Chemosensory-Driven Foraging and Nocturnal Activity in the Freshwater Snail Rivomarginella morrisoni (Gastropoda, Marginellidae): A Laboratory-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Daily Foraging Behavior Observation
2.3. Food Type and Freshness Preference Experiments
2.4. Locomotion Under Light and Dark Conditions
2.5. Chemosensory Food Detection
2.6. Visual Food Detection
2.7. Statistical Analysis
3. Results
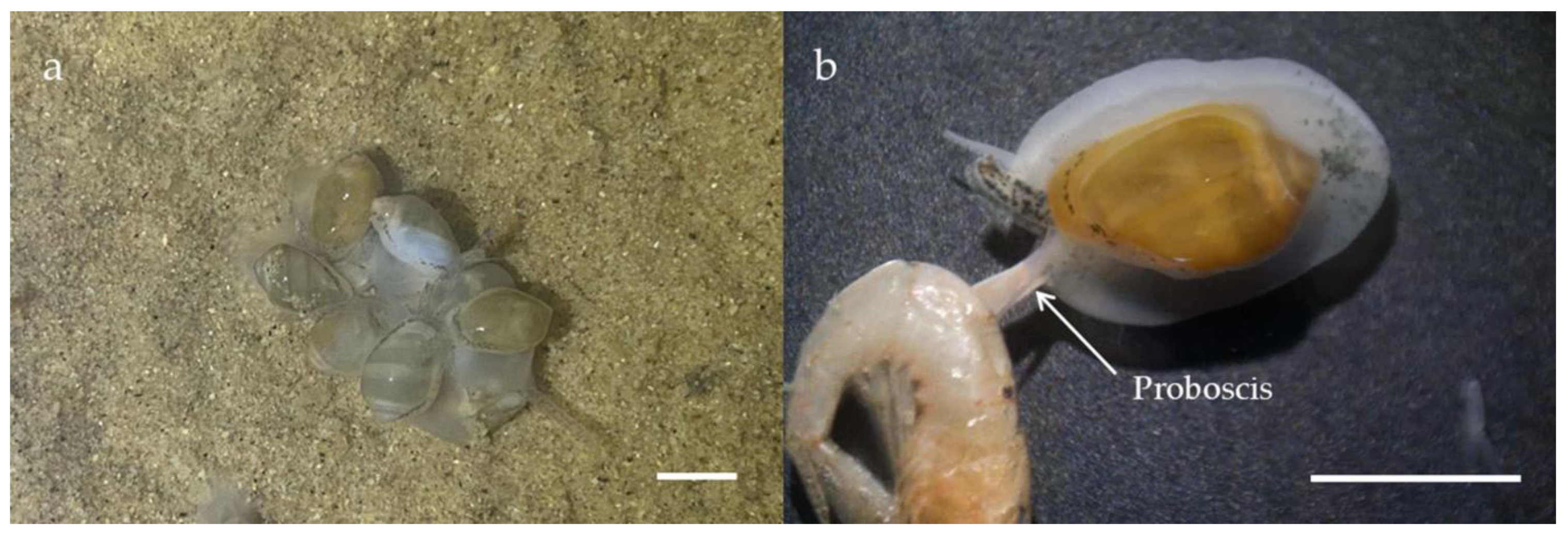
3.1. Specimen Information
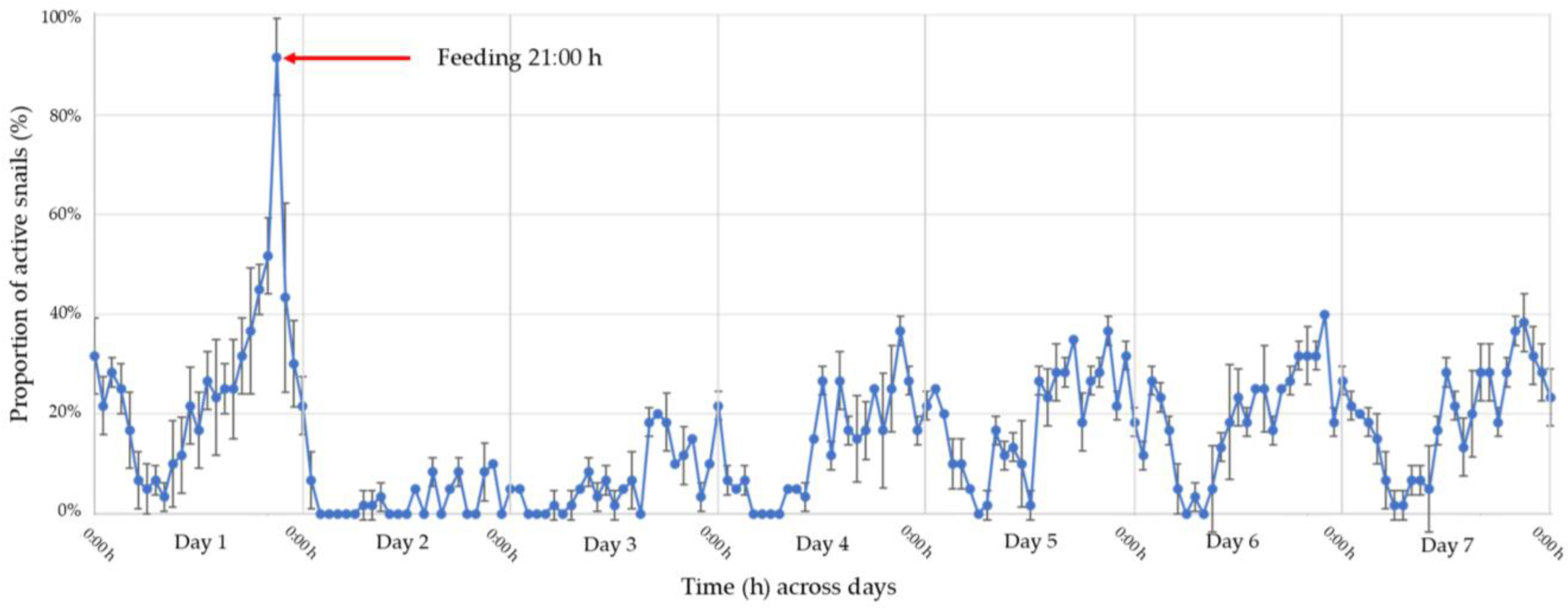
3.2. Feeding Behavior and Daily Activity
