Inter-Regional Comparisons of Gut Microbiota of Endangered Ring-Tailed Lemurs in Captivity: Insights into Environmental Adaptation and Implications for Ex Situ Conservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sample Collection
2.2. DNA Extraction and Sequencing
2.3. Data Analysis
3. Results
3.1. Sequencing Information
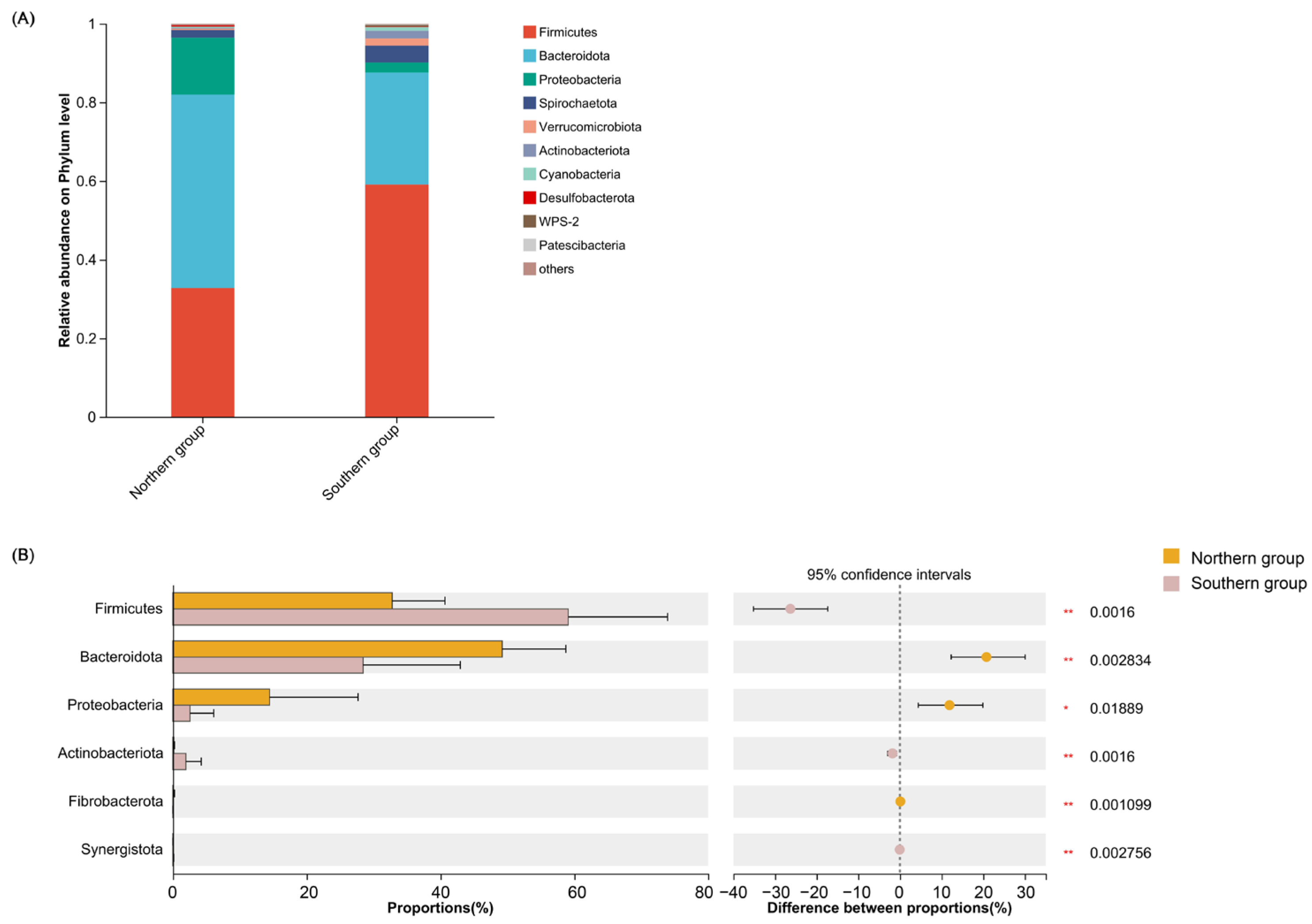
3.2. Composition of Intestinal Flora from L. catta
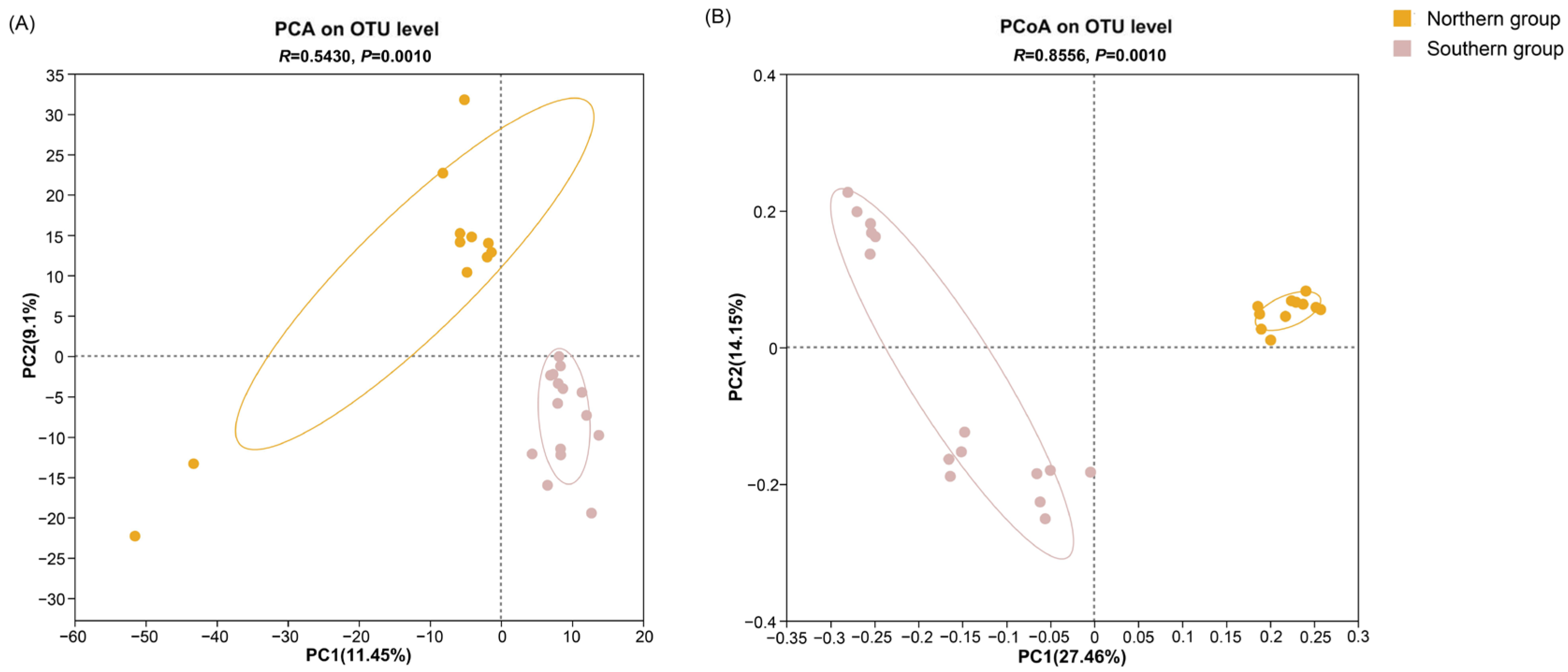
3.3. Alpha Diversity Analysis and Beta Diversity Analysis
3.4. Functional Prediction Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Amato, K.R.; Sanders, J.G.; Song, S.J.; Nute, M.; Metcalf, J.L.; Thompson, L.R.; Morton, J.T.; Amir, A.; McKenzie, V.J.; Humphrey, G.; et al. Evolutionary trends in host physiology outweigh dietary niche in structuring primate gut microbiomes. ISME J. 2019, 13, 576–587. [Google Scholar] [CrossRef]
- Zhernakova, D.V.; Wang, D.; Liu, L.; Andreu-Sánchez, S.; Zhang, Y.; Ruiz-Moreno, A.J.; Peng, H.; Plomp, N.; Del Castillo-Izquierdo, Á.; Gacesa, R.; et al. Host genetic regulation of human gut microbial structural variation. Nature 2024, 625, 813–821. [Google Scholar] [CrossRef]
- Pinacho-Guendulain, B.; Montiel-Castro, A.J.; Ramos-Fernández, G.; Pacheco-López, G. Social complexity as a driving force of gut microbiota exchange among conspecific hosts in non-human primates. Front. Integr. Neurosci. 2022, 16, 876849. [Google Scholar] [CrossRef] [PubMed]
- Raulo, A.; Allen, B.E.; Troitsky, T.; Husby, A.; Firth, J.A.; Coulson, T.; Knowles, S.C.L. Social networks strongly predict the gut microbiota of wild mice. ISME J. 2021, 15, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Hanski, I. Habitat fragmentation and species richness. J. Biogeogr. 2015, 42, 989–993. [Google Scholar] [CrossRef]
- Rija, A.A.; Critchlow, R.; Thomas, C.D.; Beale, C.M. Global extent and drivers of mammal population declines in protected areas under illegal hunting pressure. PLoS ONE 2020, 15, e0227163. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; He, X.; Zeng, B.; Meng, X.; Xu, H.; Li, Y.; Yang, M.; Li, D.; Yao, Y.; Zhang, M.; et al. Variation in gut microbiota of captive Bengal slow lorises. Curr. Microbiol. 2020, 77, 2623–2632. [Google Scholar] [CrossRef]
- Bornbusch, S.L.; Greene, L.K.; Rahobilalaina, S.; Calkins, S.; Rothman, R.S.; Clarke, T.A.; LaFleur, M.; Drea, C.M. Gut microbiota of ring-tailed lemurs (Lemur catta) vary across natural and captive populations and correlate with environmental microbiota. Anim. Microbiome 2022, 4, 29. [Google Scholar] [CrossRef]
