Abstract
Birds kept in zoological institutions are highly exposed to gastrointestinal (GI) parasitism caused by coccidia and nematodes. The current research aimed to characterize the avian GI parasitic fauna in several zoological collections in Portugal and Spain. During the full year of 2022, a total of 120 fecal samples were collected from four zoological institutions: Lisbon Zoo, Olivais Pedagogical Farm, and Avian Biodiversity Center (Lisbon, Portugal), and Avifauna park (Lugo, Spain). Analysis was conducted in domestic bird species (autochthonous and exotic poultry breeds), and 18 different exotic bird species like Galliformes (peacock, pheasant), Anseriformes (duck), Psittaciformes (parrot, macaw, cockatiel, parakeet, cockatoo), Coraciiformes (motmot), Charadriiformes (avocet), Strigiformes (owl), Phoenicopteriformes (flamingo), Struthioniformes (ostrich), Rheiformes (rhea), and Casuariiformes (emu, cassowary). Feces were processed using Mini-FLOTAC (MF), to identify parasitic forms and quantify their shedding (oocysts or eggs per gram of feces). Moreover, 15 fecal samples from pheasants were also processed using the McMaster method (McM), to compare the parasite shedding and frequencies between techniques. MF implementation allowed identification of coccidia infections in all bird collections. Also, peacocks of the Lisbon Zoo tested positive for Trichostrongylus tenuis and Strongyloides pavonis, and the exotic birds from Avifauna park were also positive for several nematode species, with Ascaridia sp., Capillaria sp., Strongyloides sp., and Syngamus trachea eggs being detected in pheasants’ feces. Moreover, the analysis of pheasants’ feces with MF detected prevalences of 33% for coccidia oocysts, and 47% for Capillaria sp. and Ascaridia sp. eggs, while McM detected prevalences of 13%, 27%, and 40% for the respective parasite taxa, with no differences being observed between methods (p = 0.39, p = 0.45, and p = 0.50, respectively). This research provided more scientific support regarding the importance of using Mini-FLOTAC in routine parasitological diagnosis in birds kept at zoological institutions.
1. Introduction
Zoological institutions have several important roles in society and for animal wildlife, particularly visitors’ education, research, and ex situ or in situ animal conservation, being of extreme importance especially for Vulnerable, Endangered, or Critically Endangered animal species or breeds [1]. According to the most recent edition of IUCN’s Red List of Threatened Species, 13% of bird species are classified as “Threatened” and 6% as “Near Threatened”, at European level [2], which highlights the importance of their conservation.
Among avian species, chickens account for the highest number of breeds at the risk of extinction (29%) [3], with examples including the Portuguese chicken breeds “Preta Lusitânica” (Lusitanian Black chicken), “Pedrês Portuguesa” (Portuguese Pedrês chicken), “Branca” (White chicken), and “Amarela” (Yellow chicken), which are currently kept at small-scale farms and zoological institutions [4,5]. In addition, the proportion of avian species with unknown status of conservation is even higher (64%) than the observed for mammals (55%) [3].
Several biotic and abiotic factors challenge the conservation of avian genetic resources. Despite all noteworthy efforts developed by zoological institutions to mimic natural habitats and maximize animals’ welfare, their contact with the same environment for long periods of time and irregularity or absence of antiparasitic drug treatments may lead to a high exposure to several pathogens, particularly gastrointestinal (GI) parasites like coccidia, capillarids, ascarids, and strongylids [6,7,8,9,10].
Gastrointestinal parasites like coccidia (e.g., Eimeria spp. and Isospora spp.), and nematodes such as Capillaria spp., ascarids and heterakids (e.g., Ascaridia spp. and Heterakis spp., respectively), Trichostrongylus tenuis, and Strongyloides spp., as well as respiratory nematodes such as syngamids (e.g., Syngamus trachea), whose eggs are swallowed from the tracheal mucosa into the GI tract and expelled with feces, constitute the most commonly diagnosed endoparasites in domestic and exotic birds. Previous studies described their occurrence in Galliformes, Struthioniformes, Rheiformes, Casuariiformes, Anseriformes, Psittaciformes, Columbiformes, Passeriformes, Falconiformes, Accipitriformes, and Strigiformes kept at several ornithological collections across the globe, particularly in Portugal [11,12,13], Spain [14], Italy [9], Serbia [8], UK [6], Egypt [15], Brazil [16,17,18], Iran [19], and China [20].
Their coprological diagnosis can be performed using quantitative techniques like the McMaster and Modified McMaster methods [21,22], qualitative techniques such as Willis Flotation, Natural Sedimentation, and Fecal Cultures [21], and quantitative–qualitative techniques like FLOTAC and Mini-FLOTAC [23,24]. All these methodologies allow the dynamics of oocyst or egg shedding in animal collections to be followed, and thus to optimize antiparasitic drug treatments [21,25]. The two former techniques have shown very interesting results in the identification and quantification of coccidia and helminth infections in several animal species, particularly horses, ruminants, and pets, with overall higher sensitivity and precision in comparison with the McMaster method [23,24,26,27]. Mini-FLOTAC in particular has been recently tested in birds [28,29,30], and successfully implemented at field level for the routine diagnosis of GI parasitic infections in poultry, peacocks, and ratites [10,31].
The current study aimed to (i) implement Mini-FLOTAC in the diagnosis of GI parasitic infections in four ornithological collections in Portugal and Spain and (ii) compare the diagnosis outcomes between McMaster and Mini-FLOTAC techniques.
2. Materials and Methods
2.1. Zoological Institutions and Sampling Procedures
Between January–December 2022, a total of 120 fresh fecal samples of clinically normal birds were collected at three Portuguese zoological institutions located in Lisbon, specifically the Lisbon Zoo (38°44′38.535″ N; 9°10′16.793″ W), Olivais Pedagogical Farm (38°45′47.186″ N; 9°6′42.666″ W), and Avian Biodiversity Centre (38°42′26.933″ N; 9°10′56.264″ W), as well as at one Spanish ornithological institution located in Lugo, the Avifauna park (43°3′14.554″ N; 7°41′22.903″ W).
At the Lisbon Zoo, a total of 24 fecal samples were collected from individual enclosures containing chickens (Gallus gallus domesticus; n = 10 samples), turkeys (Meleagris gallopavo domesticus; n = 2), peacocks (Pavo cristatus; n = 5), greater rheas (Rhea americana; n = 4), emus (Dromaius novaehollandiae, n = 2) and cassowaries (Casuarius casuarius; n = 1).
