Dual Role of Tenebrio molitor Frass in Sustainable Agriculture: Effects on Free-Living Nematodes and Suppression of Meloidogyne incognita
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Frass Collection
2.2. Experimental Design and Justification
2.3. Free-Living Nematodes in Soil Bioassays
2.4. Nematode Extraction
2.5. Meloidogyne incognita Rearing
2.6. Meloidogyne incognita Paralysis Bioassays on the Effect of Water Extract of Collected Frass from Tenebrio molitor
2.7. Statistical Analysis
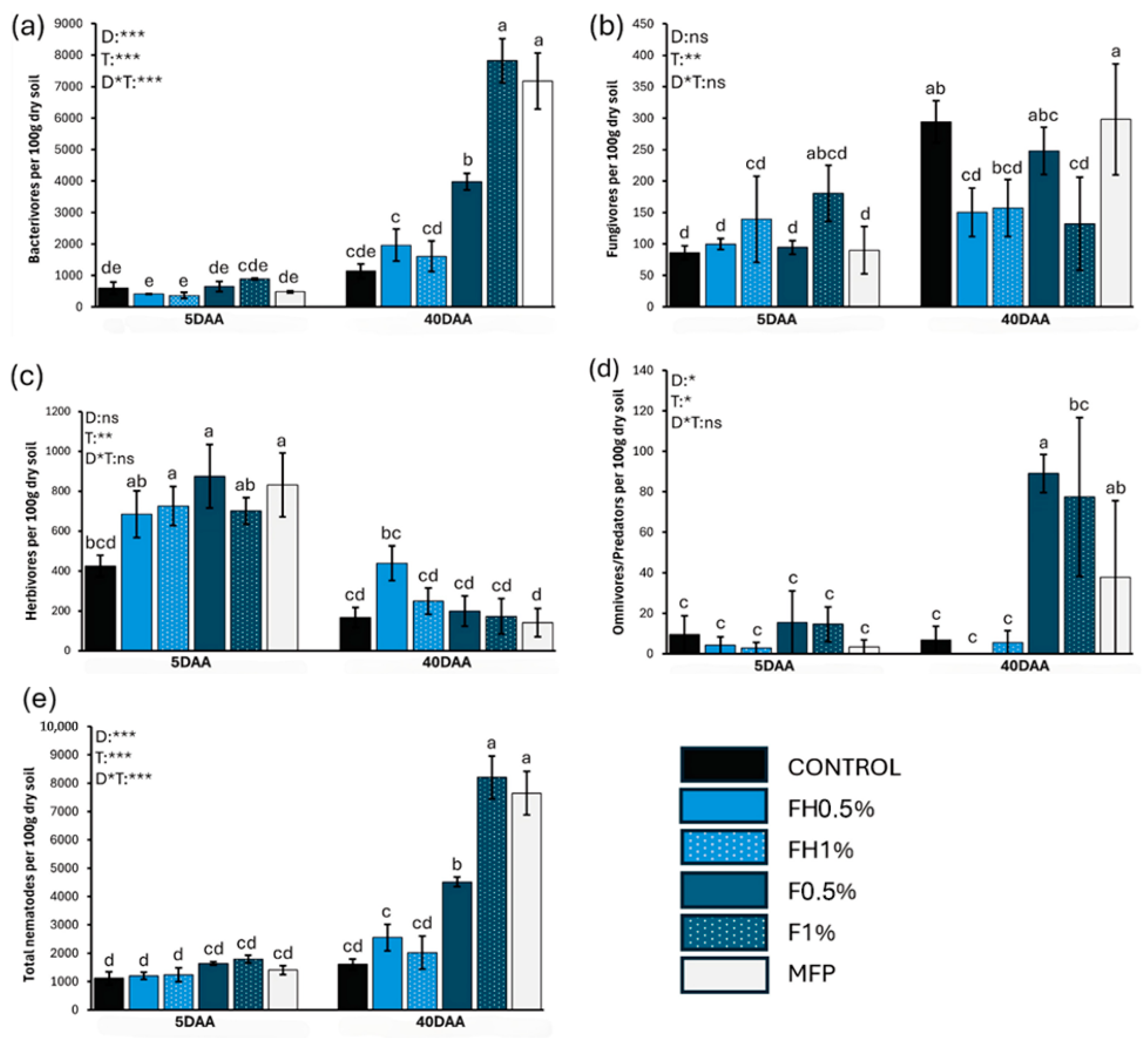
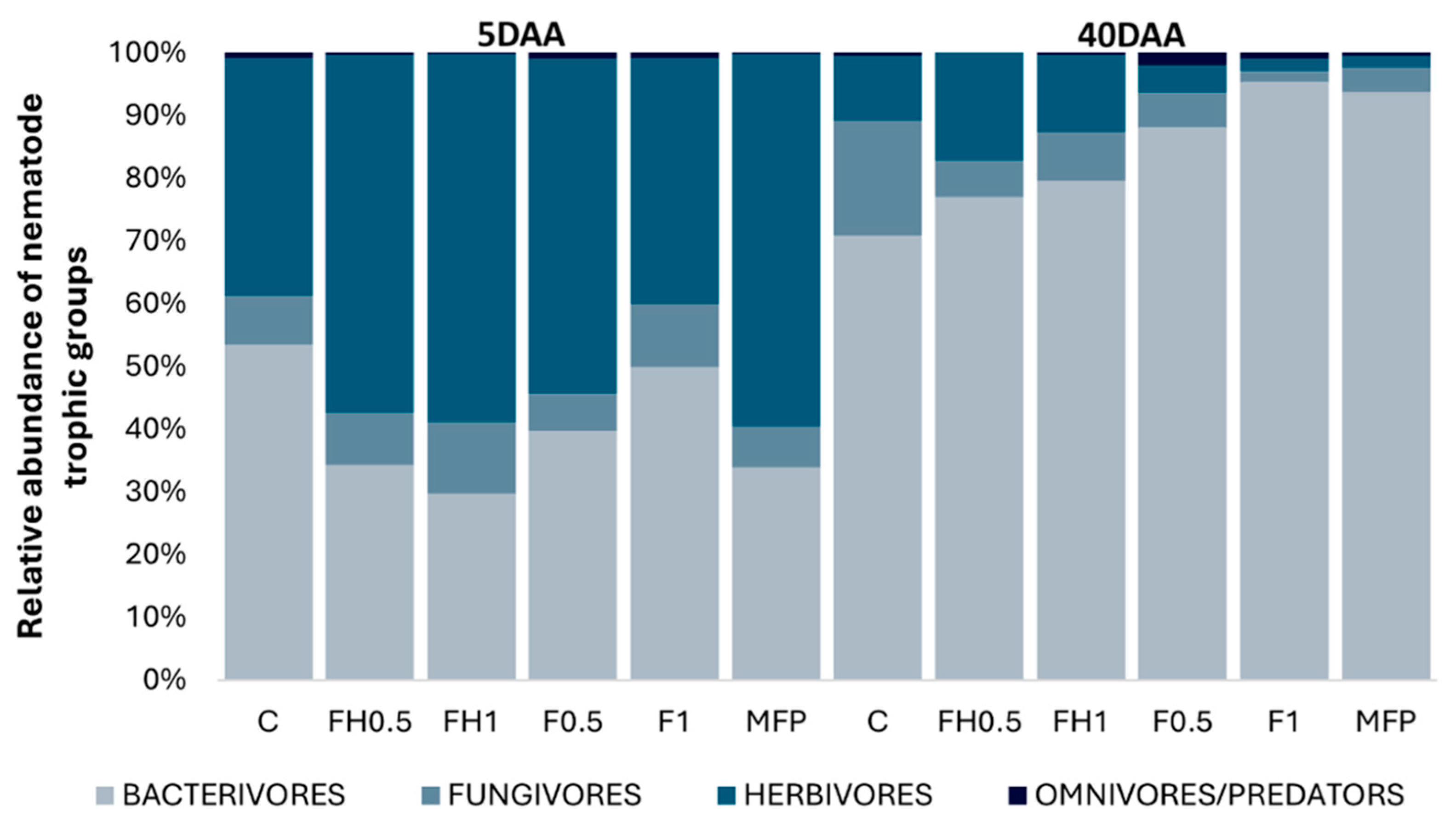
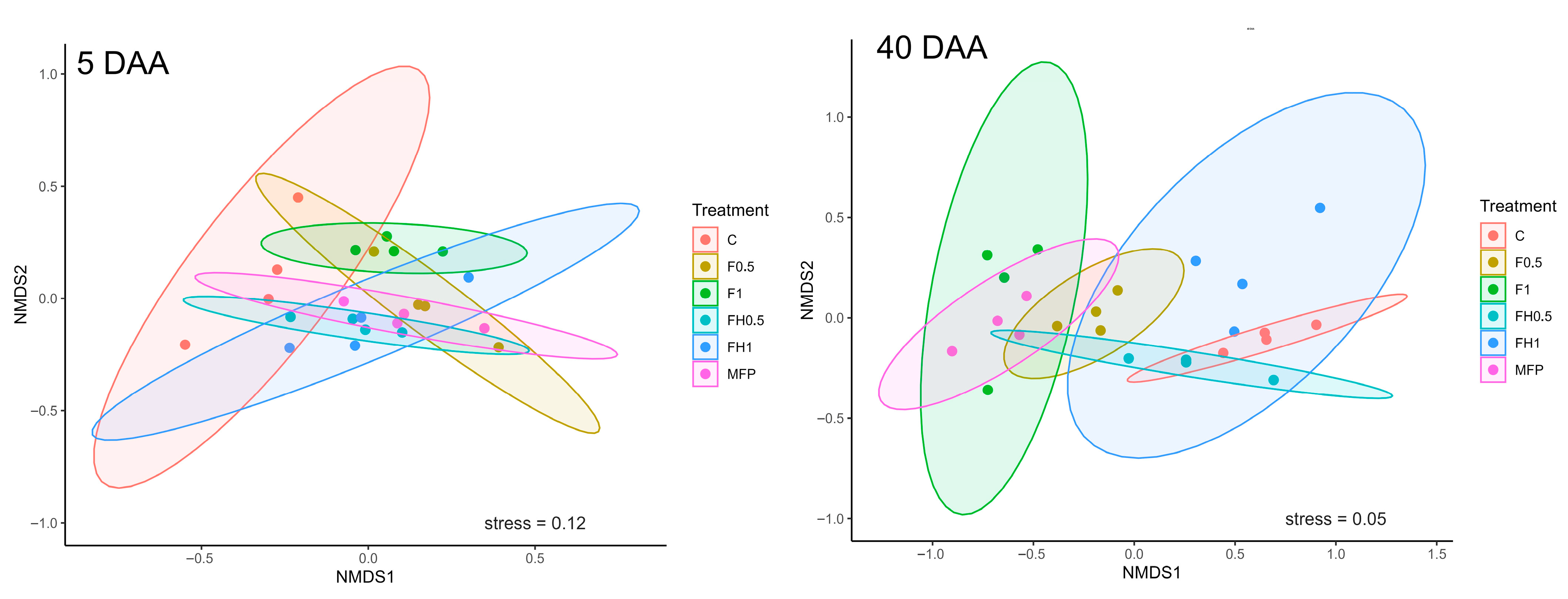
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurice, M.; Perry, R.N.; Starr, J.L. Meloidogyne species—A diverse group of novel and important plant parasites. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; CAB International: Wallingford, UK, 2010; pp. 1–17. [Google Scholar]
- Onkendi, E.M.; Kariuki, G.M.; Marais, M.; Moleleki, L.N. The threat of root-knot nematodes (Meloidogyne spp.) in Africa: A review. Plant Pathol. 2014, 63, 727–737. [Google Scholar] [CrossRef]
- Manzanilla-López, H.R.; Starr, L.J. Interactions with other pathogens. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; CAB International: Wallingford, UK, 2010; pp. 223–245. [Google Scholar]
- Ralmi, N.H.A.A.; Khandaker, M.M.; Mat, N. Occurrence and control of root knot nematode in crops: A review. Aust. J. Crop Sci. 2016, 10, 1649–1654. [Google Scholar] [CrossRef]
- Dutta, T.K.; Phani, V. The pervasive impact of global climate change on plant-nematode interaction continuum. Front. Plant Sci. 2023, 14, 1143889. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Bongers, T. Nematode indicators of organic enrichment. J. Nematol. 2006, 38, 3–12. [Google Scholar] [PubMed]
- Neher, D.A. Ecology of plant and free-living nematodes in natural and agricultural soil. Annu. Rev. Phytopathol. 2010, 48, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, U.N.; Ayres, E.; Wall, D.H.; Li, G.; Bardgett, R.D.; Wu, T.; Garey, J.R. Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties. Glob. Ecol. Biogeogr. 2014, 23, 968–978. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, T.; Wang, N.; Dou, Z.; Wang, K.; Zuo, Y. A review of soil nematodes as biological indicators for the assessment of soil health. Front. Agric. Sci. Eng. 2020, 7, 275–281. [Google Scholar] [CrossRef]