- Yang, H.; Leng, X.; Du, H.; Luo, J.; Wu, J.; Wei, Q. Adjusting the prerelease gut microbial community by diet training to improve the postrelease fitness of captive-bred Acipenser dabryanus. Front. Microbiol. 2020, 11, 488. [Google Scholar] [CrossRef] [PubMed]
- Kistler, L.; Ratan, A.; Godfrey, L.R.; Crowley, B.E.; Hughes, C.E.; Lei, R.; Cui, Y.; Wood, M.L.; Muldoon, K.M.; Andriamialison, H.; et al. Comparative and population mitogenomic analyses of Madagascar’s extinct, giant ‘subfossil’ lemurs. J. Hum. Evol. 2015, 79, 45–54. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, M.; Gould, L. Lemur catta. The IUCN Red List of Threatened Species 2020: E.T11496A115565760. 2020. Available online: https://www.iucnredlist.org/species/11496/115565760 (accessed on 9 February 2025).
- Reute, K.E.; Eppley, T.M.; Hending, D.; Pacifici, M.; Semel, B.; Zaonarivelo, J. Eulemur coronatus (Amended Version of 2020 Assessment). The IUCN Red List of Threatened Species 2020: E.T8199A182239524. 2020. Available online: https://www.iucnredlist.org/species/8199/182239524 (accessed on 9 February 2025).
- Eppley, T.M.; Ferguson, B.; Louis, E.E.; Rakotondranary, S.J.; Ganzhorn, J. Lepilemur leucopus. The IUCN Red List of Threatened Species 2020: E.T11618A115566334. 2020. Available online: https://www.iucnredlist.org/species/11618/115566334 (accessed on 9 February 2025).
- McKenzie, V.J.; Song, S.J.; Delsuc, F.; Prest, T.L.; Oliverio, A.M.; Korpita, T.M.; Alexiev, A.; Amato, K.R.; Metcalf, J.L.; Kowalewski, M.; et al. The effects of captivity on the mammalian gut microbiome. Integr. Comp. Biol. 2017, 57, 690–704. [Google Scholar] [CrossRef] [PubMed]
- McKenney, E.A.; Rodrigo, A.; Yoder, A.D. Patterns of gut bacterial colonization in three primate species. PLoS ONE 2015, 10, e0124618. [Google Scholar] [CrossRef]
- Greene, L.K.; McKenney, E.A.; Gasper, W.; Wrampelmeier, C.; Hayer, S.; Ehmke, E.E.; Clayton, J.B. Gut site and gut morphology predict microbiome structure and function in ecologically diverse lemurs. Microb. Ecol. 2023, 85, 1608–1619. [Google Scholar] [CrossRef]
- Springer, A.; Fichtel, C.; Al-Ghalith, G.A.; Koc, F.; Amato, K.R.; Clayton, J.B.; Knights, D.; Kappeler, P.M. Patterns of seasonality and group membership characterize the gut microbiota in a longitudinal study of wild Verreaux’s sifakas (Propithecus verreauxi). Ecol. Evol. 2017, 7, 5732–5745. [Google Scholar] [CrossRef]
- Bennett, G.; Malone, M.; Sauther, M.L.; Cuozzo, F.P.; White, B.; Nelson, K.E.; Stumpf, R.M.; Knight, R.; Leigh, S.R.; Amato, K.R. Host age, social group, and habitat type influence the gut microbiota of wild ring-tailed lemurs (Lemur catta). Am. J. Primatol. 2016, 78, 883–892. [Google Scholar] [CrossRef]
