, the patient received appropriate chemotherapy but had frequent hospitalizations due to recurrent nosocomial infections. The following case demonstrated an overt cHL after a history of partial involvement of cHL developing within the context of MBC hyperplasia.
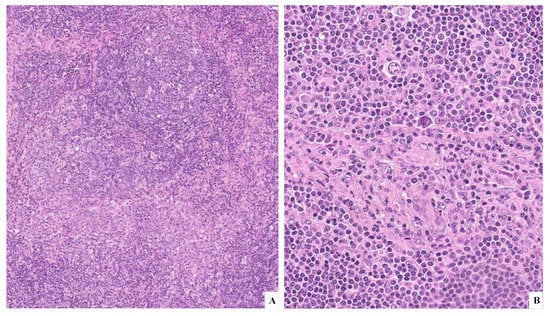
Figure 4.
(A) H&E original magnification 10× displaying follicular hyperplasia with polarized germinal centers and, in the perifollicular areas, aggregates of MBC. (B) H&E original magnification 20× showing, in the perifollicular areas, MBC mixed with rare large mononucleated or binucleated cells containing an eosinophilic nucleolus and basophilic cytoplasm.
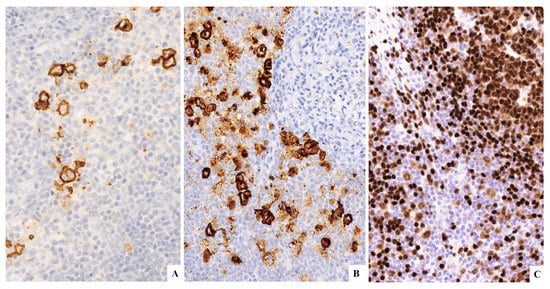
Figure 5.
(A) CD30+. (B) CD15+. (C) PAX5+ weak.
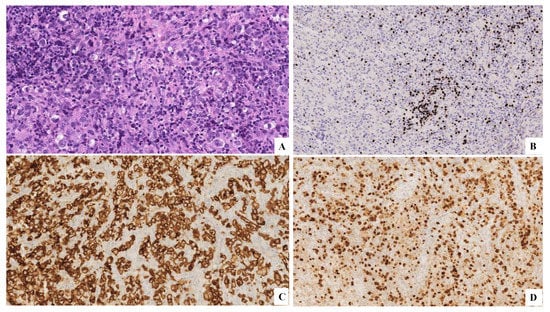
Figure 6.
(A) H&E original magnification 20× that displays small T lymphocytes, eosinophils, histiocytes, classical Reed–Sternberg cells, and Hodgkin cells. (B) PAX5-. (C) CD30+. (D) MUM1+.
5. Submitted Cases of Early Lymphoproliferative Lesions Associated with Infectious Diseases and Chronic Inflammation
5.1. Case n. 7
Dr. Vittone presented the case of a 76-year-old man with weight loss and low-grade persisting fever for many months. The PET-CT scan revealed multiple lymphoadenomegaly on both sides of the diaphragm with high spleen, bone, and left colon uptake. Histological examination of an axillary lymph node showed an area with well-formed granulomas composed of epithelioid histiocytes and occasional Langhans-type giant cells with central eosinophilic necrosis (Figure 7A). A PCR analysis performed on the paraffin-embedded tissue confirmed the presence of Mycobacterium tuberculosis DNA. The residual portion of the lymph node showed predominant follicular hyperplasia; herein, immunohistochemical investigations highlighted two follicles with strong staining for CD10, BCL2 (Figure 7C,D) with a low proliferation index suggestive of ISFN. Therefore, a diagnosis of necrotizing granulomatous lymphadenitis due to Mycobacterium tuberculosis infection associated with ISFN was made.
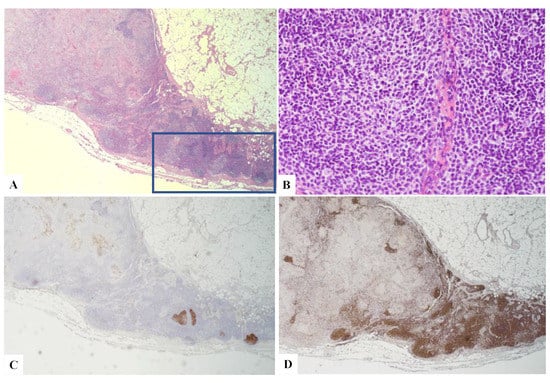
Figure 7.