At the Olivais Pedagogical Farm (OPF), a zoological institution which harbors autochthonous farm animal breeds for conservation and educational purposes, a total of 26 fecal samples were collected from laying hens of the four Portuguese autochthonous breeds: “Branca” (n = 6), “Amarela” (n = 6), “Preta Lusitânica” (n = 6), and “Pedrês Portuguesa” (n = 8), with each breed being kept in separate enclosures.
At the Avian Biodiversity Centre (ABC), a newly established ornithological collection belonging to the Higher Institute of Agronomy, University of Lisbon, a total of 24 fecal samples were collected from each of the following enclosures containing autochthonous and exotic G. gallus domesticus breeds (two samples per enclosure): enclosure 1—“Amarela” breed; 2—“Preta Lusitânica” breed; 3—“Pedrês Portuguesa” breed; 4—“Pedrês Portuguesa” and “Amarela” breeds (mixed); 5—“Branca” and “Phoenix” breeds (mixed); 6 – “Araucana” and “Japanese Silkie” breeds (mixed); 7—“White Phoenix” breed; 8—“Light Brahma” breed; 9—common backyard chickens; 10—“Dark Brahma” breed; 11—domestic quails; 12—“White Leghorn” and “Ayam Cemani” breeds (mixed).
Finally, a total of 46 fecal samples were collected at Avifauna park from individual enclosures containing the following exotic and wild bird species: greater rhea (Rhea americana; n = 3 samples), curassow (Crax fasciolata; n = 3), flamingo (Phoenicopterus spp.; n = 3), ruddy shelduck (Tadorna ferruginea; n = 2), western capercaillie (Tetrao urogallus; n = 3), black swan (Cygnus atratus; n = 3), Monk parakeet (Myiopsitta monachus; n = 1), eagle-owl (Bubo bubo; n = 1), cockatiel (Nymphicus hollandicus; n = 2), macaw (Ara spp.; n = 2), motmot (Momotus momota; n = 1), superb parrot (Polytelis swainsonii; n = 1), cockatoo (Cacatua alba; n = 1), eastern rosella (Platycercus eximius; n = 1), avocet (Recuvirostra avosetta; n = 2), and pheasants (Phasianinae; n = 17) (Figure 1).

Figure 1.
Some of the domestic and exotic bird species included in this study: (A) cassowary, (B) ostrich, (C) emu, (D) cockatoo, (E) ruddy shelducks, (F) flamingos, (G) pheasant, (H) “Araucana” breed, (I) “Brahma” breed, (J) “Ayam Cemani” breed, (K) “Japanese Silkie” breed, (L) “White Phoenix” breed, (M) “Pedrês Portuguesa” breed (Portuguese Pedrês chicken), (N) “Amarela” breed (Yellow chicken), (O) “Preta Lusitânica” breed (Lusitanian Black chicken), (P) “Branca” breed (White chicken); photo credits: João Lozano (A–G,K,O,P) and Madalena Lordelo (H–J,L–N).
Birds’ fecal samples were collected from the environment, with no direct manipulation of the animals. Feces were collected using individual plastic bags, which were then labelled and immediately transported inside a cooling bag to two laboratories: (i) feces from the Portuguese zoological institutions were stored and processed at the Laboratory of Parasitology and Parasitic Diseases of the Faculty of Veterinary Medicine, University of Lisbon (Portugal); feces from Avifauna park were analyzed at the Laboratory of Zoonoses of the Faculty of Veterinary Medicine, University of Santiago de Compostela (Spain). In both cases, feces were stored at 4 °C for a maximum of one week.
2.2. Coprological Techniques
2.2.1. Phase 1—General Coprological Diagnosis Using Mini-FLOTAC
All birds’ feces were processed using the Mini-FLOTAC (MF) protocol for exotic animals (1:20 dilution), following the procedures previously described for birds [10,24]. For each sample, 2 g of feces were mixed with 38 mL of saturated sucrose solution (specific gravity 1.2) using the Fill-FLOTAC device. Then, the fecal suspension was directly transferred to the previously assembled reading chamber, and all coccidia oocysts and helminth eggs were identified and counted using an analytic sensitivity of 10 oocysts or eggs per gram of feces (OPG or EPG, respectively). The identification of parasitic forms was based on the descriptions from McDougald and Fitz-Coy [32], Yazwinski and Tucker [33], and Zajac and Conboy [34]. Coccidia oocysts’ incubation in potassium dichromate was not performed in the current research, and thus their identification at genus level was only performed in oocysts which were already sporulated when feces were collected, being based on the oocyst size and number of sporocysts and sporozoites inside [32].
2.2.2. Phase 2—Using Pheasants’ Feces to Compare Mini-FLOTAC and McMaster Methods
A total of 15 fecal samples from pheasants of the Avifauna park, which were processed with the Mini-FLOTAC method in phase 1 of this study, were also analyzed using the McMaster method (McM) to compare parasite prevalences and shedding obtained with both techniques. For each sample, a total of 3 g of feces were weighed and placed into a plastic flask, to which 42 mL of water were added (dilution 1:15). Feces were vigorously mixed and then filtered through a mesh with 150 μm pore diameter. The filtered suspension was transferred to 12 mL tubes and centrifuged at 2000 rpm for 5 min. Then, the supernatant was discarded, and the sediment mixed with 6 mL of saturated sodium chloride solution (specific gravity 1.2). The final suspension was transferred to a McMaster chamber, to count parasitic forms using an analytic sensitivity of 50 OPG/EPG, in a light microscope [21].
In this phase of the study, the fecal dilutions chosen for the Mini-FLOTAC and McMaster methods were in accordance with the manufacturer instructions and published literature for both methods [21,24], allowing reproduction of their expected analytical performances.
Since feces from all domestic and exotic birds included in this study had a maximum of 2–5 g, only one repetition was performed for each sample. Thus, prior to their mixing with the flotation solutions, feces were gently homogenized inside the respective plastic bags, to promote a complete distribution of all parasitic forms. Moreover, the general low quantity of individual feces allowed for the use of only 15 fecal samples from pheasants to compare the analytical performances of the Mini-FLOTAC and McMaster methods in the study’s second phase. Out of 17 pheasant feces, only two samples had less than three grams remaining after being processed with the Mini-FLOTAC method, and thus were not used in this study’s phase.