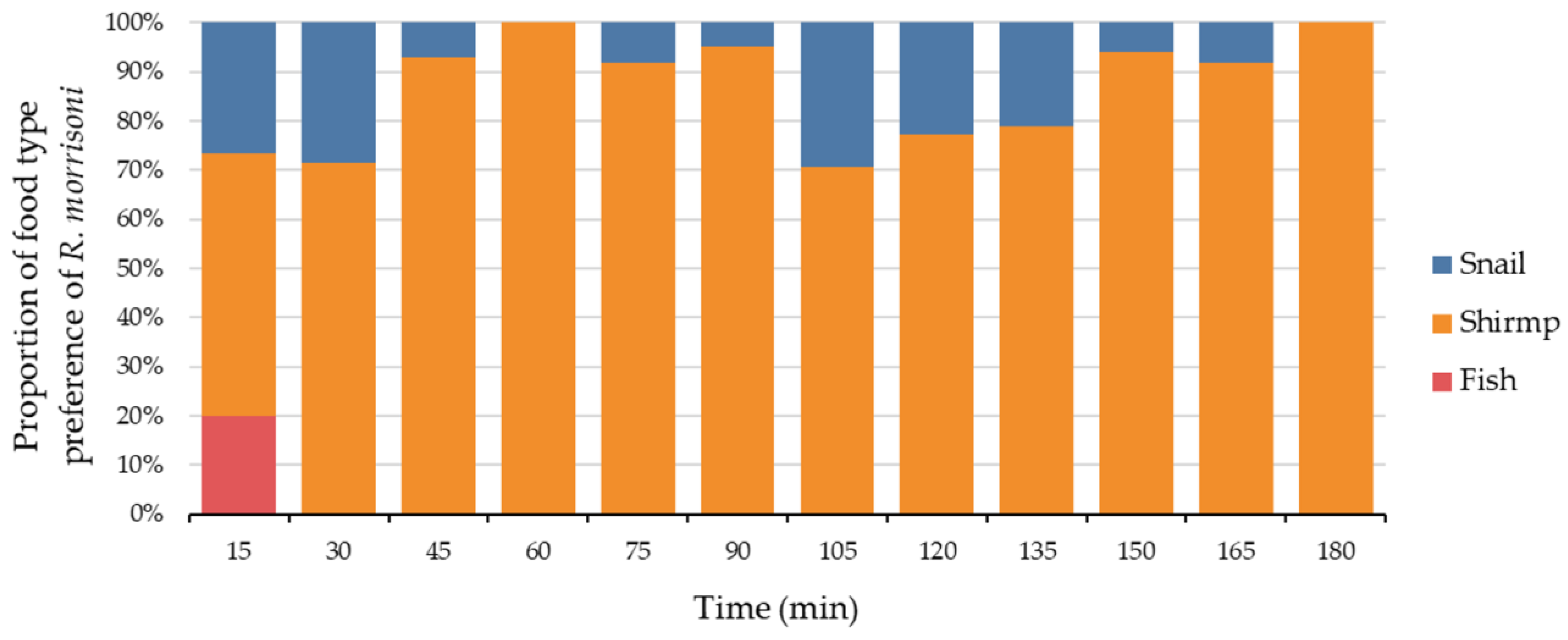
3.3. The Food Type and Food Freshness Preferences
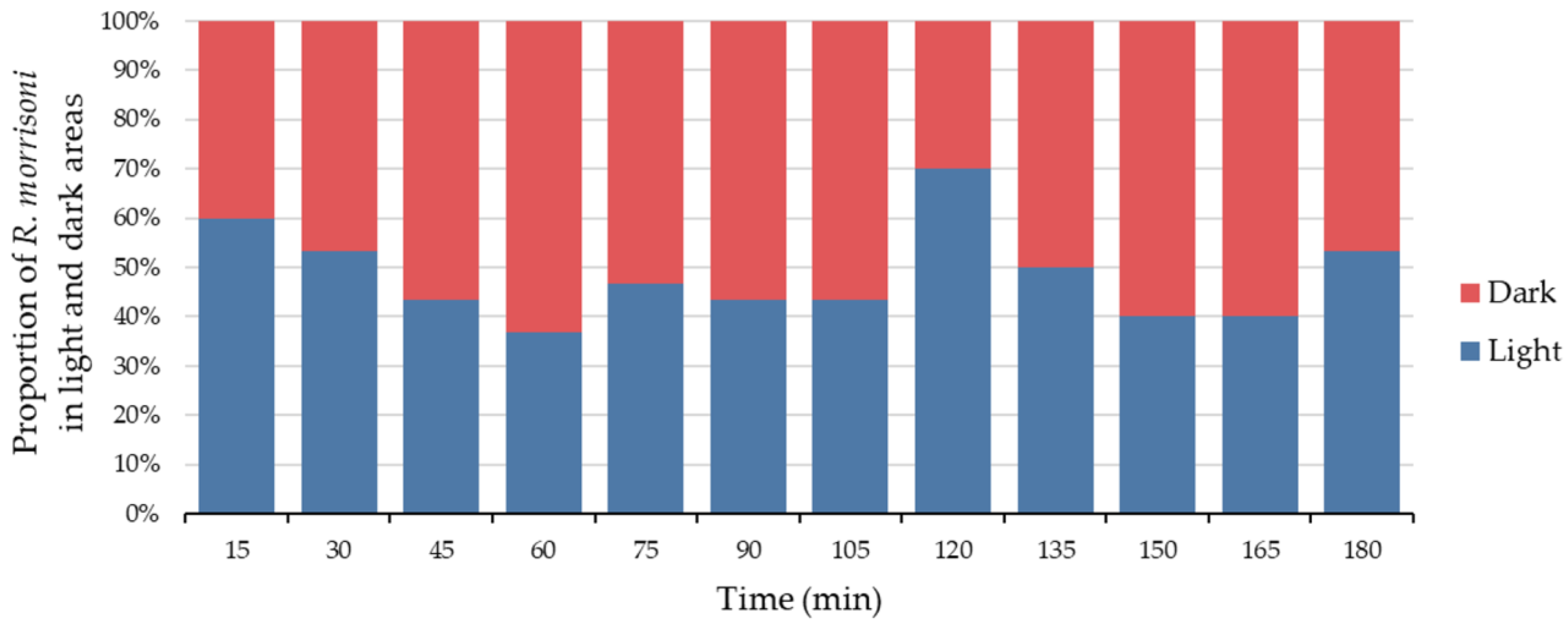
3.4. Light and Dark Locomotion Behavior
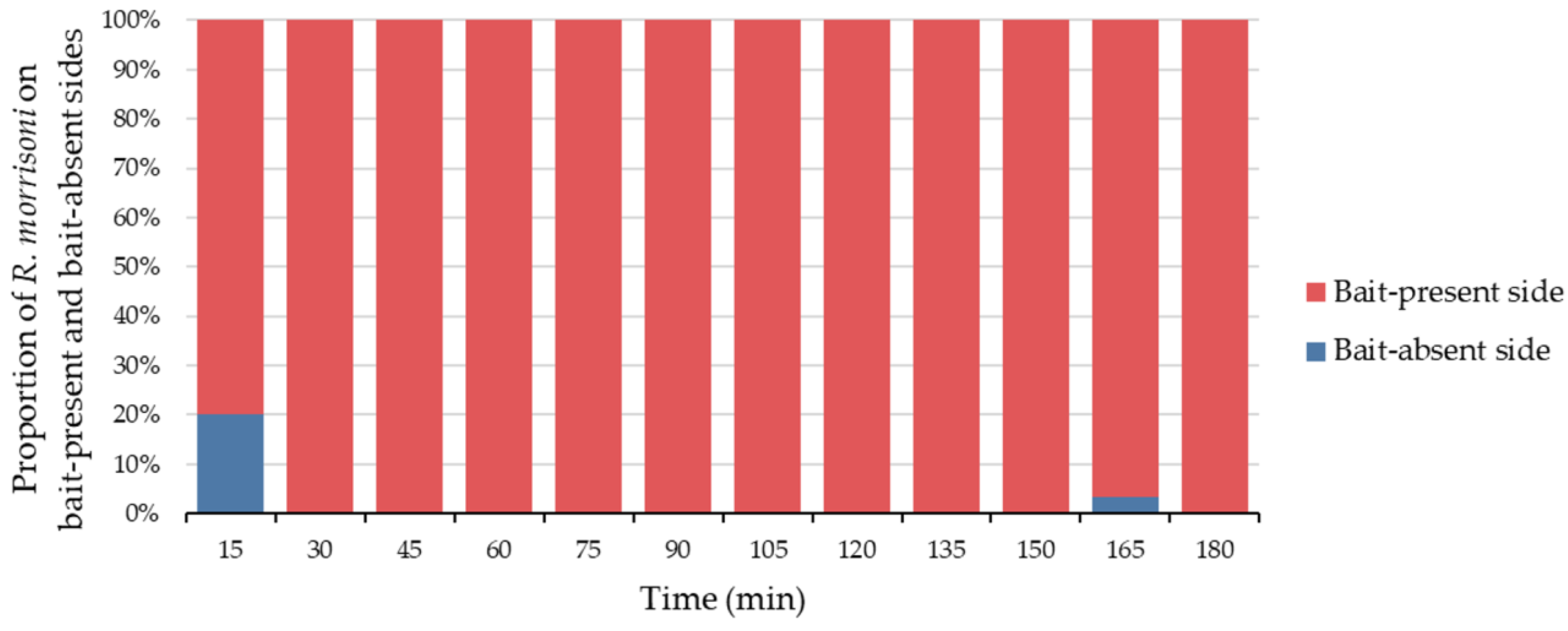
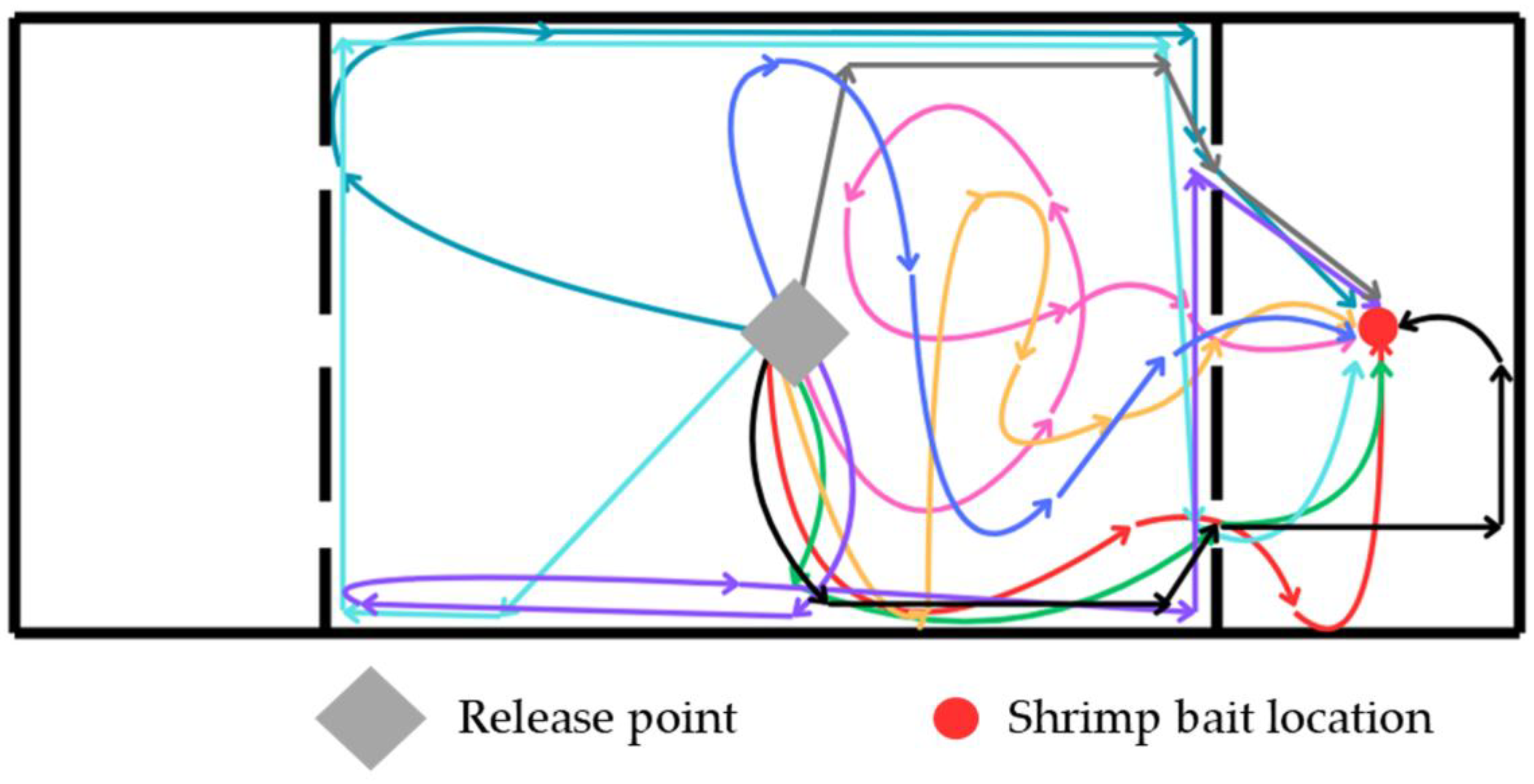
3.5. Feeding Behavior and Chemosensory Response
3.6. Visual Food Detection Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandt, R.A.M. Description of New Non-Marine Mollusk from Asia. Arch. Für Molluskenkd. 1968, 98, 275–277. [Google Scholar]
- Coomans, E.H.; Clover, W.P. The Genus Rivomarginella (Gastropoda, Marginellidae). Beaufortia 1972, 20, 69–75. [Google Scholar]
- Köhler, F.; Richter, K. Rivomarginella morrisoni. The IUCN Red List of Threatened Species 2012. Available online: https://www.iucnredlist.org/species/184928/1766366 (accessed on 19 September 2025).
