The Contribution of Genetic and Genomic Tools in Diversity Conservation: The Case of Endemic Plants of Greece
Abstract
1. Introduction
2. Genetic/Genomic Tools Used in Conservation
3. Conservation Genetics of Greek Endemic Plant Species (GEPs)
3.1. The Studied Area
3.2. Methods
- (a)
- Phytogeographical region(s) of Greece that they are distributed in;
- (b)
- Presence on island and/or mainland;
- (c)
- Ecological factors such as substrates, main habitats occupied, and altitudinal range;
- (d)
- Growth forms sensu Raunkiaer (1934) (i.e., Phanerophyte, Chamaephyte, Hemicryptophyte, Geophyte, Therophyte), which constitute a good proxy for classification into broad functional groups [101];
- (e)
- Karyology, with indications of the chromosome number and the ploidy level according to the Chromosome Counts Database (CCDB, version 1.66);
- (f)
- Genetic data: genetic markers used, mean population genetic diversity (He) and its standard deviation, plastid haplotype number, genetic differentiation (Fst, PhiST, or equivalent);
- (g)
- Number of known populations and putative extinct populations;
- (h)
- IUCN categories according to the IUCN Red List of threatened taxa;
- (i)
| Taxon | Family | Phytogeographical Region | Elevation (m) | Substrate | Habitat | Life Form | Chromosome Number | IUCN Category | Genetic Markers | Reference (s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Abies cephalonica Loudon | Pinaceae | IoI, Pe, StE, SPi, NPi, NC, NE, WAe, EAe | 600–1700 | various (limestone, flysch) | W | Phanerophyte | 2n = 2x = 24 | LC | SSRs | Parducci et al. 2001 [103] |
| Aethionema retsina Phitos & Snogerup | Brassicaceae | WAe | 0–300 | limestone | C | Chamaephyte | 2n = 2x = 24 | CR | ISSRs | Kougioumoutzis et al. 2021 [68] |
| Allium iatrouinum Trigas | Amaryllidaceae | WAe | 0–1020 | metamorphic | C | Geophyte | 2n = 2x = 14 | CR | ISSRs | Kougioumoutzis et al. 2021 [68] |
| Asperula naufraga Ehrend. & Gutermann | Rubiaceae | IoI | 2–265 | limestone | C | Chamaephyte | 2n = 2x = 20 | EN | SSRs | Valli et al. 2021 [104] |
| Brassica cretica Lam. subsp. cretica | Brassicaceae | Pe, KK | 0–1100 | limestone | C | Chamaephyte | 2n = 2x = 18 | NE | SSRs | Edh et al. 2007 [105] |
| Centaurea chrysocephala Phitos & T. Georgiadis | Asteraceae | SPi, NPi | 350–450 | various | C | Hemicryptophyte | 2n = 2x = 18 | NE | SSRs, cpDNA region | Lopez-Vinyallonga et al. 2015 [106] |
| Centaurea heldreichii Halácsy | Asteraceae | StE | 3–600 | limestone | C | Hemicryptophyte | 2n = 2x = 18 | VU | SSRs, cpDNA region | Lopez-Vinyallonga et al. 2015 [106] |
| Centaurea litochorea T. Georgiadis & Phitos | Asteraceae | NC | 830–1800 | limestone | C | Hemicryptophyte | 2n = 2x = 16 | VU | SSRs, cpDNA region | Lopez-Vinyallonga et al. 2015 [106] |
| Centaurea messenicolasiana T. Georgiadis & al. | Asteraceae | SPi | 500–800 | flysch | R | Hemicryptophyte | 2n = 2x = 18 | VU | SSRs, cpDNA region | Lopez-Vinyallonga et al. 2015 [106] |
| Centaurea princeps Boiss. & Heldr. | Asteraceae | SPi, StE | 1100–1850 | limestone | G, C | Hemicryptophyte | 2n = 2x = 18 | EN | SSRs, cpDNA region | Lopez-Vinyallonga et al. 2015 [106] |
| Centaurea raphanina Sm. subsp. raphanina | Asteraceae | KK, Cyc | 0–2200 | mainly limestone | P, W, H | Hemicryptophyte | 2n = 2x = 20 | NE | RAPDs | Psaroudaki et al. 2015 [107] |
| Cicer graecum Boiss. | Fabaceae | Pe | 400–1400 | limestone | P, W | Hemicryptophyte | unknown | EN | ISSRs, AFLPs | Stathi et al. 2020 [108] |
| Convolvulus argyrothamnos Greuter | Convolvulaceae | KK | 450–650 | limestone | C | Chamaephyte | unknown | CR | ISSRs | Kougioumoutzis et al. 2021 [68] |
| Crocus cartwrightianus Herb. | Iridaceae | Pe, StE, WAe, Cyc, KK, EAe | 0–900 | various | P | Geophyte | 2n = 2x = 16 | NE | SSRs, AFLPs | Larsen et al. 2015 [109] |
| Crocus oreocreticus B.L. Burtt | Iridaceae | KK | 700–1900 | various | P, H | Geophyte | 2n = 2x = 16 | NE | SSRs, AFLPs | Larsen et al. 2015 [109] |
| Cyclamen creticum Hildebr. | Primulaceae | KK | 0–1350 | various | W | Geophyte | 2n = 2x = 22 | NE | isoenzymes | Affre and Thompson 1997 [110] |
| Minuartia dirphya Trigas & Iatrou | Caryophyllaceae | WAe | 900–1000 | serpentine | P | Hemicryptophyte | 2n = 2x = 26 | CR | SSRs, REMAPs | Augustinos et al. 2014 [111] |