2.3. Statistical Analysis
The software Microsoft® Excel® (v2405; Microsoft Corporation, Redmond, WA, USA, 2023) was used for storing all parasitological data recorded for each ornithological institution, as well as for table and chart editing. Also, the software IBM® SPSS® Statistics (v27 for Windows; IBM Corporation, Armonk, NY, USA) was used for descriptive statistics (mean and standard errors), and to perform the following analysis: OPG and EPG data recorded in all bird collections were first subjected to normality analysis, using the Shapiro–Wilk test (n < 50 samples in each collection), which allowed for the conclusion that the data were not normally distributed (p < 0.001). Thus, parasite shedding data and prevalences obtained with Mini-FLOTAC and McMaster methods were compared using the Wilcoxon and Chi-square tests, respectively, with a significance level of p < 0.05.
3. Results
The implementation of the Mini-FLOTAC method allowed identification of coccidia oocysts in avian feces from all ornithological collections: the poultry and peacocks from the Lisbon Zoo (156 ± 104 OPG and 60 ± 60 OPG, respectively); the Portuguese autochthonous chicken breeds kept at OPF—“Preta Lusitânica” (88 ± 83 OPG), “Branca” (48 ± 25 OPG), “Amarela” (43 ± 34 OPG), and “Pedrês Portuguesa” (13 ± 6 OPG); the poultry of the ABC collection (663 ± 196 OPG), with emphasis on enclosures containing backyards chickens (5230 ± 1710 OPG), “Pedrês Portuguesa” (700 ± 40 OPG), “Amarela” (665 ± 155 OPG) and “Preta Lusitânica” (630 ± 110 OPG), and to a lesser extent at Avifauna park (Spain), particularly in avocets (20 ± 20 OPG), pheasants (11 ± 6 OPG), and western capercaillies (7 ± 3 OPG) (Table 1).

Table 1.
Coccidia and helminths’ fecal shedding levels (mean ± standard error) identified in different poultry breeds at two ornithological collections, Olivais Pedagogical Farm and Avian Biodiversity Centre, using the Mini-FLOTAC method.
Moreover, nematode eggs were also detected in feces from all bird collections, especially at Avifauna park, where the highest shedding of Capillaria sp. eggs was detected, particularly in Monk parakeets (260 EPG) and western capercaillies (103 ± 74 EPG), as well as Ascaridia sp. (72 ± 33 EPG), and a single sample containing Syngamus trachea eggs (10 EPG) was recorded in pheasants. Also, Strongyloides sp. infections were mostly detected in curassows of Avifauna park (33 ± 18 EPG), and peacocks of the Lisbon Zoo tested positive for T. tenuis (8 ± 8 EPG) (Table 2; Figure 2).

Table 2.
Coccidia and helminths’ fecal shedding levels (mean ± standard error) identified in different avian species kept at the Lisbon Zoo and Avifauna park, using the Mini-FLOTAC method.
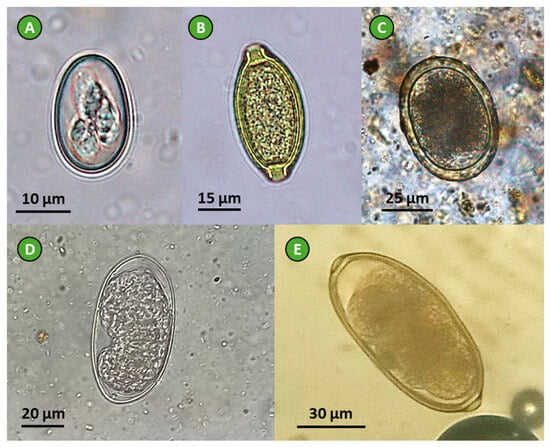
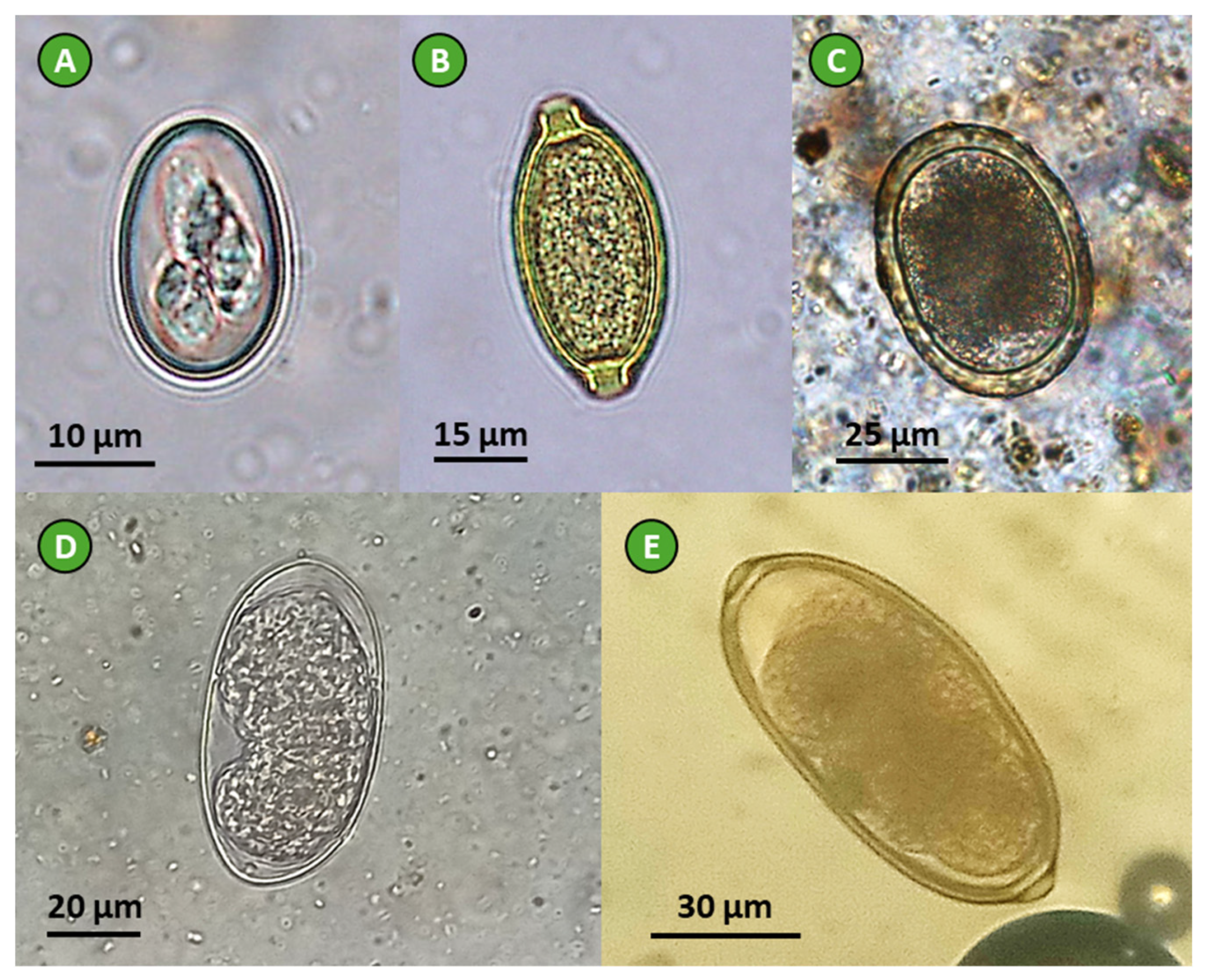
Figure 2.
