Transformed Waldenström Macroglobulinemia: Update on Diagnosis, Prognosis and Treatment
Abstract
1. Introduction
2. Epidemiology and Risk Factors
3. Clinical Presentation and Diagnosis
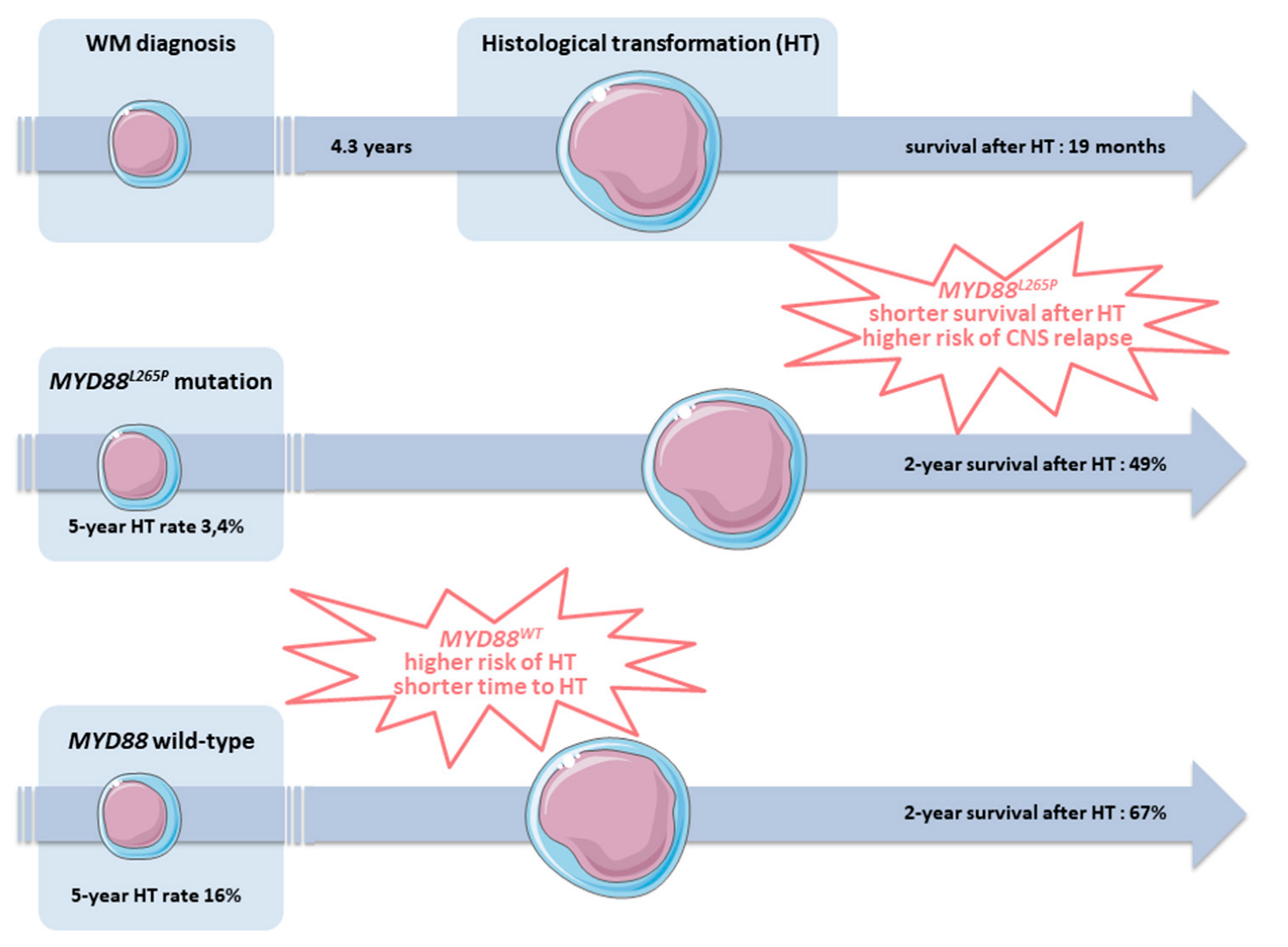
4. Morphology and Clonal Evolution
5. Prognosis
| Clinical presentation |
|
| Diagnosis |
|
| Prognosis |
|
6. Treatment Options
6.1. Chemo-Immunotherapy
6.2. Central Nervous System Prophylaxis
6.3. Stem Cell Transplantation
|
6.4. Novel Agents
6.5. CAR T-Cells
7. Proposed Management of HT in WM
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, T.A.; Frenkel, E.P. An unusual case of macroglobulinemia. Arch. Intern. Med. 1967, 119, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Osterberg, G.; Rausing, A. Reticulum cell sarcoma in Waldenström’s macroglobulinemia after chlorambucil treatment. Acta Med. Scand. 1970, 188, 497–504. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, M.R.; Fudenberg, H.H. Macroglobulinemia: An analysis for forty patients. Blood 1972, 39, 874–889. [Google Scholar] [CrossRef]
- Skarin, A.T.; Long, J.C. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 6-1978. N. Engl. J. Med. 1978, 298, 387–396. [Google Scholar]
- Choi, Y.J.; Yeh, G.; Reiner, L.; Spielvogel, A. Immunoblastic sarcoma following Waldenström’s macroglobulinemia. Am. J. Clin. Pathol. 1979, 71, 121–124. [Google Scholar] [CrossRef]
- Leonhard, S.A.; Muhleman, A.F.; Hurtubise, P.E.; Martelo, O. J Emergence of immunoblastic sarcoma in Waldenstrom’s macroglobulinemia. Cancer 1980, 45, 3102–3107. [Google Scholar] [CrossRef]
- García, R.; Hernández, J.M.; Caballero, M.D.; González, M.; San Miguel, J.F. Immunoblastic lymphoma and associated non-lymphoid malignancies following two cases of Waldenström’s macroglobulinemia. A review of the literature. Eur. J. Haematol. 1993, 50, 299–301. [Google Scholar] [CrossRef]
- Durot, E.; Tomowiak, C.; Michallet, A.S.; Dupuis, J.; Hivert, B.; Leprêtre, S.; Toussaint, E.; Godet, S.; Merabet, F.; Van Den Neste, E.; et al. Transformed Waldenström macroglobulinaemia: Clinical presentation and outcome. A multi-institutional retrospective study of 77 cases from the French Innovative Leukemia Organization (FILO). Br. J. Haematol. 2017, 179, 439–448. [Google Scholar] [CrossRef]
- Castillo, J.J.; Gustine, J.; Meid, K.; Dubeau, T.; Hunter, Z.R.; Treon, S.P. Histological transformation to diffuse large B-cell lymphoma in patients with Waldenström macroglobulinemia. Am. J. Hematol. 2016, 91, 1032–1035. [Google Scholar] [CrossRef]
- Zanwar, S.; Abeykoon, J.P.; Durot, E.; King, R.; Perez Burbano, G.E.; Kumar, S.; Gertz, M.A.; Quinquenel, A.; Delmer, A.; Gonsalves, W.; et al. Impact of MYD88L265P mutation status on histological transformation of Waldenström Macroglobulinemia. Am. J. Hematol. 2020, 95, 274–281. [Google Scholar] [CrossRef]