- Dudgeon, D. The Ecology of Tropical Asian Rivers and Streams in Relation to Biodiversity Conservation. Annu. Rev. Ecol. Syst. 2000, 31, 239–263. [Google Scholar] [CrossRef]
- Sutherland, W.J. The Importance of Behavioral Studies in Conservation Biology. Anim. Behav. 1998, 56, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Bell-Pedersen, D.; Cassone, V.M.; Earnest, D.J.; Golden, S.S.; Hardin, P.E.; Thomas, T.L.; Zoran, M.J. Circadian Rhythms from Multiple Oscillators: Lessons from Diverse Organisms. Nat. Rev. Genet. 2005, 6, 544–556. [Google Scholar] [CrossRef]
- Ademolu, K.O.; Jayeola, O.A.; Idowu, A.B.; Elemide, I.O. Circadian Variation in Locomotor and Feeding Periods of Two Land Snail Species. Arch. Zootec. 2011, 60, 1323–1326. [Google Scholar] [CrossRef]
- Mahanayak, B. Ex-situ and In-situ Conservation of Wild Life. World J. Biol. Pharm. Health Sci. 2024, 18, 277–282. [Google Scholar] [CrossRef]
- Kohn, A.J. Chemoreception in Gastropod Molluscs. Biol. Bull. 1961, 120, 251–268. [Google Scholar] [CrossRef]
- Croll, R.P. Gastropod Chemoreception. Biol. Rev. Camb. Philos. Soc. 1983, 58, 293–319. [Google Scholar] [CrossRef]
- Padilla, D.K. Form and Function of Radular Teeth of Herbivorous Molluscs: Focus on the Future. Am. Malacol. Bull. 2003, 18, 163–168. [Google Scholar]
- Souza, P.J.S.J.; Simone, L.R.L. Cladistic Analysis of the Family Marginellidae (Mollusca, Gastropoda) Based on Phenotypic Features. Zootaxa 2019, 4648, 201–240. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, D.A.; Salice, C.J. Dietary Acclimation Affects Dietary Selection in the Freshwater Snail Planorbella trivolvis. J. Mollus. Stud. 2012, 78, 226–232. [Google Scholar] [CrossRef]
- Lombardo, P.; Miccoli, F.P.; Giustini, M.; Cicolani, B. Diel Activity Cycles of Freshwater Gastropods Under Natural Light: Patterns and Ecological Implications. Ann. Limnol.—Int. J. Limnol. 2010, 46, 29–40. [Google Scholar] [CrossRef]
- Ng, T.P.T.; Lau, S.L.Y.; Davies, M.S.; Stafford, R.; Seuront, L.; Hutchinson, N.; Hui, T.T.Y.; Williams, G.A. Behavioral Repertoire of High-Shore Littorinid Snails Reveals Novel Adaptations to an Extreme Environment. Ecol. Evol. 2021, 11, 11645–11658. [Google Scholar] [CrossRef]
- Will, I. Host Preference, Detection, and Dependence: The Ectoparasitic Gastropods Melanella acicula and Peasistilifer nitidula (Eulimidae) on Holothurian Hosts. Student Research Papers. 2009. Available online: https://api.semanticscholar.org/CorpusID:83267321 (accessed on 6 November 2025).