Parasites identified in feces of the selected avian species: (A) Eimeria sp. oocyst (chicken feces), (B) Capillaria sp. egg (pheasant feces), (C) Ascaridia galli egg (chicken feces), (D) T. tenuis egg (peacock feces), and (E) Syngamus trachea egg (pheasant feces); photos taken at 400× total magnification (credits: João Lozano).
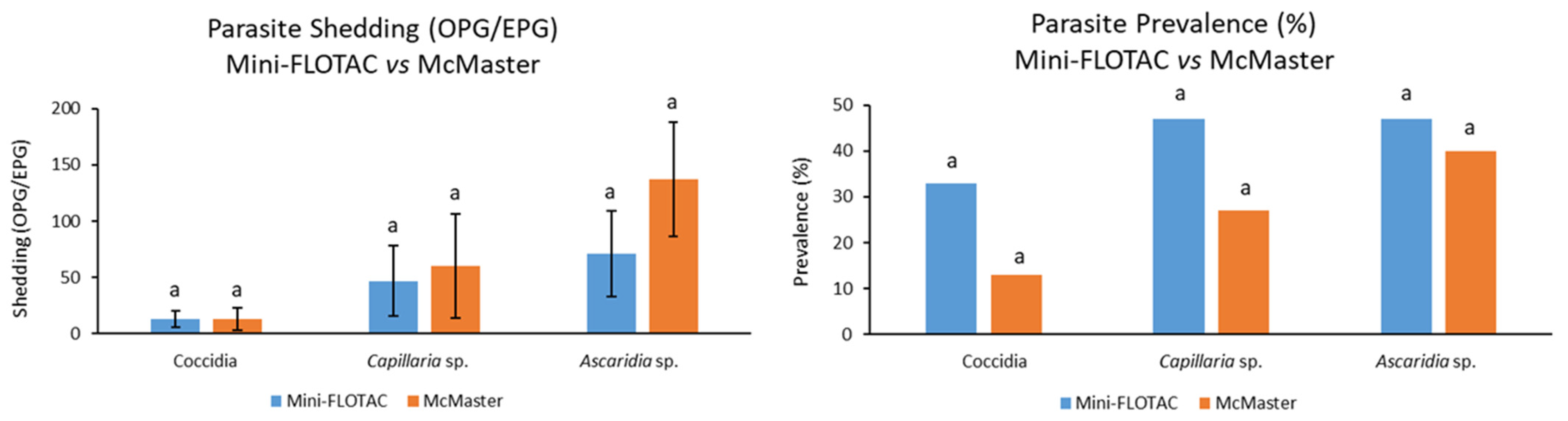
Regarding the comparisons of results obtained with both coprological methods, it was found that the coccidia, Capillaria sp., and Ascaridia sp. overall shedding detected in 15 pheasants achieved 13 ± 7 OPG and 13 ± 10 OPG, 47 ± 31 EPG and 60 ± 46 EPG, and 71 ± 38 EPG and 137 ± 51 EPG, using the Mini-FLOTAC and McMaster methods, respectively, with no significant differences between techniques (p = 0.89, p = 0.94, and p = 0.13, respectively).
The same scenario was observed for these parasites’ prevalences, with the implementation of Mini-FLOTAC and McMaster methods allowing identification of prevalences of 33% and 13% for coccidia, 47% and 27% for Capillaria sp., and 47% and 40% for Ascaridia sp., respectively, with their differences being once again not significant (p = 0.39, p = 0.45, and p = 0.50, respectively) (Figure 3).
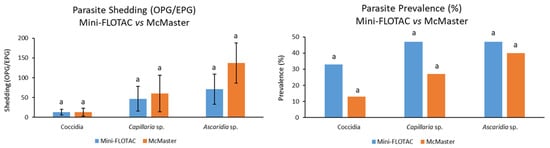
Figure 3.
Comparison of coccidia, Capillaria sp., and Ascaridia sp. fecal shedding and prevalences between Mini-FLOTAC and McMaster methods; error bars on the left chart correspond to standard errors; equal letters on each bar mean no significant difference between techniques (p > 0.05).
4. Discussion
The control of animal GI parasitic infections still mainly relies in using anticoccidial and anthelminthic drugs, as well as live non-attenuated or live attenuated vaccines, with no previous laboratorial diagnosis [35,36,37,38]. However, this kind of approach is unsustainable, especially in zoological institutions, since antiparasitic drugs target mainly the endogenous stages of the parasites’ life cycles and thus do not act on oocysts, eggs, and larvae accumulated in feces, soil, and/or pasture, and are also linked to drug resistance and bioaccumulation of pharmacological residues in soil and groundwaters [39,40,41]. Moreover, designing treatment protocols for exotic animal species is challenging for zoo veterinarians, since it often relies on adapting the guidelines reported for domestic animals, which can lead to low treatment efficacies, drug resistance, and even toxic effects in animals due to differences in the pharmacokinetics and pharmacodynamics of antiparasitic molecules between domestic and exotic animal species [42].