- Renčo, M.; Kováčik, P. Assessment of the nematicidal potential of vermicompost, vermicompost tea, and urea application on the potato-cyst nematodes Globodera rostochiensis and Globodera pallida. J. Plant Prot. Res. 2015, 55, 187–192. [Google Scholar] [CrossRef]
- Kekelis, P.; Papatheodorou, E.M.; Terpsidou, E.; Dimou, M.; Aschonitis, V.; Monokrousos, N. Free-living nematodes as indicators of soil quality in relation to clay content when coffee waste is applied. Agronomy 2022, 12, 2702. [Google Scholar] [CrossRef]
- Kekelis, P.; Argyropoulou, M.D.; Theofilidou, A.; Papatheodorou, E.M.; Aschonitis, V.; Monokrousos, N. Differentiations in the soil nematode community in an agricultural field after soil amendment using composted coffee waste in various concentrations. Agronomy 2023, 13, 2831. [Google Scholar] [CrossRef]
- Kekelis, P.; Pantazi, C.; Mourouzidou, S.; Theofilidou, A.; Dimou, M.D.; Aschonitis, V.; Monokrousos, N. Effect of varying olive mill wastewater concentrations on soil free-living nematode communities and lettuce growth. Sustainability 2024, 16, 3848. [Google Scholar] [CrossRef]
- Dimou, M.D.; Monokrousos, N.; Katapodis, P.; Diamantopoulou, P.A.; Argyropoulou, M.D.; Papatheodorou, E.M. Use of microbially treated olive mill wastewaters as soil organic amendments: Their short-term effects on the soil nematode community. Diversity 2023, 15, 497. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M. Optimizing safe approaches to manage plant-parasitic nematodes. Plants 2021, 10, 1911. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, G.; Haris, M.; Khan, A.A. Bio-organics management: Novel strategies to manage root-knot nematode Meloidogyne incognita, pest of vegetable crops. Gesunde Pflanz. 2023, 75, 193–209. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Dulaurent, A.-M. Assessment of the short-term fertilizer potential of mealworm frass using a pot experiment. Front. Sustain. Food Syst. 2021, 5, 714596. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; van Wickeren, N.; Hosseini, P.S.; De Neve, S. The impacts of black soldier fly frass on nitrogen availability, microbial activities, C sequestration, and plant growth. Front. Sustain. Food Syst. 2022, 6, 795950. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.Y.; Nurfikari, A.; van de Zande, E.M.; Wantulla, M.; van Loon, J.J.A.; de Boer, W.; Dicke, M. Insect frass and exuviae to promote plant growth and health. Trends Plant Sci. 2022, 27, 646–654. [Google Scholar] [CrossRef]
- Xiang, F.M.; Sheng, J.L.; Li, G.; Ma, J.J.; Wang, X.Z.; Jiang, C.L.; Zhang, Z.J. Black soldier fly larvae vermicompost alters soil biochemistry and bacterial community composition. Appl. Microbiol. Biotechnol. 2022, 106, 4315–4328. [Google Scholar] [CrossRef]
- Safitri, D.; Vandeweyer, D.; Deruytter, N.; Meijer, C.L.; Coudron, J.L.; Banach, J.L.; van der Fels-Klerx, H.J. Exploring potential uses of insect frass for agricultural production considering its nutrients and chemical and microbiological safety. J. Insects Food Feed 2024, 11, 167–190. [Google Scholar] [CrossRef]
- European Commission. EU Commission Regulation 2021/1925 of 5 November 2021 amending certain annexes to Regulation (EU) No 142/2011 as regards the requirements for placing on the market of certain insect products and the adaptation of a containment method. Off. J. Eur. Union 2021, 393, 4–8. [Google Scholar]
- Beesigamukama, D.; Subramanian, S.; Tanga, C.M. Nutrient quality and maturity status of frass fertilizer from nine edible insects. Sci. Rep. 2022, 12, 7182. [Google Scholar] [CrossRef]
- Fischer, H.; Romano, N. Fruit, vegetable, and starch mixtures on the nutritional quality of black soldier fly (Hermetia illucens) larvae and resulting frass. J. Insects Food Feed 2021, 7, 319–327. [Google Scholar] [CrossRef]
- Mostafaie, A.; Silva, A.R.R.; Pinto, J.N.; Prodana, M.; Lopes, I.G.; Murta, D.; Brooks, B.W.; Loureiro, S.; Cardoso, D.N. Towards circularity for agro-waste: Minimal soil hazards of olive pomace bioconverted frass by insect larvae as an organic fertilizer. J. Environ. Manag. 2025, 375, 124151. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, C.; Prodana, M.; Patinha, C.; Morgado, R.G.; Loureiro, S. Insect frass from upcycling vegetable by-products with cereals: Effects on the soil properties, plant development and soil invertebrate fitness. J. Environ. Manag. 2024, 372, 123234. [Google Scholar] [CrossRef]