(A) H&E original magnification 2.5× of the axillary lymph node displaying an area with well-formed granulomas, and in the remaining part of the lymphoid tissue, follicular hyperplasia was observed with two atypical follicles (blue rectangle). (B) H&E original magnification 40× of one of the atypical follicles suggestive of ISFN. (C) CD10+. (D) BCL2+.
5.2. Case n. 8
Dr. Santi presented a case of a 77-year-old male with a prior history of prostatic adenocarcinoma. During follow-up, left hydroureteronephrosis due to a retroperitoneal mass of 6.5 cm in the maximum diameter developed. The histological examination of left subtotal ureterectomy displayed follicular hyperplasia and predominant mature IgG4+ interfollicular plasma cells with a high IgG4/IgG ratio within the background of septal, storiform fibrosis (Figure 8 and Figure 9). Within this background, a single atypical follicle unusually constituted by monomorphous centrocytes, few centroblasts, and attenuated mantle zones was noticed with a strong positivity for BCL2 and CD10 (Figure 10). A PCR analysis showed a monoclonal IgH rearrangement. The final diagnosis was IgG4-RD with ISFN. A few months after the diagnosis, the patient died of complications due to sepsis.
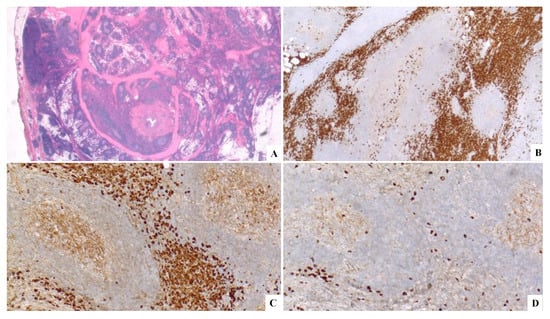
Figure 8.
(A) H&E original magnification 2× displaying a lymphoproliferative lesion in the background of septal, a storiform fibrosis. (B) CD138+. (C, D) A Kappa light chain restriction.
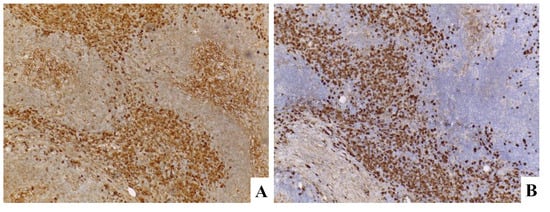
Figure 9.
(A) IgG. (B) IgG4.
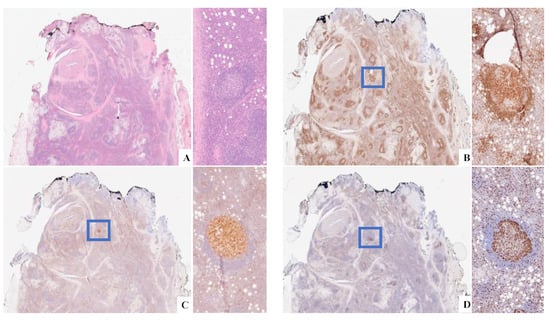
Figure 10.
(A) H&E original magnification 4× ISFN highlighted in the blue rectangle. (B) BCL2+. (C) CD10+; (D) Ki-67.
6. Conclusions
Early/in situ lymphoproliferative disorders are still an unclear subject since the evolution of these diseases at the time of the diagnosis is unpredictable on morpho-phenotypical-molecular-cytogenetic grounds. The sequence precancer–cancer in epithelial malignancies does not necessary overlap with the relationship between early lymphoproliferative lesions and overt lymphoma. In fact, most early lymphoid lesions do not progress to an overt lymphoma and may be concomitant with these latter. In the cases presented during the Workshop, the entities ISFN and ISMCN, already recognized by the 4th edition of the WHO classification, were associated with other overt lymphoproliferative processes, and therefore considered a composite lymphoma (Table 2).

Table 2.
Clinical cases presented during the Workshop.