The implementation of the Mini-FLOTAC protocol for exotic animals allowed identification of different GI parasitic populations and respective shedding and prevalences in each bird collection. The identification of Eimeria sp. infections in laying hens belonging to Portuguese poultry breeds “Pedrês Portuguesa”, “Preta Lusitânica”, “Amarela”, and “Branca”, especially those kept at the Avian Biodiversity Centre, is in accordance with previous research performed in these poultry breeds kept free-ranging in a Portuguese organic farm, where authors also identified infections by Eimeria sp. and a respective oocyst fecal shedding up to 43,500 OPG [43]. Moreover, infections by this protozoan parasite were also identified in other Galliformes included in this study, particularly in birds of exotic breeds at the Avian Biodiversity Centre, as well as in chickens and peacocks of the Lisbon Zoo, and in pheasants of the Avifauna park, highlighting the susceptibility of birds from this order to become infected by sporulated coccidian oocysts, as reported in birds from other zoological institutions [6,8,11,12,14,15,20].
Coccidia are one of the most ubiquitous GI parasites in birds, with the genera Eimeria and Isospora having the most significant health and economic impacts on ornithological collections [32,37,44,45]. The combined effect of the intrinsic biological characteristics of this protozoan parasite, particularly its monoxenous and very short life cycle, with a pre-patent period of just 3–4 days [32,37,46], and the fact that zoo birds are often exposed to same outdoor environment for long periods, and consequently to sporulated oocysts in feces, soil, feed, and drinking water [32], leads to a continuous re-infection process. Although birds from the current study did not exhibit any clinical sign of coccidiosis, the detection of oocysts in their feces justifies the need for their regular monitoring, by means of coprological diagnosis, fecal quality, feather appearance analysis, and bird behavior observation. Clinical coccidiosis can include moderate to severe diarrhea episodes, sometimes with blood coagula, and may eventually lead to death if birds are not properly treated. Subclinical disease is often difficult to diagnose, being more linked to decreased nutrient absorption and consequently weight loss, anorexia, and prostration [37,46]. Thus, its control is of most importance in zoological institutions and conservation centers, especially in those harboring native and exotic poultry breeds, whose majority is near extinction [3]. The integration of anticoccidial drugs (coccidiostatic or coccidicidal drugs) with other control solutions, particularly vaccination, improved sanitizing, and the implementation of biological solutions such as feeding birds with prebiotics and probiotics, plant extracts, essential oils, and parasiticide fungi, have been proposed for the sustainable control of poultry coccidiosis [47,48,49].
Nematodes were also detected in all bird collections, with A. galli eggs being identified in feces from laying hens of the “Amarela” breed, a result already described in previous research in autochthonous poultry breeds [43], and in peacocks as previously reported at Brazilian [18] and Iranian zoos [19]. This ascarid is the largest roundworm of Galliformes, and its pathogenicity is associated to the thickening the intestinal epithelium and appearance of hemorrhagic spots along with edema, as well as ulcerative proventriculitis, leading to diarrhea and anemia, and with the accumulation of adult forms being capable of blocking the GI lumen in more severe infections [33,50,51,52], and thus being of clinical importance for ornithological collections.
Moreover, Capillaria sp. infections were recorded in exotic bird species of the Avifauna park, and despite the overall low shedding of this nematode in the majority of the host species, its identification in the western capercaillie should be underlined. Infections by this nematode in birds may cause catarrhal inflammation and thickening of the esophagus and crop epithelia, as well as hemorrhagic enteritis in small and large intestines, and anemia in more acute cases [33,53]. Since the population trend of the Western Capercaillie is currently classified as “Decreasing”, according to the latest edition of the IUCN’s Red List [2] and data from the European Nature Information System [54], the presence of this parasite in this bird species may pose future health and conservation concerns, with the regular monitoring of this nematode’s egg excretion and detection of any clinical signs being of utmost importance for its control.
Also, pheasants of the Avifauna park tested positive for S. trachea, a nematode of the respiratory tract of birds and often responsible for serious health and economic concerns in wild and game pheasants, since nematode adult forms attach to the tracheal mucosa and, together with mucous production, lead to lumen obstruction and consequently to birds asphyxiation [33,55,56]. Thus, despite its low egg shedding, the identification of this nematode on these avian hosts justifies a higher awareness and their regular monitoring.
Results from the comparisons between Mini-FLOTAC and McMaster performances regarding parasite shedding or prevalence allowed determination of similar results for both techniques. Moreover, and despite the absence of statistical difference between the outputs obtained with each method, it must be noted that Mini-FLOTAC detected a coccidia prevalence 2.5× higher (33%) than the one obtained with the McMaster method (13%). Overall results from these comparisons allow the conclusion that Mini-FLOTAC is indeed a valuable alternative to the McMaster method for the diagnosis of coccidia and helminth infections in domestic and exotic birds, as reported in previous studies [10,11,24,31]. In addition, the fact that this method only comprises three devices for fecal processing and analysis—Fill-FLOTAC, reading chamber, and a light microscope—together with an extensively proven high sensitivity and precision [24,26] supports its use by zoo veterinarians for a simple, quick, non-expensive, and “in-house” parasitological diagnosis, as previously described in several zoological institutions [10,57,58]. Further research could also include the comparison of the McMaster method with other Mini-FLOTAC and FLOTAC dilutions [23,24], to optimize the coprological diagnosis of birds’ GI parasitism.
To the authors best knowledge, research on the GI parasitism of autochthonous poultry breeds and exotic bird species is still scarce worldwide, and overall results from this study suggest the need for further broader-scale research in more public and private zoological collections, especially those harboring avian species facing the threat of extinction, and at a multi-country level, since the integration of regular monitoring of avian parasitism with antiparasitic drug treatments is of most importance for their conservation.