- Dou, P.; Wang, X.; Lan, Y.; Cui, B.; Bai, J.; Xie, T. Benthic Macroinvertebrate Diversity as Affected by the Construction of Inland Waterways along Montane Stretches of Two Rivers in China. Water 2022, 14, 1080. [Google Scholar] [CrossRef]
- Musie, W.; Gonfa, G. Fresh Water Resource, Scarcity, Water Salinity Challenges and Possible Remedies: A Review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef]
- Bae, M.-J.; Park, Y.-S. Key Determinants of Freshwater Gastropod Diversity and Distribution: The Implications for Conservation and Management. Water 2020, 12, 1908. [Google Scholar] [CrossRef]
- Michálek, O.; Petráková, L.; Pekár, S. Capture Efficiency and Trophic Adaptations of a Specialist and Generalist Predator: A Comparison. Ecol. Evol. 2017, 7, 2756–2766. [Google Scholar] [CrossRef]
- Chatzinikolaou, E.; Grigoriou, P.; Martini, E.; Sterioti, A. Impact of Ocean Acidification and Warming on the Feeding Behaviour of Two Gastropod Species. Mediterr. Mar. Sci. 2019, 20, 669–679. [Google Scholar] [CrossRef]
- Manz, M.; Lord, J.; Morales, M. Ocean Acidification Impedes Foraging Behavior in the Mud Snail Ilyanassa obsoleta. J. Mar. Sci. Eng. 2023, 11, 623. [Google Scholar] [CrossRef]
- Hayes, K.A.; Burks, R.L.; Castro-Vazquez, A.; Darby, P.C.; Heras, H.; Martín, P.R.; Qiu, J.-W.; Thiengo, S.C.; Vega, I.A.; Wada, T.; et al. Insights from an Integrated View of the Biology of Apple Snails (Caenogastropoda: Ampullariidae). Malacologia 2015, 58, 245–302. [Google Scholar] [CrossRef]
- McQuinn, I.H.; Gendron, L.; Himmelman, J.H. Area of Attraction and Effective Area Fished by a Whelk (Buccinum undatum) Trap Under Variable Conditions. Can. J. Fish. Aquat. Sci. 1988, 45, 2054–2060. [Google Scholar] [CrossRef]
- Lapointe, V.; Sainte-Marie, B. Currents, Predators, and the Aggregation of the Gastropod Buccinum undatum Around Bait. Mar. Ecol.-Progr. Ser. 1992, 85, 245–257. Available online: http://www.jstor.org/stable/24829760 (accessed on 6 November 2025). [CrossRef]
- Lefcort, H.; Ben, A.F.; Heller, J. Terrestrial Snails use Predator-diet to Assess Danger. J. Ethol. 2006, 24, 97–102. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subpayakom, N.; Dumrongrojwattana, P.; Poeaim, S. Chemosensory-Driven Foraging and Nocturnal Activity in the Freshwater Snail Rivomarginella morrisoni (Gastropoda, Marginellidae): A Laboratory-Based Study. J. Zool. Bot. Gard. 2025, 6, 56. https://doi.org/10.3390/jzbg6040056
Subpayakom N, Dumrongrojwattana P, Poeaim S. Chemosensory-Driven Foraging and Nocturnal Activity in the Freshwater Snail Rivomarginella morrisoni (Gastropoda, Marginellidae): A Laboratory-Based Study. Journal of Zoological and Botanical Gardens. 2025; 6(4):56. https://doi.org/10.3390/jzbg6040056
Chicago/Turabian StyleSubpayakom, Navapong, Pongrat Dumrongrojwattana, and Supattra Poeaim. 2025. "Chemosensory-Driven Foraging and Nocturnal Activity in the Freshwater Snail Rivomarginella morrisoni (Gastropoda, Marginellidae): A Laboratory-Based Study" Journal of Zoological and Botanical Gardens 6, no. 4: 56. https://doi.org/10.3390/jzbg6040056
APA StyleSubpayakom, N., Dumrongrojwattana, P., & Poeaim, S. (2025). Chemosensory-Driven Foraging and Nocturnal Activity in the Freshwater Snail Rivomarginella morrisoni (Gastropoda, Marginellidae): A Laboratory-Based Study. Journal of Zoological and Botanical Gardens, 6(4), 56. https://doi.org/10.3390/jzbg6040056