The current view of the progression in lymphoproliferative lesion postulates that this process relies on a multistep sequence rather than only on a single event. This concept is exemplified by case #1, confirming that the development of a complex karyotype is the primary cause of the disease’s evolution [32], while case #2 highlights the importance of recognizing these early lesions to inform the clinician and carefully look for a possible overt and different lymphoma in another site. In case #3, the association of CLL with an atypical phenotype and ISFN was reported demonstrating that these early lesions concurrent with other lymphoproliferative processes may also be incidental findings discovered by immunohistochemistry. Nevertheless, the observation of such cases, which are usually unrelated to malignant overt counterparts, raises the question of whether there could be an underlying susceptibility for developing B lymphoproliferative processes in certain subjects [42]. The germinal centers are a fragile environment where neoplastic processes can develop. Notwithstanding the overexpression of antiapoptotic molecules such as BCL2 as a result of t(14;18) has been recognized to spy on the occurrence of FL, and case #4 displays that the histopathological examination can be sometimes the only key feature to unravel a lymphoproliferative process. In this case, the lymphoproliferative lesion was suspected on morpho-phenotypic features in two germinal centers, although there was a lack of BCL2 positivity. In this context, the finding of the overt FL on the contralateral lymph node aided the diagnosis of FL t(14;18)-negative that has been proven to be a genetically heterogeneous entity [43], and the NGS analysis performed on the case documented the presence of frequent mutations that have been detected in FL t(14;18)-negative, namely TNFAIP3 and NOTCH2 [43]. Therefore, we point out that occasional atypical follicles BCL2-negative in the context of a reactive lymph node should suggest the exclusion of an FL t(14; 18)-negative in other sites. A close correlation between molecular data and morphology is always required, since several studies have detected clonal populations in the context of classical reactive germinal centers or hyperplastic marginal zones [44,45].
In the “early” extra nodal DH-FL (case #5) [36], additional genetic hits are considered necessary for the full transformation of these ‘aggressive’ in situ neoplasms to a clinically overt disease [12,13]. Furthermore, once these lesions are recognized, clinical characteristics drive treatment decisions, and a close follow-up is mandatory to rule out overt lesions in another site.
Moving outside the germinal center, more specifically in the context of MBC hyperplasia, we notice a possible role of MBC in controlling the neoplastic disease [15,19,20]. In fact, in case #6, all the subsequent biopsies displayed RS cells closely associated with clusters of MBCs, and two previous studies have documented this aspect in overt cHL [19,20]. It has been established that the HL/RS cells originate from the germinal centers [46], and when they migrate to the perifollicular areas, they probably trigger a reaction like MBC hyperplasia. Therefore, even in cHL, MBC hyperplasia may be a physiologic antitumor mechanism of the host that counteracts the development of the full-blown disease [15,19,20].
In the presence of infectious diseases and chronic inflammation syndromes, an underlying lymphoproliferative process, may occur and should be considered. Rarely, non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma were concurrent with chronic infection by Mycobacterium tuberculosis and the Mycobacterium avium complex [47,48,49,50,51,52,53,54,55]. Setting aside patients with congenital and cell-mediated immunity defects, it has been hypothesized that Mycobacterium tuberculosis, similarly to Helicobacter pylori, might induce rearrangements more readily than other infectious agents, since they are both intracellular pathogens [53]. Much caution must be applied in the event of an IgG4-positive lymphoma or plasma cell neoplasm when numerous IgG4+ plasma cells are noticed in the context of a lymphoplasmacytic proliferation [56], and a clinical correlation might be necessary. To our knowledge, a case of necrotizing granulomatous lymphadenitis due to Mycobacterium tuberculosis infection and IgG4-RD concomitant to an early lesion have not been reported. Moreover, during the Workshop, it was suggested that the chronic inflammatory process, either created by an infectious agent or by an inflammatory disorder, could be the trigger determining an early lymphoproliferative form. However, to be added to the following hypothesis, we should also consider the well-known role of immunosurveillance of the microenvironment in B-cell lymphomas [15]; therefore, further studies could highlight the control mechanisms and the events determining the onset and progression to an overt disease.
In conclusion, pathologists should emphasize that the above-described early/in situ lesions retain an uncertain potential of malignant progression and require a strong integration with the clinical and imaging findings. Given that, presently, the therapeutic strategy relies on the diagnosis of an overt lymphoma, a conservative management accomplished by an active follow-up must be considered in these early/in situ lymphoproliferations.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Larson, D.R.; Plevak, M.F.; Offord, J.R.; Dispenzieri, A.; Katzmann, J.A.; Melton, L.J., 3rd. Prevalence of monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 2006, 354, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. (Eds.) WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues, 4th ed.; IARC: Lyon, France, 2017.