| Minuartia parnonia (Kamari) Iatroú & al. | Caryophyllaceae | Pe | 700–1200 | limestone | G | Hemicryptophyte | 2n = 2x = 26 | NT | SSRs, REMAPs | Augustinos et al. 2014 [111] |
| Minuartia wettsteinii Mattf. | Caryophyllaceae | KK | 1100–1450 | limestone | P | Chamaephyte | 2n = 2x = 26 | VU | SSRs, REMAPs | Augustinos et al. 2014 [111] |
| Odontarrhena lesbiaca P. Candargy | Brassicaceae | EAe | 0–800 | serpentine | W, G | Hemicryptophyte | unknown | NE | ISSRs | Adamidis et al. 2014 [112] |
| Origanum dictamnus L. | Lamiaceae | KK | 50–1700 | limestone | C | Chamaephyte | 2n = 2x = 30 | NT | SSRs, HRM | Papaioanou et al. 2020 [113] |
| Phlomis lanata Willd. | Lamiaceae | KK | 0–1750 | limestone | P | Chamaephyte | 2n = 2x = 20 | NE | nuDNA and cpDNA regions, AFLPs | Georgescu et al. 2016 [114] |
| Saponaria jagelii Phitos & Greuter | Caryophyllaceae | Pe | 0 | sandy | C | Therophyte | unknown | CR | ISSRs | Kougioumoutzis et al. 2021 [68] |
| Sideritis euboea Heldr. | Lamiaceae | WAe | 1000–1700 | limestone | G, H | Hemicryptophyte | 2n = 2x = 32 | EN | AFLPs | Sarrou et al. 2022 [115] |
| Sideritis syriaca L. subsp. syriaca | Lamiaceae | KK | 1000–2200 m | limestone | H | Chamaephyte | 2n = 2x = 32 | NE | DNA barcoding | Paschalidis et al. 2024 [116] |
| Tulipa bakeri A.D. Hall | Liliaceae | KK | 700–1300 | various | H, R | Geophyte | 2n = 2x = 24 | CR | DNA barcoding | Samartza et al. 2024 [117] |
| Tulipa cretica Boiss. & Heldr. | Liliaceae | KK | 0–2100 | various | P, W | Geophyte | 2n = 2x = 24 | LC | DNA barcoding | Samartza et al. 2024 [117] |
| Tulipa doerfleri Gand. | Liliaceae | KK | 330–800 | various | R | Geophyte | 2n = 2x = 36 | CR | DNA barcoding | Samartza et al. 2024 [117] |
| Tulipa goulimyi Seally & Turrill | Liliaceae | Pe, KK | 0–900 | limestone | P, R | Geophyte | 2n = 2x = 24, 3x = 36, 4x = 48 | VU | DNA barcoding | Samartza et al. 2024 [117] |
| Tulipa hageri Heldr. | Liliaceae | StE, Pe | 100–1200 | various | P, R | Geophyte | 2n = 2x = 24 | EN | DNA barcoding | Samartza et al. 2024 [117] |
| Tulipa orphanidea Heldr. sensu stricto | Liliaceae | StE, Pe | 0–1700 | various | R | Geophyte | 2n = 2x = 24, 3x = 36, 4x = 48 | EN | DNA barcoding | Samartza et al. 2024 [117] |
| Zelkova abelicea (Lam.) Boiss. | Ulmaceae | KK | 850–1800 | limestone | W | Phanerophyte | 2n = 2x = 28 | EN | AFLPs, ISSRs, nuDNA region | Fineschi et al. 2002, 2004, Christe et al. 2014 [118,119,120] |
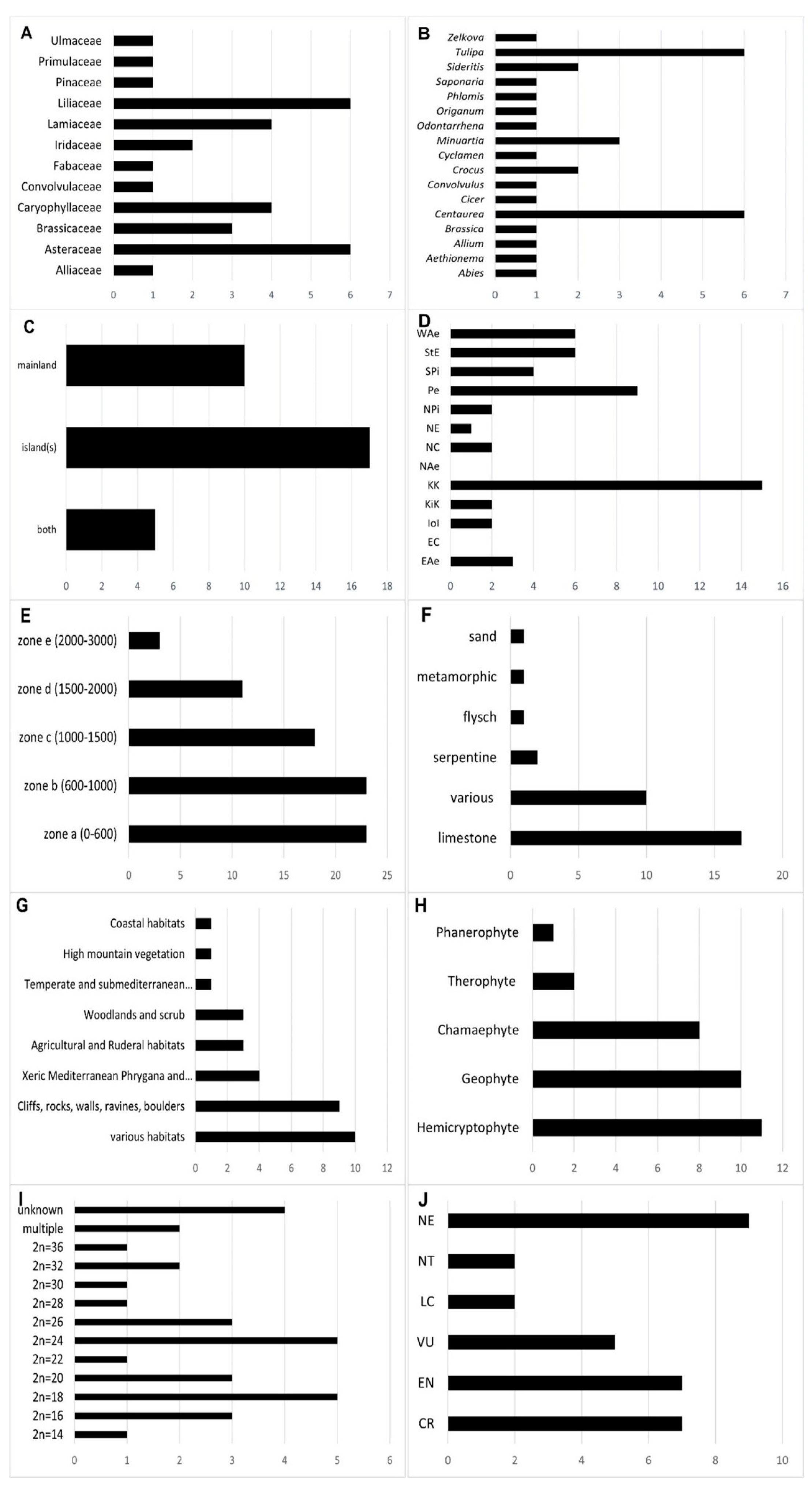
3.3. Results and Discussion
3.3.1. Insights about the Studied GEPs
3.3.2. Insights about the Molecular Markers and Methods
3.3.3. Insights about Conservation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schemske, D.W.; Husband, B.C.; Ruckelshaus, M.H.; Goodwillie, C.; Parker, I.M.; Bishop, J.G. Evaluating Approaches to the Conservation of Rare and Endangered Plants. Ecology 1994, 75, 584–606. [Google Scholar] [CrossRef]
- Van Dyke, F. Conservation Biology; Springer: Dordrecht, The Netherlands, 2008; ISBN 978-1-4020-6890-4. [Google Scholar]
- Markert, J.A.; Champlin, D.M.; Gutjahr-Gobell, R.; Grear, J.S.; Kuhn, A.; McGreevy, T.J.; Roth, A.; Bagley, M.J.; Nacci, D.E. Population Genetic Diversity and Fitness in Multiple Environments. BMC Evol. Biol. 2010, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.H.; Frankham, R. Correlation between Fitness and Genetic Diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. A Primer of Conservation Genetics; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Maxted, N.; Hunter, D.; Ortiz Ríos, R. Plant Genetic Conservation; Cambridge University Press: Cambridge, UK, 2020; ISBN 9781139024297. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2023-1. Available online: https://www.iucnredlist.org (accessed on 20 January 2024).
- Ebert, A.W.; Engels, J.M.M. Plant Biodiversity and Genetic Resources Matter! Plants 2020, 9, 1706. [Google Scholar] [CrossRef]
- Nybom, H.; Lācis, G. Recent Large-Scale Genotyping and Phenotyping of Plant Genetic Resources of Vegetatively Propagated Crops. Plants 2021, 10, 415. [Google Scholar] [CrossRef]