- Clayton, J.B.; Gomez, A.; Amato, K.; Knights, D.; Travis, D.A.; Blekhman, R.; Knight, R.; Leigh, S.; Stumpf, R.; Wolf, T.; et al. The gut microbiome of nonhuman primates: Lessons in ecology and evolution. Am. J. Primatol. 2018, 80, e22867. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Lan, L.Y.; You, Y.Y.; Hong, Q.X.; Liu, Q.X.; Xu, C.Z.; Chen, W.; Zhu, Y.; Du, X.; Fan, P. The gut microbiota of gibbons across host genus and captive site in China. Am. J. Primatol. 2022, 84, e23360. [Google Scholar] [CrossRef]
- Fogel, A.T. The gut microbiome of wild lemurs: A comparison of sympatric Lemur catta and Propithecus verreauxi. Folia Primatol. 2015, 86, 85–95. [Google Scholar] [CrossRef]
- Amato, K.R.; Yeoman, C.J.; Ken, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef]
- Clayto, J.B.; Vangay, P.; Huang, H.; Ward, T.; Hillmann, B.M.; Al-Ghalith, G.A.; Travis, D.A.; Long, H.T.; Van Tuan, B.; Van Minh, V.; et al. Captivity humanizes the primate microbiome. Proc. Natl. Acad. Sci. USA 2016, 113, 10376–10381. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Pu, Y.; Niu, L.; Deng, J.; Zeng, D.; Amato, K.; Li, Y.; Zhou, Y.; Lin, Y.; Wang, J.; et al. Comparison of gastrointestinal microbiota in golden snub-nosed monkey (Rhinopithecus roxellanae), green monkey (Chlorocebus aethiops sabaeus), and ring-tailed lemur (Lemur catta) by high throughput sequencing. Glob. Ecol. Conserv. 2022, 33, e01946. [Google Scholar] [CrossRef]
- Yasuda, K.; Oh, K.; Ren, B.; Tickle, T.L.; Franzosa, E.A.; Wachtman, L.M.; Miller, A.D.; Westmoreland, S.V.; Mansfield, K.G.; Vallender, E.J.; et al. Biogeography of the intestinal mucosal and lumenal microbiome in the rhesus macaque. Cell Host Microbe 2015, 17, 385–391. [Google Scholar] [CrossRef]
- Fre, J.C.; Rothman, J.M.; Pell, A.N.; Nizeyi, J.B.; Cranfield, M.R.; Angert, E.R. Fecal bacterial diversity in a wild gorilla. Appl. Environ. Microbiol. 2006, 72, 3788–3792. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Yang, S.; Zhou, J.; Qi, L.; Sun, X.; Fan, M.; Xu, S.; Cha, M.; Zhang, M.; et al. Comparison between the fecal bacterial microbiota of healthy and diarrheic captive musk deer. Front. Microbiol. 2018, 9, 300. [Google Scholar] [CrossRef]
- Schloss, P.D.; Handelsman, J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 2005, 71, 1501–1506. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef]
- Hess, M.; Sczyrba, A.; Egan, R.; Kim, T.W.; Chokhawala, H.; Schroth, G.; Luo, S.; Clark, D.S.; Chen, F.; Zhang, T.; et al. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 2011, 331, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, C.; Gu, Y.; Chen, L.; Ou, S.; Wan, Y.; Peng, X. Lean rats gained more body weight than obese ones from a high-fibre diet. Br. J. Nutr. 2015, 114, 1188–1194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Xia, F.; Wen, L.P.; Ge, B.C.; Li, Y.X.; Li, F.P.; Zhou, B.J. Gut microbiota as a target for prevention and treatment of type 2 diabetes: Mechanisms and dietary natural products. World J. Diabetes 2021, 12, 1146. [Google Scholar] [CrossRef]