- Cong, P.; Raffeld, M.; Teruya-Feldstein, J.; Sorbara, L.; Pittaluga, S.; Jaffe, E.S. In situ localization of follicular lymphoma: Description and analysis by laser capture microdissection. Blood 2002, 99, 3376–3382. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Santoro, A. How I treat: Diagnosing and managing “in situ” lymphoma. Blood 2011, 117, 3954–3960. [Google Scholar] [CrossRef] [PubMed]
- Jegalian, A.G.; Eberle, F.C.; Pack, S.D.; Mirvis, M.; Raffeld, M.; Pittaluga, S.; Jaffe, E.S. Follicular lymphoma in situ: Clinical implications and comparisons with partial involvement by follicular lymphoma. Blood 2011, 118, 2976–3064. [Google Scholar] [CrossRef]
- Pillai, R.K.; Surti, U.; Swerdlow, S.H. Follicular lymphoma-like B cells of uncertain significance (in situ follicular lymphoma) may infrequently progress, but precedes follicular lymphoma, is associated with other overt lymphomas and mimics follicular lymphoma in flow cytometric studies. Haematologica 2013, 98, 1571–1580. [Google Scholar] [CrossRef]
- Carvajal-Cuenca, A.; Sua, L.F.; Silva, N.M.; Pittaluga, S.; Royo, C.; Song, J.Y.; Sargent, R.L.; Espinet, B.; Climent, F.; Jacobs, S.A.; et al. In situ mantle cell lymphoma: Clinical implications of an incidental finding with indolent clinical behavior. Haematologica 2012, 97, 270–278. [Google Scholar] [CrossRef]
- Nodit, L.; Bahler, D.W.; Jacobs, S.A.; Locker, J.; Swerdlow, S.H. Indolent mantle cell lymphoma with nodal involvement and mutated immunoglobulin heavy chain genes. Hum. Pathol. 2003, 34, 1030–1034. [Google Scholar] [CrossRef]
- Adam, P.; Schiefer, A.I.; Prill, S.; Henopp, T.; Quintanilla-Martínez, L.; Bösmüller, H.C.; Chott, A.; Fend, F. Incidence of preclinical manifestations of mantle cell lymphoma and mantle cell lymphoma in situ in reactive lymphoid tissues. Mod. Pathol. 2012, 25, 1629–1636. [Google Scholar] [CrossRef][Green Version]
- Nybakken, G.E.; Bala, R.; Gratzinger, D.; Jones, C.D.; Zehnder, J.L.; Bangs, C.D.; Cherry, A.; Warnke, R.A.; Natkunam, Y. Isolated follicles enriched for centroblasts and lacking t(14;18)/BCL2 in lymphoid tissue: Diagnostic and clinical implications. PLoS ONE 2016, 11, e0151735. [Google Scholar] [CrossRef]
- Granai, M.; Lazzi, S. Early pattern of large B-cell lymphoma with IRF4 rearrangement. Blood 2020, 136, 769. [Google Scholar] [CrossRef]
- Kumar, J.; Butzmann, A.; Wu, S.; Easly, S.; Zehnder, J.L.; Warnke, R.A.; Bangs, C.D.; Jangam, D.; Cherry, A.; Lau, J.; et al. Indolent in situ B-Cell neoplasms with MYC rearrangements show somatic mutations in MYC and TNFRSF14 by Next-generation Sequencing. Am. J. Surg. Pathol. 2019, 43, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G., Jr.; Meadows, A.T.; Nichols, W.W.; Hill, R. Chromosomal deletion and retinoblastoma. N. Engl. J. Med. 1976, 295, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Ozkaya, N.; Hariharan, A. Novel insights into the early histopathogenesis of immunodeficiency-associated Burkitt lymphoma: A case report of Burkitt microlymphoma arising within HIV lymphadenitis. Histopathology 2016, 69, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Granai, M.; Lazzi, S.; Mancini, V.; Akarca, A.; Santi, R.; Vergoni, F.; Sorrentino, E.; Guazzo, R.; Mundo, L.; Cevenini, G.; et al. Burkitt lymphoma with a granulomatous reaction: An M1/Th1-polarised microenvironment is associated with controlled growth and spontaneous regression. Histopathology 2021, 80, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Camacho, F.I.; Algara, P.; Mollejo, M.; Garcìa, J.F.; Montalba, C.; Martìnez, N.; Sánchez-Beato, M.; Piris, M.A. Nodal marginal zone B-cell lymphoma: A heterogenous tumor. A comprehensive analysis of a series of 27 cases. Am. J. Surg. Pathol. 2003, 27, 762–771. [Google Scholar] [CrossRef]