- Lemoine, T.; Violle, C.; Montazeaud, G.; Isaac, M.E.; Rocher, A.; Fréville, H.; Fort, F. Plant Trait Relationships Are Maintained within a Major Crop Species: Lack of Artificial Selection Signal and Potential for Improved Agronomic Performance. New Phytol. 2023, 240, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Schatz, G.E. Plants on the IUCN Red List: Setting Priorities to Inform Conservation. Trends Plant Sci. 2009, 14, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Corlett, R.T. Plant Diversity in a Changing World: Status, Trends, and Conservation Needs. Plant Divers. 2016, 38, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Gagen, M. The Forest Pathways Report; WWF: Gland, Switzerland, 2023. [Google Scholar]
- Cuttelod, A.; García, N.; Temple, H.J.; Katariya, V. The Mediterranean: A Biodiversity Hotspot under Threat. Wildlife in a Changing World. In 2008 IUCN Red List of Threatened Species, Wildlife in a Changing World; IUCN: Gland, Switzerland, 2009; pp. 89–101. [Google Scholar]
- Reed, B.M.; Sarasan, V.; Kane, M.; Bunn, E.; Pence, V.C. Biodiversity Conservation and Conservation Biotechnology Tools. Vitr. Cell. Dev. Biol. Plant 2011, 47, 1–4. [Google Scholar] [CrossRef]
- Corlett, R.T. A Bigger Toolbox: Biotechnology in Biodiversity Conservation. Trends Biotechnol. 2017, 35, 55–65. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic Plant Species Conservation: Biotechnological Approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S. Area and Endemism. Q. Rev. Biol. 1994, 69, 451–471. [Google Scholar] [CrossRef]
- Burlakova, L.E.; Karatayev, A.Y.; Karatayev, V.A.; May, M.E.; Bennett, D.L.; Cook, M.J. Endemic Species: Contribution to Community Uniqueness, Effect of Habitat Alteration, and Conservation Priorities. Biol. Conserv. 2011, 144, 155–165. [Google Scholar] [CrossRef]
- Kruckeberg, A.R.; Rabinowitz, D. Biological Aspects of Endemism in Higher Plants. Annu. Rev. Ecol. Syst. 1985, 16, 447–479. [Google Scholar] [CrossRef]
- Gaston, K.J. What Is Rarity? In Rarity; Springer: Dordrecht, The Netherlands, 1994; pp. 1–21. [Google Scholar]
- Caesar, M.; Grandcolas, P.; Pellens, R. Outstanding Micro-Endemism in New Caledonia: More than One out of Ten Animal Species Have a Very Restricted Distribution Range. PLoS ONE 2017, 12, e0181437. [Google Scholar] [CrossRef] [PubMed]
- López-Pujol, J.; Martinell, M.C.; Massó, S.; Blanché, C.; Sáez, L. The ‘Paradigm of Extremes’: Extremely Low Genetic Diversity in an Extremely Narrow Endemic Species, Coristospermum huteri (Umbelliferae). Plant Syst. Evol. 2013, 299, 439–446. [Google Scholar] [CrossRef]
- Isik, K. Rare and Endemic Species: Why Are They Prone to Extinction? Turk. J. Bot. 2011, 35, 411–417. [Google Scholar] [CrossRef]
- Foggi, B.; Viciani, D.; Baldini, R.M.; Carta, A.; Guidi, T. Conservation Assessment of the Endemic Plants of the Tuscan Archipelago, Italy. Oryx 2015, 49, 118–126. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Chauhan, B.S. Genetic Diversity, Conservation, and Utilization of Plant Genetic Resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef]
- Maxted, N.; Ford-Lloyd, B.V.; Hawkes, J.G. Complementary Conservation Strategies. In Plant Genetic Conservation: The In Situ Approach; Maxted, N., Ford-Lloyd, B.V., Hawkes, J.G., Eds.; Chapman and Hall: London, UK, 1997; p. 15. [Google Scholar]
- Engelmann, F. Use of Biotechnologies for the Conservation of Plant Biodiversity. Vitr. Cell. Dev. Biol. Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Ogwu, M.C.; Osawaru, M.E.; Ahana, C.M. Challenges in Conserving and Utilizing Plant Genetic Resources (PGR). Int. J. Genet. Mol. Biol. 2014, 6, 16–23. [Google Scholar] [CrossRef]
- Geffen, E.; Luikart, G.; Waples, R.S. Impacts of Modern Molecular Genetic Techniques on Conservation Biology. In Key Topics in Conservation Biology; Macdonald, D.W., Service, K., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; pp. 46–63. [Google Scholar]
- Neel, M.C. Conservation Planning and Genetic Diversity. In Conservation Biology: Evolution in Action; Carrol, S.P., Fox, C.W., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 281–296. [Google Scholar]
- Moritz, C. Strategies to Protect Biological Diversity and the Evolutionary Processes That Sustain It. Syst. Biol. 2002, 51, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Neel, M.C.; Cummings, M.P. Effectiveness of Conservation Targets in Capturing Genetic Diversity. Conserv. Biol. 2003, 17, 219–229. [Google Scholar] [CrossRef]