- Durot, E.; Kanagaratnam, L.; Zanwar, S.; Kastritis, E.; D’Sa, S.; Garcia-Sanz, R.; Tomowiak, C.; Hivert, B.; Toussaint, E.; Protin, C.; et al. A prognostic index predicting survival in transformed Waldenström macroglobulinemia. Haematologica 2021, 106, 2940–2946. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Gustine, J.; Xu, L.; Manning, R.J.; Tsakmaklis, N.; Demos, M.; Meid, K.; Guerrera, M.L.; Munshi, M.; Chan, G.; et al. MYD88 wild-type Waldenstrom Macroglobulinaemia: Differential diagnosis, risk of histological transformation, and overall survival. Br. J. Haematol. 2018, 180, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Durot, E.; Kanagaratnam, L.; Zanwar, S.; Toussaint, E.; Kastritis, E.; D’Sa, S.; Alcoceba, M.; Tomowiak, C.; Hivert, B.; Protin, C.; et al. High frequency of central nervous system involvement in transformed Waldenström macroglobulinemia. Blood Adv. 2022, 6, 3655–3658. [Google Scholar] [CrossRef] [PubMed]
- Leleu, X.; Soumerai, J.; Roccaro, A.; Hatjiharissi, E.; Hunter, Z.R.; Manning, R.; Ciccarelli, B.T.; Sacco, A.; Ioakimidis, L.; Adamia, S.; et al. Increased incidence of transformation and myelodysplasia/acute leukemia in patients with Waldenström macroglobulinemia treated with nucleoside analogs. J. Clin. Oncol. 2009, 27, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Leblond, V.; Johnson, S.; Chevret, S.; Copplestone, A.; Rule, S.; Tournilhac, O.; Seymour, J.F.; Patmore, R.D.; Wright, D.; Morel, P.; et al. Results of a randomized trial of chlorambucil versus fludarabine for patients with untreated Waldenström macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic lymphoma. J. Clin. Oncol. 2013, 31, 301–307. [Google Scholar] [CrossRef]
- Tam, C.S.; Opat, S.; D’Sa, S.; Jurczak, W.; Lee, H.P.; Cull, G.; Owen, R.G.; Marlton, P.; Wahlin, B.E.; Sanz, R.G.; et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: The ASPEN study. Blood 2020, 136, 2038–2050. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Meid, K.; Gustine, J.N.; Leventoff, C.; White, T.; Flynn, C.A.; Sarosiek, S.; Demos, M.G.; Guerrera, M.L.; Kofides, A.; et al. Long-term follow-up of ibrutinib monotherapy in treatment-naive patients with Waldenstrom macroglobulinemia. Leukemia 2022, 36, 532–539. [Google Scholar] [CrossRef]
- Treon, S.P.; Meid, K.; Gustine, J.; Yang, G.; Xu, L.; Liu, X.; Patterson, C.J.; Hunter, Z.R.; Branagan, A.R.; Laubach, J.P.; et al. Long-Term Follow-Up of Ibrutinib Monotherapy in Symptomatic, Previously Treated Patients With Waldenström Macroglobulinemia. J. Clin. Oncol. 2021, 39, 565–575. [Google Scholar] [CrossRef]
- Buske, C.; Tedeschi, A.; Trotman, J.; García-Sanz, R.; MacDonald, D.; Leblond, V.; Mahe, B.; Herbaux, C.; Matous, J.V.; Tam, C.S.; et al. Ibrutinib Plus Rituximab Versus Placebo Plus Rituximab for Waldenström’s Macroglobulinemia: Final Analysis from the Randomized Phase III iNNOVATE Study. J. Clin. Oncol. 2022, 40, 52–62. [Google Scholar] [CrossRef]