- Abd Manan, F.F.; Yeoh, Y.-K.; Chai, T.-T.; Wong, F.-C. Unlocking the potential of black soldier fly frass as a sustainable organic fertilizer: A review of recent studies. J. Environ. Manag. 2024, 367, 121997. [Google Scholar] [CrossRef]
- Wantulla, M.; van Loon, J.J.A.; Dicke, M. Soil amendment with insect exuviae causes species-specific changes in the rhizosphere bacterial community of cabbage plants. Appl. Soil Ecol. 2023, 188, 104854. [Google Scholar] [CrossRef]
- Karkanis, A.; Ntatsi, G.; Vasilakakou, E.; Karavidas, I.; Ntanasi, T.; Rumbos, C.I.; Athanassiou, C.G. Combining Tenebrio molitor frass with inorganic nitrogen fertilizer to improve soil properties, growth parameters, and nutrient content of Sonchus oleraceus crop. Bioresour. Technol. 2025, 418, 131901. [Google Scholar] [CrossRef]
- Zunzunegui, I.; Martín-García, J.; Santamaría, O.; Poveda, J. Analysis of yellow mealworm (Tenebrio molitor) frass as a resource for sustainable agriculture in the context of insect farming industry growth. J. Clean. Prod. 2024, 460, 142608. [Google Scholar] [CrossRef]
- Antoniadis, V.; Molla, A.; Grammenou, A.; Apostolidis, V.; Athanassiou, C.G.; Rumbos, C.I.; Levizou, E. Insect frass as a novel organic soil fertilizer for the cultivation of spinach (Spinacia oleracea): Effects on soil properties, plant physiological parameters, and nutrient status. J. Soil Sci. Plant Nutr. 2023, 23, 5935–5944. [Google Scholar] [CrossRef]
- Urrutia, R.I.; Gutierrez, V.S.; Werdin-González, J.O. Sustainable approach to polystyrene management and bioinsecticide production: Biodegradation by Tenebrio molitor larvae co-fed with residual biomass and bioactivity of frass pyrolysis bio-oil against insect pests. Bioresour. Technol. 2025, 419, 132005. [Google Scholar] [CrossRef]
- Ntalli, N.; Monokrousos, N.; Rumbos, C.; Kontea, D.; Zioga, D.; Argyropoulou, M.D.; Menkissoglu-Spiroudi, U.; Tsiropoulos, N.G. Greenhouse biofumigation with Melia azedarach controls Meloidogyne spp. and enhances soil biological activity. J. Pest Sci. 2018, 91, 29–40. [Google Scholar] [CrossRef]
- S’Jacob, J.J.; van Bezooijen, J. A Manual for Practical Work in Nematology; Department of Nematology, Wageningen Agricultural University: Wageningen, The Netherland, 1984. [Google Scholar]