- Center for Plant Conservation Genetic Sampling Guidelines for Conservation Collections of Endangered Plants. Genetics and Conservation of Rare Plants; Falk, D.A., Holsinger, K.E., Eds.; Oxford University Press: New York, NY, USA, 1991; pp. 225–238. [Google Scholar]
- Lacy, R.C.; Ballou, J.D.; Pollak, J.P. PMx: Software Package for Demographic and Genetic Analysis and Management of Pedigreed Populations. Methods Ecol. Evol. 2012, 3, 433–437. [Google Scholar] [CrossRef]
- Che-Castaldo, J.; Gray, S.M.; Rodriguez-Clark, K.M.; Schad Eebes, K.; Faust, L.J. Expected Demographic and Genetic Declines Not Found in Most Zoo and Aquarium Populations. Front. Ecol. Environ. 2021, 19, 435–442. [Google Scholar] [CrossRef]
- Höglund, J. Evolutionary Conservation Genetics; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Marshall, D.R.; Brown, A.H.D. Optimum sampling strategies in genetic conservation. In Crop Genetic Resources for Today and Tomorrow; Frankel, O.H., Hawkes, J.G., Eds.; Cambridge University Press: Cambridge, UK, 1975; pp. 53–80. [Google Scholar]
- Crossa, J.; Hernandez, C.M.; Bretting, P.; Eberhart, S.A.; Taba, S. Statistical Genetic Considerations for Maintaining Germ Plasm Collections. Theor. Appl. Genet. 1993, 86, 673–678. [Google Scholar] [CrossRef]
- Crossa, J.; Vencovsky, R. Basic Sampling Strategies: Theory and Practice. Collect. Plant Genet. Divers. Tech. Guidel. 2011, 28, 1–28. [Google Scholar]
- Kimura, M. Rare Variant Alleles in the Light of the Neutral Theory. Mol. Biol. Evol. 1983, 1, 84–93. [Google Scholar] [CrossRef]
- Hohenlohe, P.A.; Funk, W.C.; Rajora, O.P. Population Genomics for Wildlife Conservation and Management. Mol. Ecol. 2021, 30, 62–82. [Google Scholar] [CrossRef]
- Willi, Y.; Kristensen, T.N.; Sgrò, C.M.; Weeks, A.R.; Ørsted, M.; Hoffmann, A.A. Conservation Genetics as a Management Tool: The Five Best-Supported Paradigms to Assist the Management of Threatened Species. Proc. Natl. Acad. Sci. USA 2022, 119, 6119. [Google Scholar] [CrossRef] [PubMed]
- Segelbacher, G.; Bosse, M.; Burger, P.; Galbusera, P.; Godoy, J.A.; Helsen, P.; Hvilsom, C.; Iacolina, L.; Kahric, A.; Manfrin, C.; et al. New Developments in the Field of Genomic Technologies and Their Relevance to Conservation Management. Conserv. Genet. 2022, 23, 217–242. [Google Scholar] [CrossRef]
- Fernandez, J.; Bennewitz, J. Defining genetic diversity based on genomics tools. In Genomic Management of Animal Genetic Resources, 1st ed.; Oldenbroek, J.K., Ed.; Wageningen Academic Publisher: Gelderland, The Netherlands, 2018; pp. 49–76. [Google Scholar]
- Mascher, M.; Schreiber, M.; Scholz, U.; Graner, A.; Reif, J.C.; Stein, N. Genebank Genomics Bridges the Gap between the Conservation of Crop Diversity and Plant Breeding. Nat. Genet. 2019, 51, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Browne, L.; Wright, J.W.; Fitz-Gibbon, S.; Gugger, P.F.; Sork, V.L. Adaptational Lag to Temperature in Valley Oak (Quercus lobata) Can Be Mitigated by Genome-Informed Assisted Gene Flow. Proc. Natl. Acad. Sci. USA 2019, 116, 25179–25185. [Google Scholar] [CrossRef] [PubMed]
- Funk, W.C.; Forester, B.R.; Converse, S.J.; Darst, C.; Morey, S. Improving Conservation Policy with Genomics: A Guide to Integrating Adaptive Potential into U.S. Endangered Species Act Decisions for Conservation Practitioners and Geneticists. Conserv. Genet. 2019, 20, 115–134. [Google Scholar] [CrossRef]
- Medail, F.; Quezel, P. Hot-Spots Analysis for Conservation of Plant Biodiversity in the Mediterranean Basin. Ann. Mo. Bot. Gard. 1997, 84, 112. [Google Scholar] [CrossRef]
- Médail, F.; Quézel, P. Biodiversity Hotspots in the Mediterranean Basin: Setting Global Conservation Priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Luikart, G.; Aitken, N. Conservation and the Genetics of Populations. Mammalia 2007, 7, 189–197. [Google Scholar] [CrossRef]
- Hubby, J.L.; Lewontin, R.C. A Molecular Approach to the Study of Genic Heterozygosity in Natural Populations. I. the Number of Alleles at Different Loci in Drosophila pseudoobscura. Genetics 1966, 54, 577–594. [Google Scholar] [CrossRef]
- Jefferies, R.L.; Gottlieb, L.D. Genetic Differentiation of the Microspecies Salicornia europaea L. (Sensu stricto) and S. ramosissima, J. Woods. New Phytol. 1982, 92, 123–129. [Google Scholar] [CrossRef]