- Hunter, Z.R.; Xu, L.; Tsakmaklis, N.; Demos, M.G.; Kofides, A.; Jimenez, C.; Chan, G.G.; Chen, J.; Liu, X.; Munshi, M.; et al. Insights into the genomic landscape of MYD88 wild-type Waldenström macroglobulinemia. Blood Adv. 2018, 2, 2937–2946. [Google Scholar] [CrossRef]
- Banwait, R.; Aljawai, Y.; Cappuccio, J.; McDiarmid, S.; Morgan, E.A.; Leblebjian, H.; Roccaro, A.M.; Laubach, J.; Castillo, J.J.; Paba-Prada, C.; et al. Extramedullary Waldenström macroglobulinemia. Am. J. Hematol. 2015, 90, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Stien, S.; Durot, E.; Durlach, A.; Beylot-Barry, M.; Adamski, H.; Beltraminelli, H.; Bohelay, G.; Carlotti, A.; Carpentier, O.; Cornillet, P.; et al. Cutaneous Involvement in Waldenström’s Macroglobulinaemia. Acta Derm. Venereol. 2020, 100, adv00225. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- King, R.L.; Goodlad, J.R.; Calaminici, M.; Dotlic, S.; Montes-Moreno, S.; Oschlies, I.; Ponzoni, M.; Traverse-Glehen, A.; Ott, G.; Ferry, J.A. Lymphomas arising in immune-privileged sites: Insights into biology, diagnosis, and pathogenesis. Virchows Arch. 2020, 476, 647–665. [Google Scholar] [CrossRef] [PubMed]
- Mauro, F.R.; Chauvie, S.; Paoloni, F.; Biggi, A.; Cimino, G.; Rago, A.; Gentile, M.; Morabito, F.; Coscia, M.; Bellò, M.; et al. Diagnostic and prognostic role of PET/CT in patients with chronic lymphocytic leukemia and progressive disease. Leukemia 2015, 29, 1360–1365. [Google Scholar] [CrossRef]
- Banwait, R.; O’Regan, K.; Campigotto, F.; Harris, B.; Yarar, D.; Bagshaw, M.; Leleu, X.; Leduc, R.; Ramaiya, N.; Weller, E.; et al. The role of 18F-FDG PET/CT imaging in Waldenstrom macroglobulinemia. Am. J. Hematol. 2011, 86, 567–572. [Google Scholar] [CrossRef]
- Owen, R.G.; Bynoe, A.G.; Varghese, A.; de Tute, R.M.; Rawstron, A.C. Heterogeneity of histological transformation events in Waldenström’s macroglobulinemia (WM) and related disorders. Clin. Lymphoma Myeloma Leuk. 2011, 11, 176–179. [Google Scholar] [CrossRef]
- Lin, P.; Mansoor, A.; Bueso-Ramos, C.; Hao, S.; Lai, R.; Medeiros, L.J. Diffuse large B-cell lymphoma occurring in patients with lymphoplasmacytic lymphoma/Waldenström macroglobulinemia. Clinicopathologic features of 12 cases. Am. J. Clin. Pathol. 2003, 120, 246–253. [Google Scholar] [CrossRef]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Gascoyne, R.D.; Delabie, J.; Ott, G.; Müller-Hermelink, H.K.; Campo, E.; Braziel, R.M.; Jaffe, E.S.; et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef]
- Boiza-Sánchez, M.; Manso, R.; Balagué, O.; Chamizo, C.; Askari, E.; Salgado, R.N.; Blas-López, C.; Aguirregoicoa-García, E.; Menárguez, J.; Santonja, C.; et al. Lymphoplasmacytic lymphoma associated with diffuse large B-cell lymphoma: Progression or divergent evolution? PLoS ONE 2020, 15, e0241634. [Google Scholar]