- Poppema, S.; Gilchrist, M. Monocytoid B cells and bcl-2 protein negative in contrast to marginal zone cells and monocytoid B-cell lymphoma. Int. J. Surg. Pathol. 1995, 2, 277. [Google Scholar]
- Kojima, M.; Nakamura, S.; Itoh, H.; Yoshida, K.; Shimizu, K.; Motoori, T.; Yamane, N.; Joshita, T.; Suchi, T. Occurrence of monocytoid B-cells in reactive lymph node lesions. Pathol. Res. Pract. 1998, 194, 559–565. [Google Scholar] [CrossRef]
- Mohrmann, R.L.; Nathwani, B.N.; Brynes, R.K.; Sheibani, K. Hodgkin’s disease occurring in monocytoid B-cell clusters. Am. J. Clin. Pathol. 1991, 95, 802–808. [Google Scholar] [CrossRef]
- Plank, L.; Hansmann, M.L.; Fischer, R. Monocytoid B-cells occurring in Hodgkin’s disease. Virchows Arch. 1994, 424, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, V.; Zen, Y.; Chan, J.K.; Yi, E.E.; Sato, Y.; Yoshino, T.; Klöppel, G.; Heathcote, J.G.; Khosroshahi, A.; Ferry, J.A.; et al. Consensus statement on the pathology of IgG4-related disease. Mod. Pathol. 2012, 25, 1181–1192. [Google Scholar] [CrossRef]
- Cheuk, W.; Yuen, H.K.L.; Chan, A.C.L.; Shih, L.Y.; Kuo, T.T.; Ma, M.W.; Lo, Y.F.; Chan, W.K.; Chan, J.K. Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryoadenitis: A previously undescribed complication of IgG4-related sclerosing disease. Am. J. Surg. Pathol. 2008, 32, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ohshima, K.; Ichimura, K.; Sato, M.; Yamadori, I.; Tanaka, T.; Takata, K.; Morito, T.; Kondo, E.; Yoshino, T. Ocular adnexal IgG4-related disease has uniform clinicopathology. Pathol. Int. 2008, 58, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Ferry, J.A. IgG4-related lymphadenopathy and IgG4-related lymphoma: Moving targets. Diagn. Histopathol. 2013, 19, 128–139. [Google Scholar] [CrossRef]
- Kanda, G.; Ryu, T.; Shirai, T.; Ijichi, M.; Hishima, T.; Kitamura, S.; Bandai, Y. Peripheral T-cell lymphoma that developed during the follow-up of IgG4-related disease. Intern. Med. 2011, 50, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Hodohara, K.; Yoshida, K.; Kagotani, A.; Iwai, M.; Yoshii, M.; Okuno, H.; Horinouchi, A.; Nakanishi, R.; Harada, A.; et al. Occurrence of anaplastic large cell lymphoma following IgG4-related autoimmune pancreatitis and cholecystitis and diffuse large B-cell lymphoma. Int. J. Clin. Exp. Pathol. 2013, 6, 2560–2568. [Google Scholar]
- Uehara, T.; Ikeda, S.; Hamano, H.; Kawa, S.; Moteki, H.; Matsuda, K.; Kaneko, Y.; Hara, E. A case of Mikulicz’s disease complicated by malignant lymphoma: A postmortem histopathological finding. Intern. Med. 2012, 51, 419–423. [Google Scholar] [CrossRef][Green Version]
- Takahashi, N.; Ghazale, A.H.; Smyrk, T.C.; Mandrekar, J.N.; Chari, S.T. Possible association between IgG4-associated systemic disease with or without autoimmune pancreatitis and non-Hodgkin lymphoma. Pancreas 2009, 38, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takahashi, H.; Tabeya, T.; Suzuki, C.; Naishiro, Y.; Ishigami, K.; Yajima, H.; Shimizu, Y.; Obara, M.; Yamamoto, H.; et al. Risk of malignancies in IgG4- related disease. Mod. Rheumatol. 2012, 22, 414–418. [Google Scholar] [CrossRef]