- Grigor’eva, I.N. Gallstone disease, obesity and the Firmicutes/Bacteroidetes ratio as a possible biomarker of gut dysbiosis. J. Pers. Med. 2020, 11, 13. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenk, G.; Moseiko, V.; Budovsk, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Yu, J.; Bai, G.; He, Y.; Liu, M.; Yang, X.; Li, J.; Shen, Y.; Lu, S.; Bao, W. Analysis of intestinal bacterial diversity and its gene function prediction in black-capped capuchin (Sapajus apella). J. Appl. Anim. Res. 2024, 52, 2322652. [Google Scholar] [CrossRef]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring bacteroidetes: Metabolic key points and immunological tricks of our gut commensals. Digest. Liver Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Hum. Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Honda, K. Intestinal commensal microbes as immune modulators. Cell Host Microbe 2012, 12, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S. Changes seen in gut bacteria content and distribution with obesity: Causation or association? Postgrad. Med. 2015, 127, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, M.; Mass, K.; Parks, E.J.; Wagner, D.A.; McClure, E.A.; Graf, J.; Savoye, M.; Pierpont, B.; Cline, G.; Santoro, N. Role of gut microbiota and short chain fatty acids in modulating energy harvest and fat partitioning in youth. J. Clin. Endocrinol. Metab. 2016, 101, 4367–4376. [Google Scholar] [CrossRef]
- Hart, E.; Creevey, C.; Hitch, T.; Kingston-Smith, A. Meta-proteomics of rumen microbiota indicates niche compartmentalisation and functional dominance in a limited number of metabolic pathways between abundant bacteria. Sci. Rep. 2018, 8, 10504. [Google Scholar] [CrossRef]
- Adams, N.E.; Becker, M.A.; Edmands, S. Effect of geography and captivity on scat bacterial communities in the imperiled channel island fox. Front. Microbiol. 2021, 12, 748323. [Google Scholar] [CrossRef]
- Chen, L.; Sun, M.; Xu, D.; Gao, Z.; Shi, Y.; Wang, S.; Zhou, Y. Gut microbiome of captive wolves is more similar to domestic dogs than wild wolves indicated by metagenomics study. Front. Microbiol. 2022, 13, 1027188. [Google Scholar] [CrossRef]
- Fontaine, S.S.; Novarro, A.J.; Kohl, K.D. Environmental temperature alters the digestive performance and gut microbiota of a terrestrial amphibian. J. Exp. Biol. 2018, 221, jeb187559. [Google Scholar] [CrossRef]