- Rossi, D.; Spina, V.; Deambrogi, C.; Rasi, S.; Laurenti, L.; Stamatopoulos, K.; Arcaini, L.; Lucioni, M.; Rocque, G.B.; Xu-Monette, Z.Y.; et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood 2011, 117, 3391–3401. [Google Scholar] [CrossRef] [PubMed]
- Talaulikar, D.; Biscoe, A.; Lim, J.H.; Gibson, J.; Arthur, C.; Mackinlay, N.; Saxena, K.; Cheng, Y.Y.; Chen, V.M. Genetic analysis of Diffuse Large B-cell Lymphoma occurring in cases with antecedent Waldenström Macroglobulinaemia reveals different patterns of clonal evolution. Br. J. Haematol. 2019, 185, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C.; Alonso-Álvarez, S.; Alcoceba, M.; Ordóñez, G.R.; García-Álvarez, M.; Prieto-Conde, M.I.; Chillón, M.C.; Balanzategui, A.; Corral, R.; Marín, L.A.; et al. From Waldenström’s macroglobulinemia to aggressive diffuse large B-cell lymphoma: A whole-exome analysis of abnormalities leading to transformation. Blood Cancer J. 2017, 7, e591. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; O’Brien, S.; Khouri, I.; Giles, F.J.; Kantarjian, H.M.; Champlin, R.; Wen, S.; Do, K.A.; Smith, S.C.; Lerner, S.; et al. Clinical outcomes and prognostic factors in patients with Richter’s syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J. Clin. Oncol. 2006, 24, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, C.; Anastasia, A.; Chiarenza, A.; Marcheselli, L.; Cavallo, F.; Rattotti, S.; Botto, B.; Ferrari, A.; Nassi, L.; Pagani, C.; et al. Outcome of transformed follicular lymphoma worsens according to the timing of transformation and to the number of previous therapies. A retrospective multicenter study on behalf of Fondazione Italiana Linfomi (FIL). Br. J. Haematol. 2019, 185, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Link, B.K.; Maurer, M.J.; Nowakowski, G.S.; Ansell, S.M.; Macon, W.R.; Syrbu, S.I.; Slager, S.L.; Thompson, C.A.; Inwards, D.J.; Johnston, P.B.; et al. Rates and outcomes of follicular lymphoma transformation in the immunochemotherapy era: A report from the University of Iowa/Mayo Clinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J. Clin. Oncol. 2013, 31, 3272–3278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Link, B.K.; Witzig, T.E.; Maurer, M.J.; Allmer, C.; King, R.L.; Feldman, A.L.; Habermann, T.M.; Ansell, S.M.; Slager, S.L.; et al. Impact of concurrent indolent lymphoma on the clinical outcome of newly diagnosed diffuse large B-cell lymphoma. Blood 2019, 134, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Rovira, J.; Karube, K.; Valera, A.; Colomer, D.; Enjuanes, A.; Colomo, L.; Martínez-Trillos, A.; Giné, E.; Dlouhy, I.; Magnano, L.; et al. MYD88 L265P Mutations, But No Other Variants, Identify a Subpopulation of DLBCL Patients of Activated B-cell Origin, Extranodal Involvement, and Poor Outcome. Clin. Cancer Res. 2016, 22, 2755–2764. [Google Scholar] [CrossRef]