Author Contributions
Conceptualization, J.L., M.O., A.P.-S. and L.M.d.C.; methodology, J.L., C.P., R.S., C.C.-M., M.S.A., L.R., M.O., A.P.-S. and L.M.d.C.; software, J.L.; validation, M.O., A.P.-S. and L.M.d.C.; formal analysis, J.L.; investigation, J.L., M.O., A.P.-S. and L.M.d.C.; resources, J.L., C.P., R.S., C.C.-M., M.S.A., D.C., M.L., A.B., R.B., L.R., M.O., A.P.-S. and L.M.d.C.; data curation, J.L. and C.P.; writing—original draft preparation, J.L.; writing—review and editing, M.O., A.P.-S. and L.M.d.C.; visualization, J.L.; supervision, M.S.A., A.P.-S., M.L., A.B., R.B., L.R., M.O., A.P.-S. and L.M.d.C.; project administration, L.M.d.C.; funding acquisition, J.L., M.S.A., M.O., A.P.-S. and L.M.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CIISA/FMV Project UIDB/00276/2020 and LA/P/0059/2020—AL4AnimalS (both funded by FCT), as well as by Project ED431B 2021/07 (Consellería de Cultura, Educación e Universidades, Xunta de Galicia). Also, João Lozano holds a Ph.D. Research Fellowship, 2020.09037.BD (funded by FCT).
Institutional Review Board Statement
Ethical review and approval were waived for this study since fecal samples were collected from soil, after excretion, and thus without any direct manipulation of the birds. Moreover, all procedures were integrated in the normal daily activities of each zoological institution.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to acknowledge the owners of each bird collection for allowing us to perform fecal samplings at the respective facilities, and to thank all veterinarians and technicians for all the support provided during this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Miranda, R.; Escribano, N.; Casas, M.; Pino-Del-Carpio, A.; Villarroya, A. The Role of Zoos and Aquariums in a Changing World. Annu. Rev. Anim. Biosci. 2023, 11, 287–306. [Google Scholar] [CrossRef] [PubMed]
- IUCN Red List. European Red List of Birds 2021. Available online: https://data.europa.eu/doi/10.2779/959320 (accessed on 7 December 2023).
- FAO. Status and Trends of Animal Genetic Resources. Available online: https://openknowledge.fao.org/items/1875926b-3ca0-4e9b-9012-465d7d6db9f2 (accessed on 15 May 2024).
- Carolino, I.; Lopes, S.; Silva, F.; Oliveira e Sousa, C.; Almeida, J.; Carolino, N. Galinhas de Raças Autóctones e a Sua Importância Na Agricultura Sustentável. Rev. Voz Do Campo 2014, 22–23. Available online: https://vozdocampo.pt/2022/05/15/galinhas-de-rac%CC%A7as-autoctones-e-a-sua-importa%CC%82ncia-na-agricultura-sustentavel/ (accessed on 4 June 2024).
- Brito, N.; Lopes, J.; Ribeiro, V.; Dantas, R.; Leite, J. Small Scale Egg Production: The Challenge of Portuguese Autochthonous Chicken Breeds. Agriculture 2021, 11, 818. [Google Scholar] [CrossRef]
- Carrera-Játiva, P.D.; Morgan, E.R.; Barrows, M.; Wronski, T. Gastrointestinal Parasites in Captive and Free-Ranging Birds and Potential Cross-Transmission in a Zoo Environment. J. Zoo Wildl. Med. 2018, 49, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Játiva, P.D.; Morgan, E.R.; Barrows, M.; Jiménez-Uzcátegui, G.; Armijos Tituaña, J.R. Free-Ranging Avifauna as a Source of Generalist Parasites for Captive Birds in Zoological Settings: An Overview of Parasite Records and Potential for Cross-Transmission. J. Adv. Vet. Anim. Res. 2020, 7, 482–500. [Google Scholar] [CrossRef] [PubMed]
- Ilić, T.; Becskei, Z.; Gajić, B.; Özvegy, J.; Stepanović, P.; Nenadović, K.; Dimitrijević, S. Prevalence of Endoparasitic Infections of Birds in Zoo Gardens in Serbia. Acta Parasitol. 2018, 63, 134–146. [Google Scholar] [CrossRef]
- Papini, R.; Girivetto, M.; Marangi, M.; Mancianti, F.; Giangaspero, A. Endoparasite Infections in Pet and Zoo Birds in Italy. Sci. World J. 2012, 2012, 253127. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Almeida, C.; Victório, A.; Melo, P.; Rodrigues, J.; Rinaldi, L.; Cringoli, G.; Gomes, L.; Oliveira, M.; Paz-Silva, A.; et al. Implementation of Mini-Flotac in Routine Diagnosis of Coccidia and Helminth Infections in Domestic and Exotic Birds. Vet. Sci. 2021, 8, 160. [Google Scholar] [CrossRef]
- Rosa de Almeida, C. Caraterização Do Parasitismo Gastrointestinal Em Coleções de Pavão Comum (Pavo cristatus) Inseridos No Património Cultural Na Região de Lisboa. MSc Thesis, Faculty of Veterinary Medicine, University of Lisbon, Lisbon, Portugal, 2022. [Google Scholar]
- Rocha de Castro, D. Pesquisa de Parasitas Gastrointestinais Em Aves de Rapina Mantidas Em Cativeiro Em Portugal. MSc Thesis, Faculty of Veterinary Medicine, University of Lisbon, Lisbon, Portugal, 2021. [Google Scholar]
- Lozano, J.; Palomero, A.; Anaya, A.; Lux-Hoppe, E.; Gomes, L.; Oliveira, M.; Paz-Silva, A.; Teresa Rebelo, M.; Madeira de Carvalho, L. Long-Term Survey of Eimeria spp. Prevalence and Faecal Shedding in a Traditional Portuguese Free-Range Broiler Farm. Rev. Port. Zoot 2021, 6, 13–23. [Google Scholar]
- Pérez Cordón, G.; Hitos Prados, A.; Romero, D.; Sánchez Moreno, M.; Pontes, A.; Osuna, A.; Rosales, M.J. Intestinal Parasitism in the Animals of the Zoological Garden “Peña Escrita” (Almuñecar, Spain). Vet. Parasitol. 2008, 156, 302–309. [Google Scholar] [CrossRef]