- Liu, P.Y.; Cheng, A.C.; Huang, S.W.; Chang, H.W.; Oshida, T.; Yu, H.T. Variations in gut microbiota of Siberian flying squirrels correspond to seasonal phenological changes in their Hokkaido subarctic forest ecosystem. Microb. Ecol. 2019, 78, 223–231. [Google Scholar] [CrossRef]
- Dietz, M.W.; Matson, K.D.; Versteegh, M.A.; Van Der Velde, M.; Parmentier, H.K.; Arts, J.A.; Salles, J.F.; Tieleman, B.I. Gut microbiota of homing pigeons shows summer–winter variation under constant diet indicating a substantial effect of temperature. Anim. Microb. 2022, 4, 64. [Google Scholar] [CrossRef]
- Griswold, K.E.; White, B.A.; Mackie, R.I. Diversity of extracellular proteolytic activities among prevotella species from the rumen. Curr. Microbiol. 1999, 39, 187–194. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Amato, K.R.; Leigh, S.R.; Kent, A.; Mackie, R.I.; Yeoman, C.J.; Stumpf, R.M.; Wilson, B.A.; Nelson, K.E.; White, B.A.; Garber, P.A. The gut microbiota appears to compensate for seasonal diet variation in the wild black howler monkey (Alouatta pigra). Microb. Ecol. 2015, 69, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.L.; Lee, K.J.; Couto-Rodriguez, M.; Patel, J.; Sinha, R.; Guo, C.; Olson, S.H.; Seimon, A.; Seimon, T.A.; Ondzie, A.U.; et al. Gut microbiomes of wild great apes fluctuate seasonally in response to diet. Nat. Commun. 2018, 9, 1786. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Hayakawa, T.; Kiyono, M.; Yamabata, N.; Enari, H.; Enari, H.S.; Fujita, S.; Kawazoe, T.; Asai, T.; Oi, T.; et al. Diet-related factors strongly shaped the gut microbiota of Japanese macaques. Am. J. Primatol. 2023, 85, e23555. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X. Comparative analyses of fecal microbiota in Tibetan and Chinese Han living at low or high altitude by barcoded 454 pyrosequencing. Sci. Rep. 2015, 5, 14682. [Google Scholar] [CrossRef]
- Wang, W.; Wang, F.; Li, L.; Wang, A.; Sharshov, K.; Druzyaka, A.; Lancuo, Z.; Wang, S.; Shi, Y. Characterization of the gut microbiome of black-necked cranes (Grus nigricollis) in six wintering areas in China. Arch. Microbiol. 2020, 202, 983–993. [Google Scholar] [CrossRef]
- Petrenko, V.; Sinturel, F.; Riezman, H.; Dibner, C. Lipid metabolism around the body clocks. Prog. Lipid Res. 2023, 91, 101235. [Google Scholar] [CrossRef]
- Steffansen, B.; Nielsen, C.U.; Brodin, B.; Eriksson, A.H.; Andersen, R.; Frokjaer, S. Intestinal solute carriers: An overview of trends and strategies for improving oral drug absorption. Eur. J. Pharm. Sci. 2004, 21, 3–16. [Google Scholar] [CrossRef]