- Sharma, I.K.; Jones, D.L.; Young, A.G.; French, C.J. Genetic Diversity and Phylogenetic Relatedness among Six Endemic Pterostylis Species (Orchidaceae; Series Grandiflorae) of Western Australia, as Revealed by Allozyme Polymorphisms. Biochem. Syst. Ecol. 2001, 29, 697–710. [Google Scholar] [CrossRef]
- Torres, E.; Iriondo, J.M.; Pérez, C. Genetic Structure of an Endangered Plant, Antirrhinum microphyllum (Scrophulariaceae): Allozyme and RAPD Analysis. Am. J. Bot. 2003, 90, 85–92. [Google Scholar] [CrossRef]
- Reinar, W.B.; Lalun, V.O.; Reitan, T.; Jakobsen, K.S.; Butenko, M.A. Length Variation in Short Tandem Repeats Affects Gene Expression in Natural Populations of Arabidopsis thaliana. Plant Cell 2021, 33, 2221–2234. [Google Scholar] [CrossRef]
- Morgante, M.; Hanafey, M.; Powell, W. Microsatellites Are Preferentially Associated with Nonrepetitive DNA in Plant Genomes. Nat. Genet. 2002, 30, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Paunescu, A. Biotechnology for Endangered Plant Conservation: A Critical Overview. Rom. Biotechnol. Lett. 2009, 14, 4095–4103. [Google Scholar]
- Kalia, R.K.; Rai, M.K.; Kalia, S.; Singh, R.; Dhawan, A.K. Microsatellite Markers: An Overview of the Recent Progress in Plants. Euphytica 2011, 177, 309–334. [Google Scholar] [CrossRef]
- Radosavljević, I.; Satovic, Z.; Jakse, J.; Javornik, B.; Greguraš, D.; Jug-Dujaković, M.; Liber, Z. Development of New Microsatellite Markers for Salvia officinalis L. and Its Potential Use in Conservation-Genetic Studies of Narrow Endemic Salvia brachyodon Vandas. Int. J. Mol. Sci. 2012, 13, 12082–12093. [Google Scholar] [CrossRef] [PubMed]
- Manole-Paunescu, A. Biotechnology for Endangered Plant Conservation. In Biotechnology and Biodiversity; Ahuja, M.R., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2014; pp. 181–202. [Google Scholar]
- Tani, N.; Kawahara, T.; Yoshimaru, H.; Hoshi, Y. Development of SCAR Markers Distinguishing Pure Seedlings of the Endangered Species Morus boninensis from M. boninensis × M. acidosa Hybrids for Conservation in Bonin (Ogasawara) Islands. Conserv. Genet. 2003, 4, 605–612. [Google Scholar] [CrossRef]
- Tsumura, Y.; Matsumoto, A.; Tani, N.; Ujino-Ihara, T.; Kado, T.; Iwata, H.; Uchida, K. Genetic Diversity and the Genetic Structure of Natural Populations of Chamaecyparis obtusa: Implications for Management and Conservation. Heredity 2007, 99, 161–172. [Google Scholar] [CrossRef]
- Liu, Z.; Shu, Q.; Wang, L.; Yu, M.; Hu, Y.; Zhang, H.; Tao, Y.; Shao, Y. Genetic Diversity of the Endangered and Medically Important Lycium ruthenicum Murr. Revealed by Sequence-Related Amplified Polymorphism (SRAP) Markers. Biochem. Syst. Ecol. 2012, 45, 86–97. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, P.; Singh, S.C.; Sundaresan, V. Efficiency of ISSR and RAPD Markers in Genetic Divergence Analysis and Conservation Management of Justicia adhatoda L.; a Medicinal Plant. Plant Syst. Evol. 2014, 300, 1409–1420. [Google Scholar] [CrossRef]
- da Silva, L.N.; Essi, L.; Welker, C.A.D.; de Souza-Chies, T.T. Assessing the Genetic Diversity and Population Structure of the Endangered Chascolytrum bulbosum (Poaceae, Poeae) Using AFLP Markers. Biochem. Syst. Ecol. 2016, 68, 236–242. [Google Scholar] [CrossRef]
- Sorkheh, K.; Amirbakhtiar, N.; Ercisli, S. Retracted Article: Potential Start Codon Targeted (SCoT) and Inter-Retrotransposon Amplified Polymorphism (IRAP) Markers for Evaluation of Genetic Diversity and Conservation of Wild Pistacia Species Population. Biochem. Genet. 2016, 54, 368–387. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kotsakiozi, P.; Stathi, E.; Trigas, P.; Parmakelis, A. Conservation Genetics of Four Critically Endangered Greek Endemic Plants: A Preliminary Assessment. Diversity 2021, 13, 152. [Google Scholar] [CrossRef]
- Lanes, É.C.; Pope, N.S.; Alves, R.; Carvalho Filho, N.M.; Giannini, T.C.; Giulietti, A.M.; Imperatriz-Fonseca, V.L.; Monteiro, W.; Oliveira, G.; Silva, A.R.; et al. Landscape Genomic Conservation Assessment of a Narrow-Endemic and a Widespread Morning Glory from Amazonian Savannas. Front. Plant Sci. 2018, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Manawaduge, C.; Brown, G.; Simmons, C.L.; Phillips, M.J.; Fuller, S. Molecular Systematic Analysis of the Genus Notelaea (Oleaceae): SNPs from High-density DArT-sequencing Unravel the Mystery of the Species Limits of Threatened Species of Notelaea. J. Syst. Evol. 2023, 61, 643–656. [Google Scholar] [CrossRef]