- Vermaat, J.S.; Somers, S.F.; de Wreede, L.C.; Kraan, W.; de Groen, R.A.L.; Schrader, A.M.R.; Kerver, E.D.; Scheepstra, C.G.; Berenschot, H.; Deenik, W.; et al. MYD88 mutations identify a molecular subgroup of diffuse large B-cell lymphoma with an unfavorable prognosis. Haematologica 2020, 105, 424–434. [Google Scholar] [CrossRef]
- Schmitz, N.; Zeynalova, S.; Nickelsen, M.; Kansara, R.; Villa, D.; Sehn, L.H.; Glass, B.; Scott, D.W.; Gascoyne, R.D.; Connors, J.M.; et al. CNS International Prognostic Index: A Risk Model for CNS Relapse in Patients with Diffuse Large B-Cell Lymphoma Treated With R-CHOP. J. Clin. Oncol. 2016, 34, 3150–3156. [Google Scholar] [CrossRef]
- Villa, D.; Connors, J.M.; Shenkier, T.N.; Gascoyne, R.D.; Sehn, L.H.; Savage, K.J. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: The impact of the addition of rituximab to CHOP chemotherapy. Ann. Oncol. 2010, 21, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- El-Galaly, T.C.; Cheah, C.Y.; Bendtsen, M.D.; Nowakowski, G.S.; Kansara, R.; Savage, K.J.; Connors, J.M.; Sehn, L.H.; Goldschmidt, N.; Shaulov, A.; et al. Treatment strategies, outcomes and prognostic factors in 291 patients with secondary CNS involvement by diffuse large B-cell lymphoma. Eur. J. Cancer 2018, 93, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Eyre, T.A.; Djebbari, F.; Kirkwood, A.A.; Collins, G.P. Efficacy of central nervous system prophylaxis with stand-alone intrathecal chemotherapy in diffuse large B-cell lymphoma patients treated with anthracycline-based chemotherapy in the rituximab era: A systematic review. Haematologica 2020, 105, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Noia, V.M.; Reed, D.R.; McCook, A.A.; Sen, J.M.; Barlow, C.M.; Malecek, M.K.; Watkins, M.; Kahl, B.S.; Spinner, M.A.; Advani, R.; et al. Single-route CNS prophylaxis for aggressive non-Hodgkin lymphomas: Real-world outcomes from 21 US academic institutions. Blood 2022, 139, 413–423. [Google Scholar] [CrossRef]
- Puckrin, R.; El Darsa, H.; Ghosh, S.; Peters, A.; Owen, C.; Stewart, D. Ineffectiveness of high-dose methotrexate for prevention of CNS relapse in diffuse large B-cell lymphoma. Am. J. Hematol. 2021, 96, 764–771. [Google Scholar] [CrossRef]
- Fleming, M.; Huang, Y.; Dotson, E.; Bond, D.A.; Reneau, J.; Epperla, N.; Alinari, L.; Brammer, J.; Christian, B.A.; Baiocchi, R.A.; et al. Feasibility of high-dose methotrexate administered on day 1 of (R)CHOP in aggressive non-Hodgkin lymphomas. Blood Adv. 2022, 6, 460–472. [Google Scholar] [CrossRef]
- Wilson, M.R.; Eyre, T.A.; Kirkwood, A.A.; Wong Doo, N.; Soussain, C.; Choquet, S.; Martinez-Calle, N.; Preston, G.; Ahearne, M.; Schorb, E.; et al. Timing of high-dose methotrexate CNS prophylaxis in DLBCL: A multicenter international analysis of 1384 patients. Blood 2022, 139, 2499–2511. [Google Scholar] [CrossRef]
- Villa, D.; George, A.; Seymour, J.F.; Toze, C.L.; Crump, M.; Lee, C.; Buckstein, R.; Stewart, D.A.; MacDonald, D.; Foley, R.; et al. Favorable outcomes from allogeneic and autologous stem cell transplantation for patients with transformed nonfollicular indolent lymphoma. Biol. Blood Marrow Transplant. 2014, 20, 1813–1818. [Google Scholar] [CrossRef]