- Lovegrove, B. The influence of climate on the basal metabolic rate of small mammals: A slow-fast metabolic continuum. J. Comp. Physiol. B 2003, 173, 87–112. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Cong, J.; Wang, M.; Zhao, M.; Lu, H.; Xie, C.; Yang, C.; Yuan, T.; Li, D.; et al. Variations of soil microbial community structures beneath broadleaved forest trees in temperate and subtropical climate zones. Front. Microbiol. 2017, 8, 200. [Google Scholar] [CrossRef]



| Group Name | Numbers of Taxon | |||||
|---|---|---|---|---|---|---|
| Phylum | Class | Order | Family | Genus | Species | |
| Southern group | 19 | 30 | 78 | 132 | 275 | 465 |
| Northern group | 14 | 24 | 63 | 105 | 230 | 419 |
| Total | 20 | 32 | 87 | 154 | 351 | 645 |
| Group Name | Dominant Phyla (≥1%) | Dominant Genera (≥5%) |
|---|---|---|
| Southern group | Firmicutes (59.10%) | unclassified_f__Lachnospiraceae (11.60%) Bacteroides (6.15%) Christensenellaceae_R-7_group (6.17%) |
| Bacteroidota (28.44%) | ||
| Spirochaetota (4.31%) | ||
| Proteobacteria (2.57%) | ||
| Actinobacteriota (1.96%) | ||
| Verrucomicrobiota (1.82%) | ||
| Northern group | Bacteroidota (49.22%) | Prevotella (19.20%) Prevotellaceae_NK3B31_group (8.70%) Succinivibrio (8.68%) norank_f__Prevotellaceae (5.73%) Faecalibacterium (5.17%) |
| Firmicutes (32.78%) | ||
| Proteobacteria (14.46%) | ||
| Spirochaetota (1.92%) |
| Group Name | Chao Index | Ace Index | Simpson Index | Shannon Index |
|---|---|---|---|---|
| Northern group | 546.04 | 560.64 | 0.086216 | 3.6368 |
| Southern group | 500.11 | 508.49 | 0.045837 | 4.0102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Yao, H.; Wu, N.; Wu, H.; Zhao, D. Inter-Regional Comparisons of Gut Microbiota of Endangered Ring-Tailed Lemurs in Captivity: Insights into Environmental Adaptation and Implications for Ex Situ Conservation. J. Zool. Bot. Gard. 2025, 6, 57. https://doi.org/10.3390/jzbg6040057
Sun M, Yao H, Wu N, Wu H, Zhao D. Inter-Regional Comparisons of Gut Microbiota of Endangered Ring-Tailed Lemurs in Captivity: Insights into Environmental Adaptation and Implications for Ex Situ Conservation. Journal of Zoological and Botanical Gardens. 2025; 6(4):57. https://doi.org/10.3390/jzbg6040057
Chicago/Turabian StyleSun, Menglin, Hongyu Yao, Nan Wu, Hong Wu, and Dapeng Zhao. 2025. "Inter-Regional Comparisons of Gut Microbiota of Endangered Ring-Tailed Lemurs in Captivity: Insights into Environmental Adaptation and Implications for Ex Situ Conservation" Journal of Zoological and Botanical Gardens 6, no. 4: 57. https://doi.org/10.3390/jzbg6040057
APA StyleSun, M., Yao, H., Wu, N., Wu, H., & Zhao, D. (2025). Inter-Regional Comparisons of Gut Microbiota of Endangered Ring-Tailed Lemurs in Captivity: Insights into Environmental Adaptation and Implications for Ex Situ Conservation. Journal of Zoological and Botanical Gardens, 6(4), 57. https://doi.org/10.3390/jzbg6040057