- Gupta, R.; Khosroshahi, A.; Shinagare, S.; Fernandez, C.; Ferrone, C.; Lauwers, G.Y.; Stone, J.H.; Deshpande, V. Does autoimmune pancreatitis increase the risk of pancreatic carcinoma? a retrospective analysis of pancreatic resections. Pancreas 2013, 42, 506–510. [Google Scholar] [CrossRef]
- Kussick, S.J.; Kalnoski, M.; Braziel, R.M.; Wood, B.L. Prominent clonal B-cell populations identified by flow cytometry in histologically reactive lymphoid proliferations. Am. J. Clin. Pathol. 2004, 121, 464–472. [Google Scholar] [CrossRef]
- Vivian, L.F.; Magnoli, F.; Campiotti, L.; Chini, C.; Calabrese, G.; Sessa, F.; Tibiletti, M.G.; Uccella, S. Composite follicular lymphoma and “early” (in situ and mantle zone growth pattern) mantle cell neoplasia: A rare entity with peculiar cytogenetic and clinical features. Pathol. Res. Pract. 2020, 216, 153067. [Google Scholar] [CrossRef] [PubMed]
- Fend, F.; Quintanilla-Martinez, L.; Kumar, S.; Beaty, M.W.; Blum, L.; Sorbara, L.; Jaffe, E.S.; Raffeld, M. Composite low grade B-cell lymphomas with two immunophenotypically distinct cell populations are true biclonal lymphomas. A molecular analysis using laser capture microdissection. Am. J. Pathol. 1999, 154, 1857–1866. [Google Scholar] [CrossRef]
- Criel, A.; Michaux, L.; de Wolf-Peeters, C. The concept of typical and atypical chronic lymphocytic leukaemia. Leuk. Lymphoma 1999, 33, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Matutes, E.; Oscier, D.; Garcia-Marco, J.; Ellis, J.; Copplestone, A.; Gillingham, R.; Hamblin, T.; Lens, D.; Swansbury, G.J.; Catovsky, D. Trisomy 12 defines a group of CLL with atypical morphology: Correlation between cytogenetic, clinical and laboratory features in 544 patients. Br. J. Haematol. 1996, 92, 382–388. [Google Scholar] [CrossRef]
- Lazzi, S.; Granai, M.; Capanni, M.; Fend, F. Unusual presentation of extra-nodal double-hit follicular lymphoma: A case report. BMC Gastroenterol. 2022, 22, 254. [Google Scholar] [CrossRef]
- Katsushima, H.; Fukuhara, N.; Konosu-Fukaya, S.; Himuro, M.; Kitawaki, Y.; Ichikawa, S.; Ishizawa, K.; Sasano, H.; Harigae, H.; Ichinohasama, R. Does double-hit follicular lymphoma with translocations of MYC and BCL2 change the definition of transformation? Leuk. Lymphoma 2018, 59, 758–762. [Google Scholar] [CrossRef]
- Miyaoka, M.; Kikuti, Y.Y.; Carreras, J.; Ikoma, H.; Hiraiwa, S.; Ichiki, A.; Kojima, M.; Ando, K.; Yokose, T.; Sakai, R.; et al. Clinicopathological and genomic analysis of double-hit follicular lymphoma: Comparison with high-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements. Mod. Pathol. 2018, 31, 313–326. [Google Scholar] [CrossRef]
- Miao, Y.; Hu, S.; Lu, X.; Li, S.; Wang, W.; Medeiros, L.J.; Lin, P. Double-hit follicular lymphoma with MYC and BCL2 translocations: A study of 7 cases with a review of literature. Hum. Pathol. 2016, 58, 72–77. [Google Scholar] [CrossRef]
- Tomita, N.; Tokunaka, M.; Nakamura, N.; Takeuchi, K.; Koike, J.; Motomura, S.; Miyamoto, K.; Kikuchi, A.; Hyo, R.; Yakushijin, Y.; et al. Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 and MYC translocations. Haematologica 2009, 94, 935–943. [Google Scholar] [CrossRef]
- Christie, L.; Kernohan, N.; Levison, D.; Sales, M.; Cunningham, J.; Gillespie, K.; Batstone, P.; Meiklejohn, D.; Goodlad, J. C-MYC translocation in t(14;18) positive follicular lymphoma at presentation: An adverse prognostic indicator? Leuk. Lymphoma 2008, 49, 470–476. [Google Scholar] [CrossRef]