- Kamel, A.; Abdel-Latef, G. Prevalence of Intestinal Parasites with Molecular Detection and Identification of Giardia duodenalis in Fecal Samples of Mammals, Birds and Zookeepers at Beni-Suef Zoo, Egypt. J. Parasit. Dis. 2021, 45, 695–705. [Google Scholar] [CrossRef]
- Hofstatter, P.G.; Guaraldo, A.M.A. Levantamento Parasitológico Em Aves de Alguns Zoológicos Brasileiros. Rev. Bras. De Parasitol. Vet. 2015, 24, 87–91. [Google Scholar] [CrossRef]
- Gallo, S.S.M.; Teixeira, C.S.; Ederli, N.B.; Oliveira, F.C.R. Gastrointestinal Parasites of a Population of Emus (Dromaius novaehollandiae) in Brazil. Braz. J. Biol. 2020, 80, 66–72. [Google Scholar] [CrossRef]
- Melo, Y.; Ferraz, H.; Saturnino, K.; Silva, T.; Braga, I.; Amaral, A.; Meirelles-Bartoli, R.; Ramos, D. Gastrointestinal Parasites in Captive and Free-Living Wild Birds in Goiania Zoo. Braz. J. Biol. 2022, 82, e240386. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, V.; Jameie, F. Intestinal Parasitic Infection in Wild Animals of a Zoological Garden in Alborz, Iran. J. Istanb. Vet. Sci. 2019, 3, 37–42. [Google Scholar] [CrossRef][Green Version]
- Zhang, K.; Fu, Y.; Ma, C.; Wang, Y.; Zhang, J.; Lang, J.; Han, K.; Chen, Y.; Yu, F.; Zhang, L. First Detection of Coccidia Species in Blue Peafowl (Pavo cristatus) in China via Both Microscopic and Molecular Methods. Vet. Parasitol. Reg. Stud. Rep. 2022, 36, 100807. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.; Conboy, G. Fecal Exam Procedures. In Veterinary Clinical Parasitology; Zajac, A., Conboy, G., Eds.; Wiley-Blackwell: Ames, Iowa, 2012; pp. 140–155. [Google Scholar]
- Shapiro, L. Appendix I: Important Techniques for Veterinary Technicians. In Pathology and Parasitology for Veterinary Technicians; Shapiro, L., Ed.; Delmar, Cengage Learning: Boston, MA, USA, 2010; pp. 223–241. [Google Scholar]
- Cringoli, G.; Rinaldi, L.; Maurelli, M.P.; Utzinger, J. FLOTAC: New Multivalent Techniques for Qualitative and Quantitative Copromicroscopic Diagnosis of Parasites in Animals and Humans. Nat. Protoc. 2010, 5, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, G.; Maurelli, M.P.; Levecke, B.; Bosco, A.; Vercruysse, J.; Utzinger, J.; Rinaldi, L. The Mini-FLOTAC Technique for the Diagnosis of Helminth and Protozoan Infections in Humans and Animals. Nat. Protoc. 2017, 12, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Englar, R. Quick Assessment Tests (QATS) Involving Feces. In Low-Cost Veterinary Clinical Diagnostics; Englar, R., Dial, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; pp. 159–233. [Google Scholar]
- Maurelli, M.P.; Dourado Martins, O.M.; Morgan, E.R.; Charlier, J.; Cringoli, G.; Mateus, T.L.; Bacescu, B.; Chartier, C.; Claerebout, E.; de Waal, T.; et al. A Qualitative Market Analysis Applied to Mini-FLOTAC and Fill-FLOTAC for Diagnosis of Helminth Infections in Ruminants. Front. Vet. Sci. 2020, 7, 580649. [Google Scholar] [CrossRef]
- Maurelli, M.P.; Rinaldi, L.; Alfano, S.; Pepe, P.; Coles, G.C.; Cringoli, G. Mini-FLOTAC, a New Tool for Copromicroscopic Diagnosis of Common Intestinal Nematodes in Dogs. Parasit. Vectors 2014, 7, 356. [Google Scholar] [CrossRef]
- Coker, S.M.; Pomroy, W.E.; Howe, L.; McInnes, K.; Vallee, E.; Morgan, K.J. Comparing the Mini-FLOTAC and Centrifugal Faecal Flotation for the Detection of Coccidia (Eimeria spp.) in Kiwi (Apteryx mantelli). Parasitol. Res. 2020, 119, 4287–4290. [Google Scholar] [CrossRef]
- Daş, G.; Klauser, S.; Stehr, M.; Tuchscherer, A.; Metges, C.C. Accuracy and Precision of McMaster and Mini-FLOTAC Egg Counting Techniques Using Egg-Spiked Faeces of Chickens and Two Different Flotation Fluids. Vet. Parasitol. 2020, 283, 109158. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Paras, K.L.; Applegate, T.J.; Verocai, G.G. Comparison between McMaster and Mini-FLOTAC Methods for the Enumeration of Eimeria Maxima Oocysts in Poultry Excreta. Vet. Parasitol. 2018, 254, 21–25. [Google Scholar] [CrossRef]
- Lozano, J.; Anaya, A.; Rinaldi, L.; Cringoli, G.; Gomes, L.; Oliveira, M.; Paz-Silva, A.; Rebelo, M.; Madeira de Carvalho, L. Diagnosis of Coccidiosis by Eimeria Spp. in Free-Range Chickens Using Mini-FLOTAC and McMaster Techniques-Preliminary Results. Sci. Parasitol. 2021, 22, 13–18. [Google Scholar]
- McDougald, L.; Fitz-Coy, S. Parasitic Diseases—Coccidiosis. In Diseases of Poultry; Saif, Y., Ed.; Blackwell Publishing Professional: Ames, IA, USA, 2008; pp. 1068–1085. [Google Scholar]
- Yazwinski, T.; Tucker, C. Nematodes and Acanthocephalans. In Diseases of Poultry; Saif, Y., Ed.; Blackwell Publishing Professional: Ames, IA, USA, 2008; pp. 1025–1066. [Google Scholar]
- Zajac, A.; Conboy, G. Parasites of Domestic Animals—Birds. In Veterinary Clinical Parasitology; Zajac, A., Conboy, G., Eds.; Wiley-Blackwell: Ames, IA, USA, 2012; pp. 140–155. [Google Scholar]
- Erez, M.S.; Doğan, İ.; Kozan, E.; Göksu, A. A Survey of Knowledge, Approaches, and Practices Surrounding Parasitic Infections and Antiparasitic Drug Usage by Veterinarians in Türkiye. Animals 2023, 13, 2693. [Google Scholar] [CrossRef]