- Bongers, T. De Nematoden van Nederland; Koninklijke Nederlandse Natuurhistorische Vereniging: Utrecht, The Netherland, 1994. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; De Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Hussey, R.S.; Barker, K.R. A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Rényi, A. On measures of entropy and information. In Proceedings of the Fourth Berkeley Symposium on Mathematical Statistics and Probability. Volume 1: Contributions to the Theory of Statistics, Berkeley, CA, USA, 20 June–30 July 1960; University of California Press: Berkeley, CA, USA, 1961; Volume 4, pp. 547–562. [Google Scholar]
- Patil, G.P.; Taillie, C. Diversity as a concept and its measurement. J. Am. Stat. Assoc. 1982, 77, 548–561. [Google Scholar] [CrossRef]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-logistic analysis of herbicide rate response relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Monokrousos, N.; Argyropoulou, M.D.; Tzani, K.; Menkissoglou-Spiroudi, U.; Boutsis, G.; D’Addabbo, T.; Ntalli, N. The effect of botanicals with nematicidal activity on the structural and functional characteristics of the soil nematode community. Agriculture 2021, 11, 326. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, H.; Chen, L.; Yuan, Y.; Fang, H.; Luan, L.; Chen, Y.; Wang, X.; Liu, M.; Li, H.; et al. Nematodes and microorganisms interactively stimulate soil organic carbon turnover in the macroaggregates. Front. Microbiol. 2018, 9, 2803. [Google Scholar] [CrossRef]
- Theofilidou, A.; Argyropoulou, M.D.; Ntalli, N.; Kekelis, P.; Mourouzidou, S.; Zafeiriou, I.; Tsiropoulos, N.G.; Monokrousos, N. Assessing the role of Melia azedarach botanical nematicide in enhancing the structure of the free-living nematode community. Soil Syst. 2023, 7, 80. [Google Scholar] [CrossRef]
- Gebremikael, M.T.; Steel, H.; Buchan, D.; Bert, W.; De Neve, S. Nematodes enhance plant growth and nutrient uptake under C- and N-rich conditions. Sci. Rep. 2016, 6, 32862. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, L.; Paplinska, E.; Zielinski, J. The role of nematodes in decomposition of plant material in a rye field. Pedobiologia 1981, 21, 182–191. [Google Scholar] [CrossRef]