- Supple, M.A.; Shapiro, B. Conservation of Biodiversity in the Genomics Era. Genome Biol. 2018, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Luikart, G.; Kardos, M.; Hand, B.K.; Rajora, O.P.; Aitken, S.N.; Hohenlohe, P.A. Population Genomics: Advancing Understanding of Nature. In Population Genomics; Rajora, O., Ed.; Springer: Cham, Germany, 2018; pp. 3–79. [Google Scholar]
- Rajora, O.P. Population Genomics; Rajora, O.P., Ed.; Springer International Publishing: Cham, Germany, 2019; ISBN 978-3-030-04587-6. [Google Scholar]
- Meek, M.H.; Larson, W.A. The Future Is Now: Amplicon Sequencing and Sequence Capture Usher in the Conservation Genomics Era. Mol. Ecol. Resour. 2019, 19, 795–803. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; White, V.L.; Jasper, M.; Yagui, H.; Sinclair, S.J.; Kearney, M.R. An Endangered Flightless Grasshopper with Strong Genetic Structure Maintains Population Genetic Variation despite Extensive Habitat Loss. Ecol. Evol. 2021, 11, 5364–5380. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, X.; Xu, X.; Zou, Z.; Chen, B.; Qin, Y.; Zhang, X.; Dong, J.; Liu, D.; Pan, L.; et al. Rebound in China’s Coastal Wetlands Following Conservation and Restoration. Nat. Sustain. 2021, 4, 1076–1083. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, L.; Sun, W. Transcriptome Data Analysis Provides Insights into the Conservation of Michelia lacei, a Plant Species with Extremely Small Populations Distributed in Yunnan Province, China. BMC Plant Biol. 2024, 24, 200. [Google Scholar] [CrossRef]
- Fuentes-Pardo, A.P.; Ruzzante, D.E. Whole-genome Sequencing Approaches for Conservation Biology: Advantages, Limitations and Practical Recommendations. Mol. Ecol. 2017, 26, 5369–5406. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.; García-Dorado, A. Allelic Diversity and Its Implications for the Rate of Adaptation. Genetics 2013, 195, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Perdomo, E.; Magnin-Robert, J.-B.; Raffiot, B.; Deulvot, C.; Floriot, M.; Lejeune-Hénaut, I.; Marget, P.; Burstin, J.; Tayeh, N.; Aubert, G. A QTL Approach in Faba Bean Highlights the Conservation of Genetic Control of Frost Tolerance among Legume Species. Front. Plant Sci. 2022, 13, 865. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Banks, S.C.; Edworthy, M.; Stojanovic, D.; Langmore, N.E.; Heinsohn, R. Using Conservation Genetics to Prioritise Management Options for an Endangered Songbird. Heredity 2023, 130, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Ryder, O.A. Species Conservation and Systematics: The Dilemma of Subspecies. Trends Ecol. Evol. 1986, 1, 9–10. [Google Scholar] [CrossRef]
- Waples, R.S. Definition of “species” under the Endangered Species Act: Application to Pacific Salmon. Act. Mar. Fish. Rev. 1991, 53, 11–22. [Google Scholar]
- Moritz, C. Defining ‘Evolutionarily Significant Units’ for Conservation. Trends Ecol. Evol. 1994, 9, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C. Conservation Units and Translocations: Strategies for Conserving Evolutionary Processes. Hereditas 1999, 130, 217–228. [Google Scholar] [CrossRef]
- Fraser, D.J.; Bernatchez, L. Adaptive Evolutionary Conservation: Towards a Unified Concept for Defining Conservation Units. Mol. Ecol. 2001, 10, 2741–2752. [Google Scholar] [CrossRef]
- Weeks, A.R.; Stoklosa, J.; Hoffmann, A.A. Conservation of Genetic Uniqueness of Populations May Increase Extinction Likelihood of Endangered Species: The Case of Australian Mammals. Front. Zool. 2016, 13, 31. [Google Scholar] [CrossRef]
- Coleman, R.A.; Weeks, A.R.; Hoffmann, A.A. Balancing Genetic Uniqueness and Genetic Variation in Determining Conservation and Translocation Strategies: A Comprehensive Case Study of Threatened Dwarf Galaxias, Galaxiella pusilla (Mack) (Pisces: Galaxiidae). Mol. Ecol. 2013, 22, 1820–1835. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist; Botanic Garden and Botanical Museum Berlin-Dahlem: Berlin, Germany, 2013. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist. Supplement. Willdenowia 2016, 46, 301–347. [Google Scholar] [CrossRef]
- Flora of Greece. Available online: https://portal.cybertaxonomy.org/flora-greece/intro (accessed on 7 February 2024).