- Landgren, O.; Albitar, M.; Ma, W.; Abbasi, F.; Hayes, R.B.; Ghia, P.; Marti, G.E.; Caporaso, N. B-cell clones as early markers for chronic lymphocytic leukemia. N. Engl. J. Med. 2009, 360, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Nann, D.; Ramis-Zaldivar, J.E.; Müller, I.; Gonzalez-Farre, B.; Schmidt, J.; Egan, C.; Salmeron-Villalobos, J.; Clot, G.; Mattern, S.; Otto, F.; et al. Follicular lymphoma t(14;18)-negative is genetically a heterogeneous disease. Blood Adv. 2020, 4, 5652–5665. [Google Scholar] [CrossRef] [PubMed]
- Nam-Cha, S.H.; San-Millan, B.; Mollejo, M.; Garcia-Cosio, M.; Garijo, G.; Gomez, M.; Warnke, R.A.; Jaffe, E.S.; Piris, M.A. Light-chain-restricted germinal centres in reactive lymphadenitis: Report of eight cases. Histopathology 2008, 52, 436–444. [Google Scholar] [CrossRef]
- Attygalle, A.D.; Liu, H.; Shirali, S.; Diss, T.C.; Loddenkemper, C.; Stein, H.; Dogan, A.; Du, M.Q.; Isaacson, P.G. Atypical marginal zone hyperplasia of mucosa-associated lymphoid tissue: A reactive condition of childhood showing immunoglobulin lambda light-chain restriction. Blood 2004, 104, 3343–3348. [Google Scholar] [CrossRef]
- Weniger, M.A.; Küppers, R. Molecular biology of Hodgkin lymphoma. Leukemia 2021, 35, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, D.; Sherigar, J. Concurrent small bowel lymphoma and mycobacterial infection: The use of adesonosine deaminase activity and polymerase chain reaction to facilitated rapid diagnosis and treatment. Eur. J. Gastroenterol. Hepatol. 2006, 18, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, M.; Ouedraogo, S.M.; Cisse, R.; Lougue, C.; Badoum, G.; Sigani, A.; Drabo, Y.J. Active tuberculosis in a patient with Hodgkin’s disease. A case report. Rev. Pneumol. Clin. 2000, 56, 33–35. [Google Scholar] [PubMed]
- Audebert, F.; Schneidewind, A.; Hartmann, P.; Kullmann, F.; Schölmerich, J. Lymph node tuberculosis as primary manifestation of Hodgkin’s disease. Med. Klin. 2006, 101, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.O.; Soll, B.A.; Issel, B.F.; Fraser, C. Bronchus-associated lymphoid tissue lymphoma and mycobacterium tuberculosis infection: An unusual case and a review of the literature. Respir. Care 2007, 52, 755–758. [Google Scholar]
- Inadome, Y.; Ikezawa, T.; Oyasu, R.; Noguchi, M. Malignant lymphoma of bronchus-associated lymphoid tissue (BALT) coexistent with pulmonary tuberculosis. Pathol. Int. 2001, 51, 807–811. [Google Scholar] [CrossRef]
- Centkowski, P.; Sawczuk-Chabin, J.; Prochorec, M.; Warzocha, K. Hodgkin’s lymphoma and tuberculosis coexistence in cervical lymph nodes. Leuk. Lymphoma 2005, 46, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Bellido, M.C.; Martino, R.; Martínez, C.; Sureda, A.; Brunet, S. Extrapulmonary tuberculosis and non- Hodgkin’s lymphoma: Coexistence in an abdominal lymph node. Haematologica 1995, 80, 482–483. [Google Scholar] [PubMed]
- Fanourgiakis, P.; Mylona, E.; Androulakis, I.I.; Eftychiou, C.; Vryonis, E.; Georgala, A.; Skoutelis, A.; Aoun, M. Non-Hodgkin’s lymphoma and tuberculosis coexistence in the same organs: A report of two cases. Postgrad. Med. J. 2008, 84, 276–277. [Google Scholar] [CrossRef]
- Gaur, S.; Trayner, E.; Aish, L.; Weinstein, R. Bronchus-associated lymphoid tissue lymphoma arising in a patient with bronchiectasis and chronic Mycobacterium avium infection. Am. J. Hematol. 2004, 77, 22–25. [Google Scholar] [CrossRef]
- Geyer, J.T.; Niesvizky, R.; Jayabalan, D.S.; Mathew, S.; Subramaniyam, S.; Geyer, A.I.; Orazi, A.; Ely, S.A. IgG4 plasma cell myeloma: New insights into the pathogenesis of IgG4-related disease. Mod. Pathol. 2014, 27, 375–381. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).