- Maina, S.; Karuri, H.; Ng’endo, R.N. Free-living nematode assemblages associated with maize residues and their ecological significance. J. Nematol. 2021, 53, e2021–e2038. [Google Scholar] [CrossRef]
- Ferris, H.; Pocasangre, L.E.; Serrano, E.; Muñoz, J.; Garcia, S.; Perichi, G.; Martinez, G. Diversity and complexity complement apparent competition: Nematode assemblages in banana plantations. Acta Oecol. 2012, 40, 11–18. [Google Scholar] [CrossRef]
- Yeates, G.W.; Wardle, D.A. Nematodes as predators and prey: Relationships to biological control and soil processes. Pedobiologia 1996, 40, 43–50. [Google Scholar] [CrossRef]
- Waldo, B.D.; Grabau, Z.J.; Mengistu, T.M.; Crow, W.T. Nematicide effects on non-target nematodes in bermudagrass. J. Nematol. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Du Preez, G.; Daneel, M.; De Goede, R.; Du Toit, M.J.; Ferris, H.; Fourie, H.; Geisen, S.; Kakouli-Duarte, T.; Korthals, G.; Sánchez-Moreno, S.; et al. Nematode-based indices in soil ecology: Application, utility, and future directions. Soil Biol. Biochem. 2022, 169, 108640. [Google Scholar] [CrossRef]
- Ferris, H. Contribution of nematodes to the structure and function of the soil food web. J. Nematol. 2010, 42, 63–67. [Google Scholar]
- Yang, L.; Sun, R.; Li, J.; Zhai, L.; Cui, H.; Fan, B.; Liu, H. Combined organic-inorganic fertilization builds higher stability of soil and root microbial networks than exclusive mineral or organic fertilization. Soil Ecol. Lett. 2023, 5, 220142. [Google Scholar] [CrossRef]
- Ling, N.; Zhu, C.; Xue, C.; Chen, H.; Duan, Y.; Peng, C.; Guo, S.; Shen, Q. Insight into how organic amendments can shape the soil microbiome in long-term field experiments as revealed by network analysis. Soil Biol. Biochem. 2016, 99, 137–149. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef]
- Borrett, S.R.; Moody, J.; Edelmann, A. The rise of network ecology: Maps of the topic diversity and scientific collaboration. Ecol. Model. 2014, 293, 111–127. [Google Scholar] [CrossRef]
- Kisaakye, J.; Beesigamukama, D.; Haukeland, S.; Subramanian, S.; Thiongo, P.K.; Kelemu, S.; Tanga, C.M. Chitin-Enriched Insect Frass Fertilizer as a Biorational Alternative for Root-Knot Nematode (Meloidogyne incognita) Management. Front. Plant Sci. 2024, 15, 1361739. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, X.F.; Wei, M.; Shi, Q.H.; Yang, F.J. Eupolyphaga frass and its extracts protected tomato from Meloidogyne incognita infestation. Ying Yong Sheng Tai Xue Bao 2015, 26, 2511–2517. (In Chinese) [Google Scholar] [PubMed]