- Strid, A.; Tan, K.; Matricaria, L. Mountain Flora of Greece; Edinburgh University Press: Edinburgh, UK, 1991; Volume 2, pp. 450–452. [Google Scholar]
- Triantis, K.A.; Mylonas, M. Greek islands biology. In Encyclopedia of Islands; Gillespie, R., Glague, D.A., Eds.; University of California Press: Berkeley, CA, USA, 2009; pp. 388–392. [Google Scholar]
- Kapsimalis, V.P. Geoarchaeological Challenges in the Cyclades Continental Shelf (Aegean Sea). Z. Für Geomorphol. Suppl. Issues 2009, 53, 169–190. [Google Scholar] [CrossRef]
- Lykousis, V. Sea-Level Changes and Shelf Break Prograding Sequences during the Last 400ka in the Aegean Margins: Subsidence Rates and Palaeogeographic Implications. Cont. Shelf Res. 2009, 29, 2037–2044. [Google Scholar] [CrossRef]
- Sakellariou, D.; Galanidou, N. Pleistocene Submerged Landscapes and Palaeolithic Archaeology in the Tectonically Active Aegean Region. Geol. Soc. Lond. Spec. Publ. 2016, 411, 145–178. [Google Scholar] [CrossRef]
- Fassoulas, C. Biogeography and Biodiversity of the Aegean. In Honour of Prof. Moysis Mylonas; Sfenthourakis, S., Pafilis, P., Parmakelis, A., Poulakakis, N., Triantis, K., Eds.; Broken Hill Publishers Ltd: Nicosia, Cyprus, 2018; pp. 25–46. [Google Scholar]
- Kougioumoutzis, K.; Kokkoris, I.; Panitsa, M.; Kallimanis, A.; Strid, A.; Dimopoulos, P. Plant Endemism Centres and Biodiversity Hotspots in Greece. Biology 2021, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Phitos, D.; Strid, A.; Snogerup, S.; Greuter, W. The Red Data Book of Rare and Threatened Plants of Greece; WWF: Athens, Greece, 1995. [Google Scholar]
- Strid, A. Atlas of the Aegean Flora; Botanischer Garten und Botanisches Museum Berlin-Dahlem: Berlin, Germany, 2016; Volume 33, p. 1578. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: London, UK, 1934. [Google Scholar]
- Phitos, D.; Constantinidis, T.; Kamari, G. The Red Data Book of Rare and Threatened Plants of Greece; Hellenic Botanical Society: Patras, Greece, 2009; Volume 1–2. [Google Scholar]
- Parducci, L.; Szmidt, A.E.; Madaghiele, A.; Anzidei, M.; Vendramin, G.G. Genetic Variation at Chloroplast Microsatellites (CpSSRs) in Abies nebrodensis (Lojac.) Mattei and Three Neighboring Abies Species. Theor. Appl. Genet. 2001, 102, 733–740. [Google Scholar] [CrossRef]
- Valli, A.-T.; Koumandou, V.L.; Iatrou, G.; Andreou, M.; Papasotiropoulos, V.; Trigas, P. Conservation Biology of Threatened Mediterranean Chasmophytes: The Case of Asperula naufraga Endemic to Zakynthos Island (Ionian Islands, Greece). PLoS ONE 2021, 16, e0246706. [Google Scholar] [CrossRef]
- Edh, K.; Widen, B.; Ceplitis, A. Nuclear and Chloroplast Microsatellites Reveal Extreme Population Differentiation and Limited Gene Flow in the Aegean Endemic Brassica cretica (Brassicaceae). Mol. Ecol. 2007, 16, 4972–4983. [Google Scholar] [CrossRef]
- López-Vinyallonga, S.; López-Pujol, J.; Constantinidis, T.; Susanna, A.; Garcia-Jacas, N. Mountains and Refuges: Genetic Structure and Evolutionary History in Closely Related, Endemic Centaurea in Continental Greece. Mol. Phylogenetics Evol. 2015, 92, 243–254. [Google Scholar] [CrossRef]
- Psaroudaki, A.; Nikoloudakis, N.; Skaracis, G.; Katsiotis, A. Genetic Structure and Population Diversity of Eleven Edible Herbs of Eastern Crete. J. Biol. Res. Thessalon. 2015, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Stathi, E.; Kougioumoutzis, K.; Abraham, E.M.; Trigas, P.; Ganopoulos, I.; Avramidou, E.V.; Tani, E. Population Genetic Variability and Distribution of the Endangered Greek Endemic Cicer graecum under Climate Change Scenarios. AoB Plants 2020, 12, 7. [Google Scholar] [CrossRef]
- Larsen, B.; Orabi, J.; Pedersen, C.; Ørgaard, M. Large Intraspecific Genetic Variation within the Saffron-Crocus Group (Crocus L.; Series Crocus; Iridaceae). Plant Syst. Evol. 2015, 301, 425–437. [Google Scholar] [CrossRef]
- Affre, L.; Thompson, J.D. Population Genetic Structure and Levels of Inbreeding Depression in the Mediterranean Island Endemic Cyclamen creticum (Primulaceae). Biol. J. Linn. Soc. 1997, 60, 527–549. [Google Scholar] [CrossRef]
- Augustinos, A.; Sotirakis, K.; Trigas, P.; Kalpoutzakis, E.; Papasotiropoulos, V. Genetic Variation in Three Closely Related Minuartia (Caryophyllaceae) Species Endemic to Greece: Implications for Conservation Management. Folia Geobot. 2014, 49, 603–621. [Google Scholar] [CrossRef]
- Adamidis, G.C.; Dimitrakopoulos, P.G.; Manolis, A.; Papageorgiou, A.C. Genetic Diversity and Population Structure of the Serpentine Endemic Ni Hyperaccumulator Alyssum lesbiacum. Plant Syst. Evol. 2014, 300, 2051–2060. [Google Scholar] [CrossRef]
- Papaioannou, C.; Zeliou, K.; Trigas, P.; Papasotiropoulos, V. High Resolution Melting (HRM) Genotyping in the Genus Origanum: Molecular Identification and Discrimination for Authentication Purposes. Biochem. Genet. 2020, 58, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, L.; Stefanakis, M.K.; Kokkini, S.; Katerinopoulos, H.E.; Pirintsos, S.A. Chemical and Genetic Characterization of Phlomis Species and Wild Hybrids in Crete. Phytochemistry 2016, 122, 91–102. [Google Scholar] [CrossRef]