- Peek, H.W.; Landman, W.J.M. Coccidiosis in Poultry: Anticoccidial Products, Vaccines and Other Prevention Strategies. Vet. Q. 2011, 31, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Pineda, C.; Navarro-Ruíz, J.L.; López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Chicken Coccidiosis: From the Parasite Lifecycle to Control of the Disease. Front. Vet. Sci. 2021, 8, 787653. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, T.; Abbas, R.Z.; Imran, M.; Abbas, A.; Butt, A.; Aslam, S.; Ahmad, J. Vaccines against Chicken Coccidiosis with Particular Reference to Previous Decade: Progress, Challenges, and Opportunities. Parasitol. Res. 2022, 121, 2749–2763. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.Z.; Iqbal, Z.; Blake, D.; Khan, M.N.; Saleemi, M.K. Anticoccidial Drug Resistance in Fowl Coccidia: The State of Play Revisited. Worlds Poult. Sci. J. 2011, 67, 337–349. [Google Scholar] [CrossRef]
- Mooney, D.; Richards, K.G.; Danaher, M.; Grant, J.; Gill, L.; Mellander, P.E.; Coxon, C.E. An Analysis of the Spatio-Temporal Occurrence of Anthelmintic Veterinary Drug Residues in Groundwater. Sci. Total Environ. 2021, 769, 144804. [Google Scholar] [CrossRef]
- Martins, R.R.; Silva, L.J.G.; Pereira, A.M.P.T.; Esteves, A.; Duarte, S.C.; Pena, A. Coccidiostats and Poultry: A Comprehensive Review and Current Legislation. Foods 2022, 11, 2738. [Google Scholar] [CrossRef] [PubMed]
- Panayotova-Pencheva, M.S. Experience in the Ivermectin Treatment of Internal Parasites in Zoo and Captive Wild Animals: A Review. Zool. Gart. 2016, 85, 280–308. [Google Scholar] [CrossRef]
- Carvalho, I.; Castanheira, F.; Mateus, T. Parasitas Gastrointestinais Em Aves de Produção Biológica e de Raça Autóctone Portuguesa. Agrotec. Cuid. Veterinários 2017, 23, 22–25. [Google Scholar]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-Calculating the Cost of Coccidiosis in Chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- Panayotova-Pencheva, M.S. Parasites in Captive Animals: A Review of Studies in Some European Zoos. Zool. Gart. 2013, 82, 60–71. [Google Scholar] [CrossRef]
- Lozano, J.; Anaya, A.; Palomero Salinero, A.; Lux-Hoppe, E.; Gomes, L.; Paz-Silva, A.; Teresa Rebelo, M.; Madeira de Carvalho, L. Gastrointestinal Parasites of Free-Range Chickens—A Worldwide Issue. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Vet. Med. 2019, 76, 110. [Google Scholar] [CrossRef]
- Attree, E.; Sanchez-Arsuaga, G.; Jones, M.; Xia, D.; Marugan-Hernandez, V.; Blake, D.; Tomley, F. Controlling the Causative Agents of Coccidiosis in Domestic Chickens; an Eye on the Past and Considerations for the Future. CABI Agric. Biosci. 2021, 2, 37. [Google Scholar] [CrossRef]
- Jamil, M.; Aleem, M.T.; Shaukat, A.; Khan, A.; Mohsin, M.; Rehman, T.U.; Abbas, R.Z.; Saleemi, M.K.; Khatoon, A.; Babar, W.; et al. Medicinal Plants as an Alternative to Control Poultry Parasitic Diseases. Life 2022, 12, 449. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Louro, M.; Almeida, C.; Victório, A.C.; Melo, P.; Rodrigues, J.P.; Oliveira, M.; Paz-Silva, A.; Madeira de Carvalho, L. Isolation of Saprophytic Filamentous Fungi from Avian Fecal Samples and Assessment of Its Predatory Activity on Coccidian Oocysts. Sci. Rep. 2023, 13, 8965. [Google Scholar] [CrossRef] [PubMed]
- Brar, R.S.; Kumar, R.; Leishangthem, G.D.; Banga, H.S.; Singh, N.D.; Singh, H. Ascaridia galli Induced Ulcerative Proventriculitis in a Poultry Bird. J. Parasit. Dis. 2016, 40, 562–564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shohana, N.N.; Rony, S.A.; Ali, M.H.; Hossain, M.S.; Labony, S.S.; Dey, A.R.; Farjana, T.; Alam, M.Z.; Alim, M.A.; Anisuzzaman. Ascaridia galli Infection in Chicken: Pathobiology and Immunological Orchestra. Immun. Inflamm. Dis. 2023, 11, e1001. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.; Daş, G.; Tarbiat, B.; Geldhof, P.; Jansson, D.S.; Gauly, M. Ascaridia galli—An Old Problem That Requires New Solutions. Int. J. Parasitol. Drugs Drug Resist. 2023, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Groves, P.J. Impact of Parasites on Australian Laying Hen Welfare. Anim. Prod. Sci. 2021, 61, 1031–1036. [Google Scholar] [CrossRef]
- European Nature Information System. Capercaillie—Tetrao Urogallus (Linnaeus, 1758). Available online: https://eunis.eea.europa.eu/species/1315 (accessed on 26 January 2024).
- Martin, K.A.; Jesudoss Chelladurai, J.R.J.; Sato, Y.; Brewer, M.T. Syngamus Asphyxiation in a Captive Ring-Necked Pheasant. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100493. [Google Scholar] [CrossRef] [PubMed]
- Friend, M.; Franson, J. Field Manual of Wildlife Diseases—General Field Procedures and Diseases of Birds, 1st ed.; USGS: Madison, WI, USA, 1999; ISBN 0607880961. [Google Scholar]
- Capasso, M.; Maurelli, M.P.; Ianniello, D.; Alves, L.C.; Amadesi, A.; Laricchiuta, P.; Silvestre, P.; Campolo, M.; Cringoli, G.; Rinaldi, L. Use of Mini-FLOTAC and Fill-FLOTAC for Rapidly Diagnosing Parasitic Infections in Zoo Mammals. Rev. Bras. De Parasitol. Vet. 2019, 28, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, M. Survey of Gastrointestinal Parasites of Non-Human Primates from Two Iberian Zoos and Perspectives of Their Integrated Control. MSc Thesis, Faculty of Veterinary Medicine, University of Lisbon, Lisbon, Portugal, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).