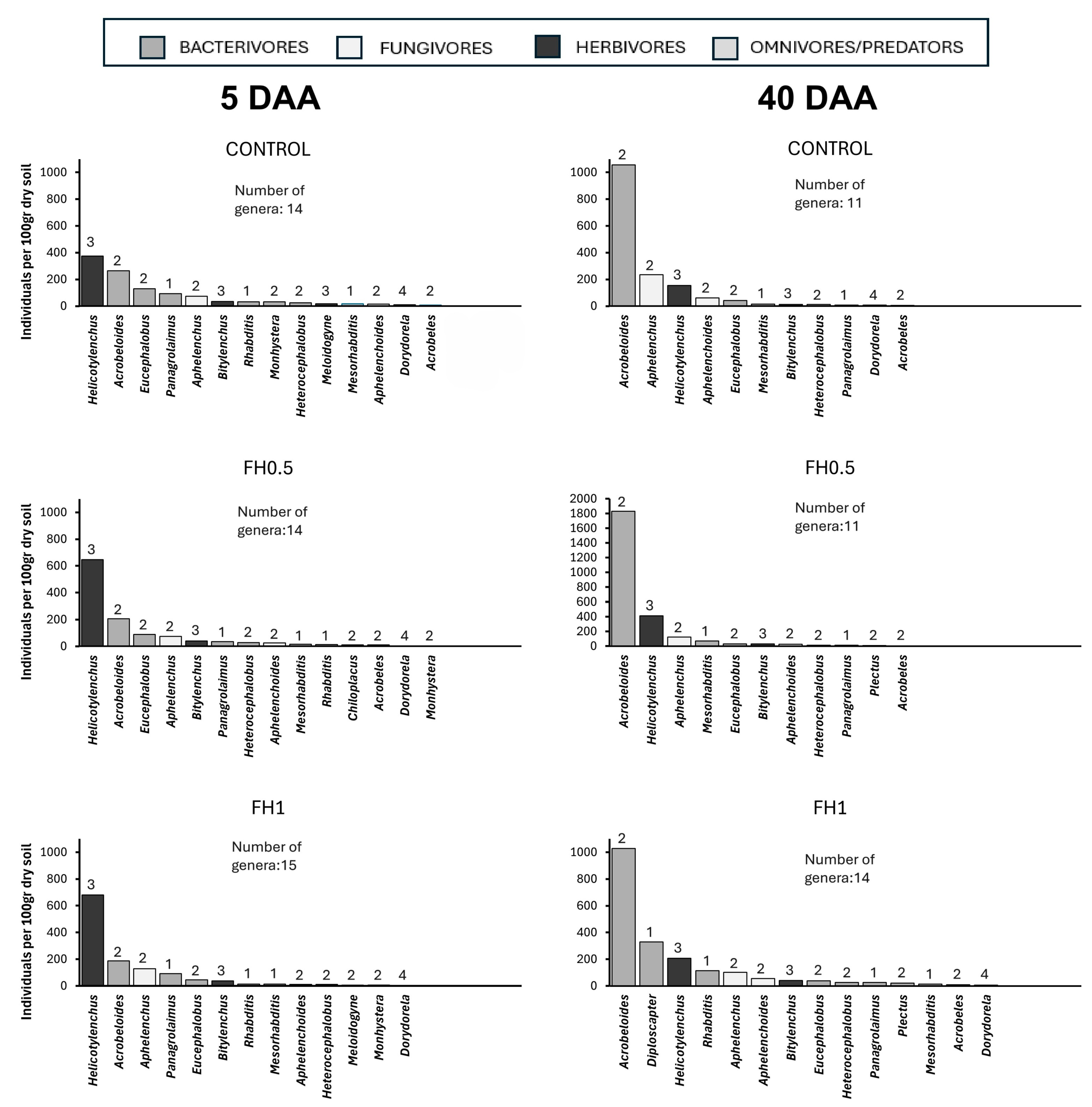
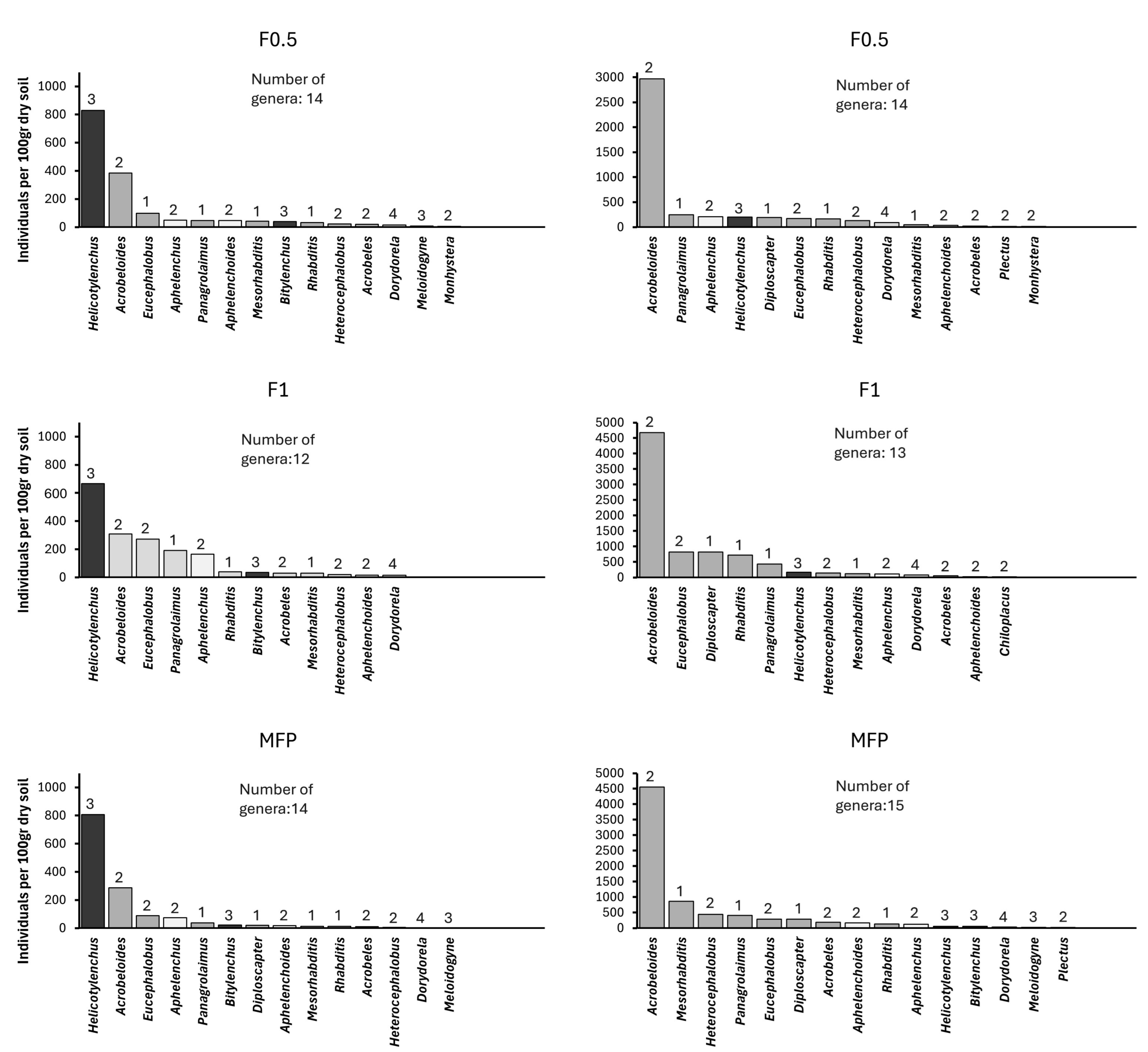
| Genera | C-p Value | Trophic Group |
|---|---|---|
| Acrobeles | 2 | Bacterivore |
| Acrobeloides | 2 | Bacterivore |
| Aphelenchoides | 2 | Fungivore |
| Aphelenchus | 2 | Fungivore |
| Bitylenchus | 3 | Ectoparasitic Herbivore |
| Helicotylenchus | 3 | Semi-Endoparasitic Herbivore |
| Chiloplacus | 2 | Bacterivore |
| Diploscapter | 1 | Bacterivore |
| Dorydorela | 4 | Omnivore |
| Eucephalobus | 2 | Bacterivore |
| Heterocephalobus | 2 | Bacterivore |
| Panagrolaimus | 1 | Bacterivore |
| Plectus | 2 | Bacterivore |
| Rhabditis | 1 | Bacterivore |
| Mesorhabditis | 1 | Bacterivore |
| Meloidogyne | 3 | Sedentary endoparasitic Herbivore |
| Monhystera | 2 | Bacterivore |
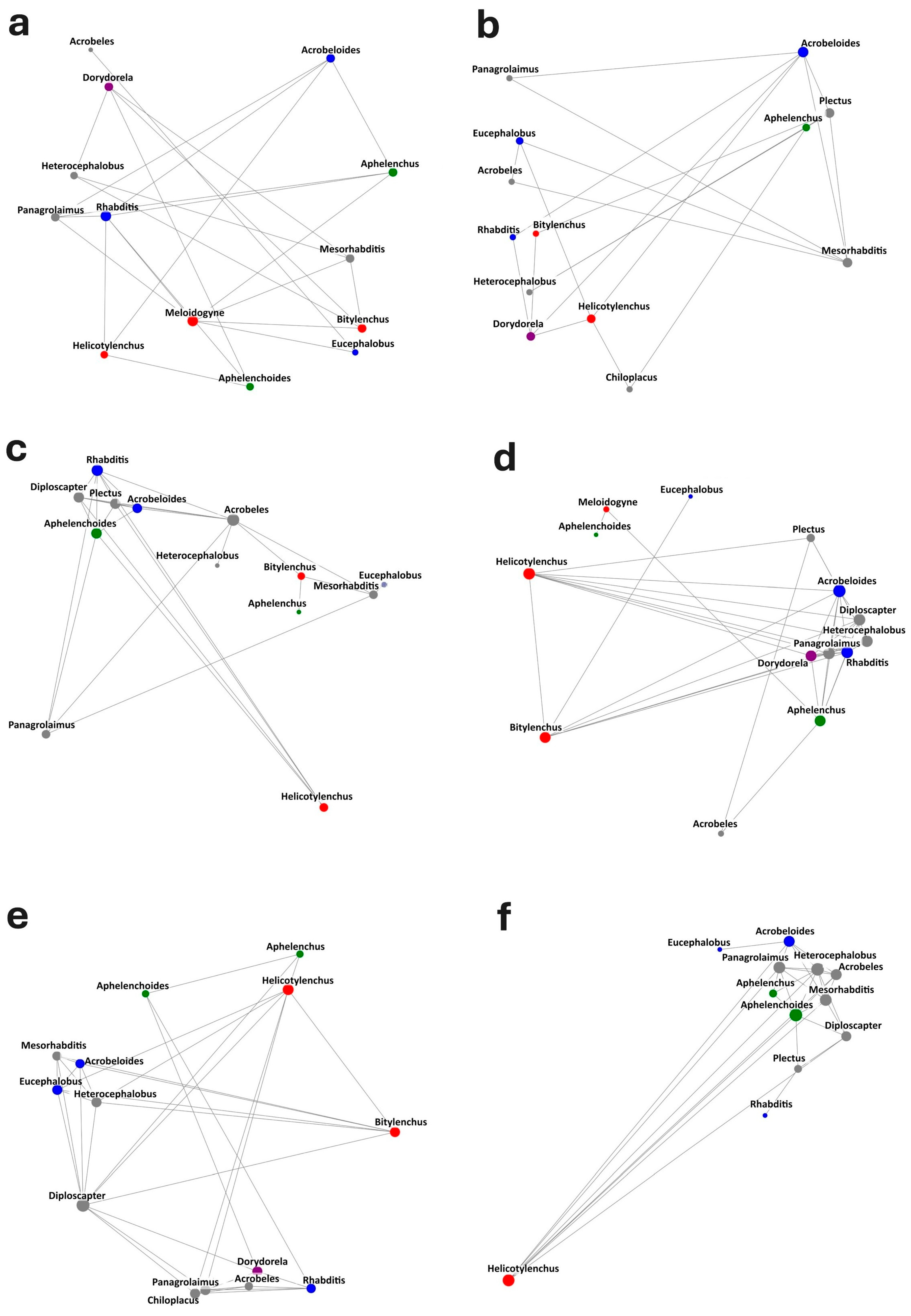
| Co-Occurrence Network Metrics | |||||
|---|---|---|---|---|---|
| Treatment | Nodes | Edges | Average Degree | Density | Average Clustering |
| C | 13 | 24 | 3.692 | 0.308 | 0.531 |
| FH0.5 | 13 | 21 | 3.231 | 0.269 | 0.456 |
| FH1 | 13 | 28 | 4.308 | 0.359 | 0.486 |
| F0.5 | 14 | 40 | 5.714 | 0.44 | 0.533 |
| F1 | 14 | 39 | 5.571 | 0.429 | 0.7 |
| MFP | 12 | 31 | 5.167 | 0.47 | 0.605 |
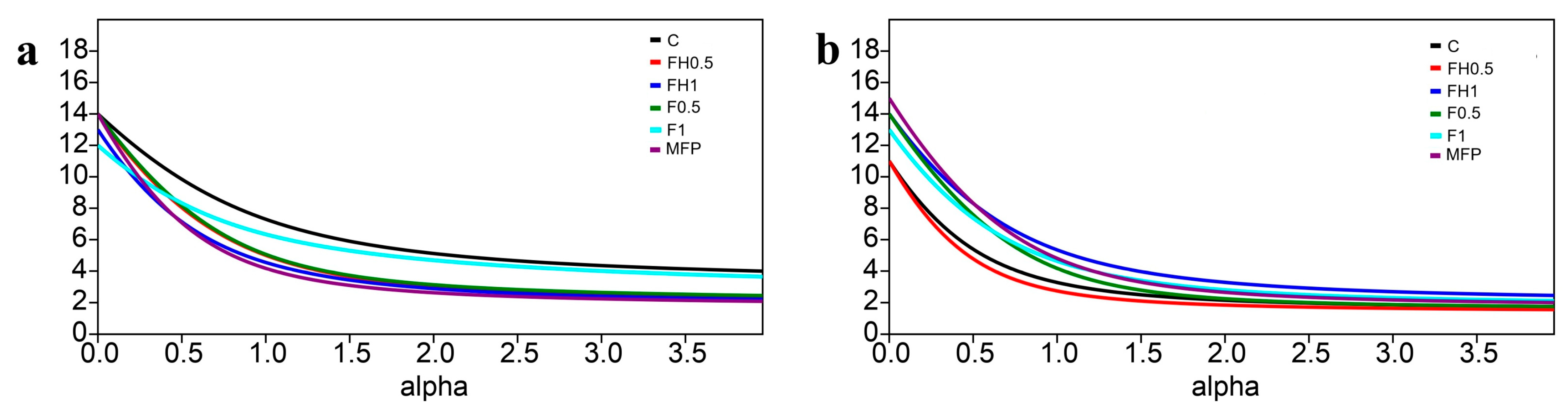
| MI | EI | SI | CI | PPI | ||
|---|---|---|---|---|---|---|
| 5DAA | C | 1.85 ± 0.09 abc | 49.53 ± 9.87 a | 5.33 ± 5.33 a | 21.87 ± 8.37 bcd | 1.00 ± 1.00 a |
| FH0.5 | 1.89 ± 0.03 abc | 43.82 ± 7.02 ab | 3.51 ± 3.51 a | 29.77 ± 5.12 b | 2.00 ± 1.00 a | |
| FH1 | 1.79 ± 0.04 bc | 59.51 ± 3.70 a | 3.60 ± 3.60 a | 21.40 ± 2.35 bcd | 2.00 ± 1.00 a | |
| F0.5 | 1.91 ± 0.10 abc | 45.51 ± 6.10 ab | 9.52 ± 9.52 a | 23.84 ± 11.67 bc | 3.00 ± 0.00 a | |
| F1 | 1.79 ± 0.04 bc | 59.10 ± 4.32 a | 6.34 ± 3.51 a | 14.40 ± 1.24 bcd | 2.00 ± 1.00 a | |
| MFP | 1.87 ± 0.04 abc | 45.99 ± 3.41 ab | 2.84 ± 2.84 a | 21.27 ± 7.30 bcd | 3.00 ± 0.00 a | |
| 40DAA | C | 2.00 ± 0.02 a | 21.78 ± 1.07 bc | 2.42 ± 2.42 a | 77.31 ± 9.35 a | 3.00 ± 0.00 a |
| FH0.5 | 1.96 ± 0.01 ab | 19.17 ± 2.64 c | 0.00 ± 0.00 a | 32.03 ± 1.31 b | 3.00 ± 0.00 a | |