- Sarrou, E.; Doukidou, L.; Avramidou, E.V.; Martens, S.; Angeli, A.; Stagiopoulou, R.; Fyllas, N.M.; Tourvas, N.; Abraham, E.; Maloupa, E.; et al. Chemodiversity Is Closely Linked to Genetic and Environmental Diversity: Insights into the Endangered Populations of the Local Endemic Plant Sideritis euboea Heldr. of Evia Island (Greece). J. Appl. Res. Med. Aromat. Plants 2022, 31, 100426. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tsichlas, I.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Ipsilantis, I.; Grigoriadou, K.; Samartza, I.; et al. DNA Barcoding and Fertilization Strategies in Sideritis syriaca subsp. syriaca, a Local Endemic Plant of Crete with High Medicinal Value. Int. J. Mol. Sci. 2024, 25, 1891. [Google Scholar] [CrossRef]
- Samartza, I.; Tsaballa, A.; Sakellariou, M.; Mahnev, P.; Tsiripidis, I.; Krigas, N.; Tsoktouridis, G. Taxonomic and Molecular Characterization of 15 Wild-Growing Tulip Species of Greece Using the Internal Transcribed Spacer (ITS) Nuclear Marker in Combination with the psb A-trn H and trn L/trn F Plastid Markers. Biotechnol. Biotechnol. Equip. 2024, 38, 2337694. [Google Scholar] [CrossRef]
- Fineschi, S.; Anzidei, M.; Cafasso, D.; Cozzolino, S.; Garfì, G.; Pastorelli, R.; Salvini, D.; Taurchini, D.; Vendramin, G.G. Molecular Markers Reveal a Strong Genetic Differentiation between Two European Relic Tree Species: Zelkova abelicea (Lam.) Boissier and Z. sicula Di Pasquale, Garfì & Quézel (Ulmaceae). Conserv. Genet. 2002, 3, 145–153. [Google Scholar] [CrossRef]
- Fineschi, S.; Cozzolino, S.; Migliaccio, M.; Vendramin, G.G. Genetic Variation of Relic Tree Species: The Case of Mediterranean Zelkova abelicea (Lam.) Boisser and Z. sicula Di Pasquale, Garfì and Quézel (Ulmaceae). For. Ecol. Manag. 2004, 197, 273–278. [Google Scholar] [CrossRef]
- Christe, C.; Kozlowski, G.; Frey, D.; Fazan, L.; Bétrisey, S.; Pirintsos, S.; Gratzfeld, J.; Naciri, Y. Do Living Ex Situ Collections Capture the Genetic Variation of Wild Populations? A Molecular Analysis of Two Relict Tree Species, Zelkova abelica and Zelkova carpinifolia. Biodivers. Conserv. 2014, 23, 2945–2959. [Google Scholar] [CrossRef]
- Georghiou, K.; Delipetrou, P. Patterns and Traits of the Endemic Plants of Greece. Bot. J. Linn. Soc. 2010, 162, 130–422. [Google Scholar] [CrossRef]
- Médail, F.; Baumel, A. Using Phylogeography to Define Conservation Priorities: The Case of Narrow Endemic Plants in the Mediterranean Basin Hotspot. Biol. Conserv. 2018, 224, 258–266. [Google Scholar] [CrossRef]
- Kalendar, R.; Grob, T.; Regina, M.; Suoniemi, A.; Schulman, A. IRAP and REMAP: Two New Retrotransposon-Based DNA Fingerprinting Techniques. Theor. Appl. Genet. 1999, 98, 704–711. [Google Scholar] [CrossRef]
- Biswas, M.K.; Xu, Q.; Deng, X. Utility of RAPD, ISSR, IRAP and REMAP Markers for the Genetic Analysis of Citrus spp. Sci. Hortic. 2010, 124, 254–261. [Google Scholar] [CrossRef]
- Boronnikova, S.V.; Kalendar, R.N. Using IRAP Markers for Analysis of Genetic Variability in Populations of Resource and Rare Species of Plants. Russ. J. Genet. 2010, 46, 36–42. [Google Scholar] [CrossRef]
- D’Onofrio, C.; De Lorenzis, G.; Giordani, T.; Natali, L.; Cavallini, A.; Scalabrelli, G. Retrotransposon-Based Molecular Markers for Grapevine Species and Cultivars Identification. Tree Genet. Genomes 2010, 6, 451–466. [Google Scholar] [CrossRef]
- Abdollahi Mandoulakani, B.; Piri, Y.; Darvishzadeh, R.; Bernoosi, I.; Jafari, M. Retroelement Insertional Polymorphism and Genetic Diversity in Medicago sativa Populations Revealed by IRAP and REMAP Markers. Plant Mol. Biol. Report. 2012, 30, 286–296. [Google Scholar] [CrossRef]
- Spielman, D.; Brook, B.W.; Frankham, R. Most Species Are Not Driven to Extinction before Genetic Factors Impact Them. Proc. Natl. Acad. Sci. USA 2004, 101, 15261–15264. [Google Scholar] [CrossRef] [PubMed]
- Bobo-Pinilla, J.; Salmerón-Sánchez, E.; Mota, J.F.; Peñas, J. Genetic Conservation Strategies of Endemic Plants from Edaphic Habitat Islands: The Case of Jacobaea auricula (Asteraceae). J. Nat. Conserv. 2021, 61, 126004. [Google Scholar] [CrossRef]
- Zavodna, M.; Abdelkrim, J.; Pellissier, V.; Machon, N. A Long-Term Genetic Study Reveals Complex Population Dynamics of Multiple-Source Plant Reintroductions. Biol. Conserv. 2015, 192, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liveri, E.; Passa, K.; Papasotiropoulos, V. The Contribution of Genetic and Genomic Tools in Diversity Conservation: The Case of Endemic Plants of Greece. J. Zool. Bot. Gard. 2024, 5, 276-293. https://doi.org/10.3390/jzbg5020019
Liveri E, Passa K, Papasotiropoulos V. The Contribution of Genetic and Genomic Tools in Diversity Conservation: The Case of Endemic Plants of Greece. Journal of Zoological and Botanical Gardens. 2024; 5(2):276-293. https://doi.org/10.3390/jzbg5020019
Chicago/Turabian StyleLiveri, Eleni, Kondylia Passa, and Vasileios Papasotiropoulos. 2024. "The Contribution of Genetic and Genomic Tools in Diversity Conservation: The Case of Endemic Plants of Greece" Journal of Zoological and Botanical Gardens 5, no. 2: 276-293. https://doi.org/10.3390/jzbg5020019
APA StyleLiveri, E., Passa, K., & Papasotiropoulos, V. (2024). The Contribution of Genetic and Genomic Tools in Diversity Conservation: The Case of Endemic Plants of Greece. Journal of Zoological and Botanical Gardens, 5(2), 276-293. https://doi.org/10.3390/jzbg5020019