- Blaker, Y.N.; Eide, M.B.; Liestøl, K.; Lauritzsen, G.F.; Kolstad, A.; Fosså, A.; Smeland, E.B.; Holte, H. High dose chemotherapy with autologous stem cell transplant for patients with transformed B-cell non-Hodgkin lymphoma in the rituximab era. Leuk Lymphoma 2014, 55, 2319–2327. [Google Scholar] [CrossRef]
- Villa, D.; Crump, M.; Keating, A.; Panzarella, T.; Feng, B.; Kuruvilla, J. Outcome of patients with transformed indolent non-Hodgkin lymphoma referred for autologous stem-cell transplantation. Ann. Oncol. 2013, 24, 1603–1609. [Google Scholar] [CrossRef]
- Ban-Hoefen, M.; Vanderplas, A.; Crosby-Thompson, A.L.; Abel, G.A.; Czuczman, M.S.; Gordon, L.I.; Kaminski, M.S.; Kelly, J.; Millenson, M.; Nademanee, A.P.; et al. Transformed non-Hodgkin lymphoma in the rituximab era: Analysis of the NCCN outcomes database. Br. J. Haematol. 2013, 163, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.; Pedersen, M.B.; Vase, M.Ø.; Bendix, K.; Møller, M.B.; Johansen, P.; Jensen, B.A.; Jensen, P.; Munksgaard, L.; Brown, P.D.; et al. Outcome determinants for transformed indolent lymphomas treated with or without autologous stem-cell transplantation. Ann. Oncol. 2015, 26, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, J.; MacDonald, D.A.; Kouroukis, C.T.; Cheung, M.; Olney, H.J.; Turner, A.R.; Anglin, P.; Seftel, M.; Ismail, W.S.; Luminari, S.; et al. Salvage chemotherapy and autologous stem cell transplantation for transformed indolent lymphoma: A subset analysis of NCIC CTG LY12. Blood 2015, 126, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.K.; Lim, K.J.; Lewis, K.; Jain, P.; Qing, Y.; Feng, L.; Cheah, C.Y.; Seymour, J.F.; Ritchie, D.; Burbury, K.; et al. Autologous stem cell transplantation for untreated transformed indolent B-cell lymphoma in first remission: An international, multi-centre propensity-score-matched study. Br. J. Haematol. 2020, 191, 806–815. [Google Scholar] [CrossRef]
- Castillo, J.J.; Allan, J.N.; Siddiqi, T.; Advani, R.H.; Meid, K.; Leventoff, C.; White, T.P.; Flynn, C.A.; Sarosiek, S.; Branagan, A.R.; et al. Venetoclax in Previously Treated Waldenström Macroglobulinemia. J. Clin. Oncol. 2022, 40, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Sehn, L.H.; Johnson, P.; Zinzani, P.L.; Hong, X.; Zhu, J.; Patti, C.; Belada, D.; Samoilova, O.; Suh, C.; et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 1285–1295. [Google Scholar] [CrossRef]
- Morschhauser, F.; Feugier, P.; Flinn, I.W.; Gasiorowski, R.; Greil, R.; Illés, Á.; Johnson, N.A.; Larouche, J.F.; Lugtenburg, P.J.; Patti, C.; et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood 2021, 137, 600–609. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.A.; Kersten, M.J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef]