| FH1 | 1.75 ± 0.11 c | 56.19 ± 14.12 a | 1.42 ± 1.42 a | 13.82 ± 8.80 bcd | 2.00 ± 1.00 a | |
| F0.5 | 1.88 ± 0.06 abc | 43.02 ± 10.03 abc | 9.36 ± 1.73 a | 10.28 ± 3.11 bd | 3.00 ± 0.00 a | |
| F1 | 1.75 ± 0.10 c | 54.36 ± 17.68 a | 6.16 ± 3.10 a | 5.09 ± 4.60 d | 3.00 ± 0.00 a | |
| MFP | 1.79 ± 0.02 bc | 54.91 ± 1.62 a | 2.67 ± 2.67 a | 4.40 ± 1.36 d | 2.00 ± 1.00 a | |
| D | ns | ns | ns | * | ns | |
| T | ** | ** | ns | ** | ns | |
| D × T | ns | ns | ns | ** | ns |
| Insect name | 24 h | 48 h | 72 h | ||||||
| EC50 (% v/v) | SE | 95% CI | EC50 (% v/v) | SE | 95% CI | EC50 (% v/v) | SE | 95% CI | |
| Tenebrio molitor | 10.66 | 0.47 | 9.09–11.05 | 10.66 | 0.47 | 9.10–11.15 | 10.04 | 0.92 | 8.12–11.96 |
| Tenebrio molitor heated | 13.84 | 0.29 | 13.23–14.44 | 20.85 | 0.32 | 20.18–21.51 | 20.66 | 0.46 | 19.69–21.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizou, E.; Monokrousos, N.; Kardami, T.; Baliota, G.V.; Rumbos, C.I.; Athanassiou, C.G.; Tsiropoulos, N.; Ntalli, N. Dual Role of Tenebrio molitor Frass in Sustainable Agriculture: Effects on Free-Living Nematodes and Suppression of Meloidogyne incognita. BioTech 2025, 14, 71. https://doi.org/10.3390/biotech14030071
Rizou E, Monokrousos N, Kardami T, Baliota GV, Rumbos CI, Athanassiou CG, Tsiropoulos N, Ntalli N. Dual Role of Tenebrio molitor Frass in Sustainable Agriculture: Effects on Free-Living Nematodes and Suppression of Meloidogyne incognita. BioTech. 2025; 14(3):71. https://doi.org/10.3390/biotech14030071
Chicago/Turabian StyleRizou, Evgenia, Nikolaos Monokrousos, Triantafyllia Kardami, Georgia V. Baliota, Christos I. Rumbos, Christos G. Athanassiou, Nikolaos Tsiropoulos, and Nikoletta Ntalli. 2025. "Dual Role of Tenebrio molitor Frass in Sustainable Agriculture: Effects on Free-Living Nematodes and Suppression of Meloidogyne incognita" BioTech 14, no. 3: 71. https://doi.org/10.3390/biotech14030071
APA StyleRizou, E., Monokrousos, N., Kardami, T., Baliota, G. V., Rumbos, C. I., Athanassiou, C. G., Tsiropoulos, N., & Ntalli, N. (2025). Dual Role of Tenebrio molitor Frass in Sustainable Agriculture: Effects on Free-Living Nematodes and Suppression of Meloidogyne incognita. BioTech, 14(3), 71. https://doi.org/10.3390/biotech14030071