- Bishop, M.R.; Dickinson, M.; Purtill, D.; Barba, P.; Santoro, A.; Hamad, N.; Kato, K.; Sureda, A.; Greil, R.; Thieblemont, C.; et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Palomba, M.L.; Qualls, D.; Monette, S.; Sethi, S.; Dogan, A.; Roshal, M.; Senechal, B.; Wang, X.; Rivière, I.; Sadelain, M.; et al. CD19-directed chimeric antigen receptor T cell therapy in Waldenström macroglobulinemia: A preclinical model and initial clinical experience. J. Immunother. Cancer 2022, 10, e004128. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Bansal, R.; Jurcic, J.G.; Sawas, A.; Mapara, M.Y.; Reshef, R. Chimeric antigen receptor T cells for treatment of transformed Waldenström macroglobulinemia. Leuk Lymphoma 2020, 61, 465–468. [Google Scholar] [CrossRef] [PubMed]
| Reference | 7 | 28 | 9 | 10 | 8 |
|---|---|---|---|---|---|
| Number of patients | 16 (including 14 from review of the literature) | 12 | 20 | 50 | 77 |
| Incidence of HT | NA | 13% | 2.4% at 10 years | 4.7% at 10 years | NA |
| No treatment prior to HT | 7% | 25% | 25% | 15% | 21% |
| Median time from MW to HT (years) | 4 | 3.7 | 4.4 | 4.5 | 4.6 |
| Male sex | 69% | 33% | 60% | 66% | 65% |
| Median age at HT | NA | 68 | 70 | 66 | 71 |
| Extranodal involvement | NA | 100% | 84% | 72% | 91% |
| Elevated LDH | NA | 80% | 67% | 53% | 72% |
| Front-line treatment for HT (R)-CHOP-like HyperCVAD Rituximab containing regimen Autologous SCT | NA NA NA NA | 33% 58% 42% 8% | 80% 0% 85% 30% | 80% 0% 69% NA | 85% 0% 83% 15% |
| Overall response rate Complete response | NA NA | NA NA | NA 77% | 73% 53% | 61% 48% |
| Progression-free survival (months) | NA | NA | NA | 10 | 9 |
| Survival after HT (months) | 2 | 75% died within 10 months | 32 | 38 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durot, E.; Tomowiak, C.; Toussaint, E.; Morel, P.; Talaulikar, D.; Kapoor, P.; Castillo, J.J.; Delmer, A. Transformed Waldenström Macroglobulinemia: Update on Diagnosis, Prognosis and Treatment. Hemato 2022, 3, 650-662. https://doi.org/10.3390/hemato3040044
Durot E, Tomowiak C, Toussaint E, Morel P, Talaulikar D, Kapoor P, Castillo JJ, Delmer A. Transformed Waldenström Macroglobulinemia: Update on Diagnosis, Prognosis and Treatment. Hemato. 2022; 3(4):650-662. https://doi.org/10.3390/hemato3040044
Chicago/Turabian StyleDurot, Eric, Cécile Tomowiak, Elise Toussaint, Pierre Morel, Dipti Talaulikar, Prashant Kapoor, Jorge J. Castillo, and Alain Delmer. 2022. "Transformed Waldenström Macroglobulinemia: Update on Diagnosis, Prognosis and Treatment" Hemato 3, no. 4: 650-662. https://doi.org/10.3390/hemato3040044
APA StyleDurot, E., Tomowiak, C., Toussaint, E., Morel, P., Talaulikar, D., Kapoor, P., Castillo, J. J., & Delmer, A. (2022). Transformed Waldenström Macroglobulinemia: Update on Diagnosis, Prognosis and Treatment. Hemato, 3(4), 650-662. https://doi.org/10.3390/hemato3040044







