Computational Investigation of Methoxy Radical-Driven Oxidation of Dimethyl Sulfide: A Pathway Linked to Methane Oxidation
Abstract
1. Introduction
2. Computational Methods
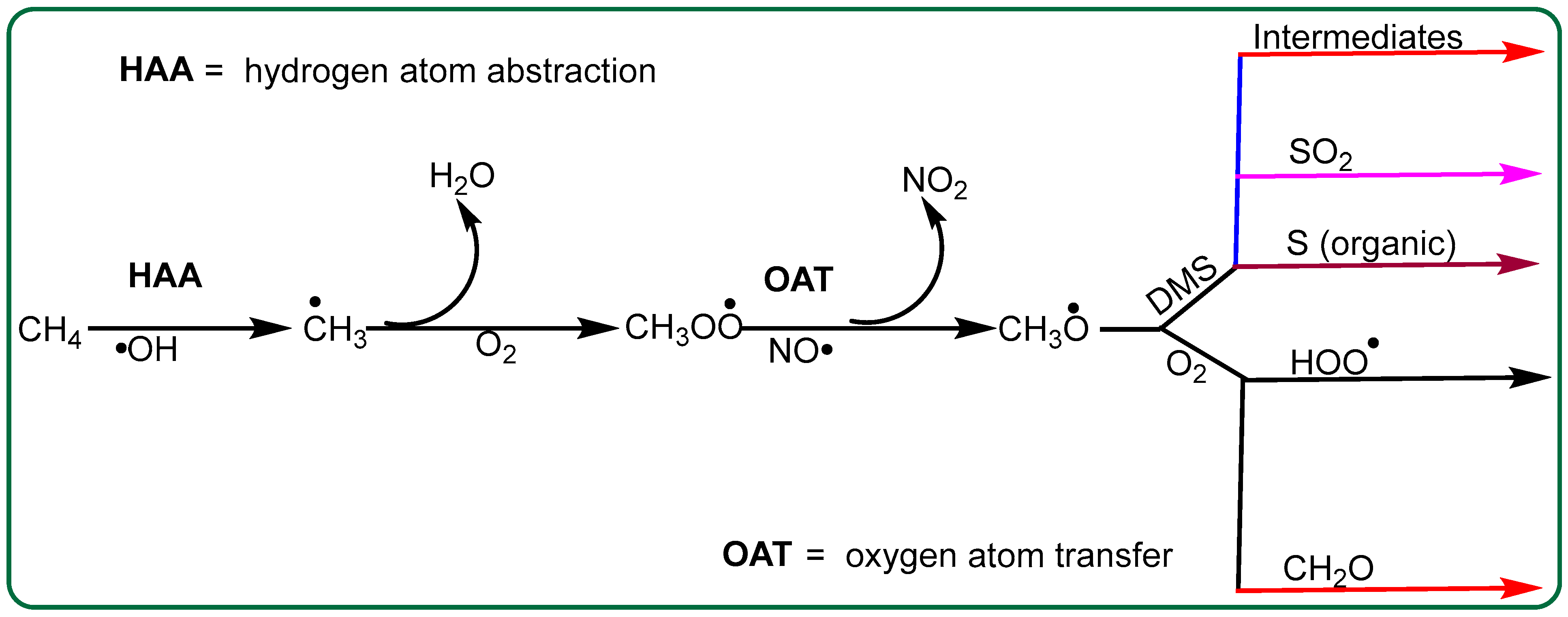
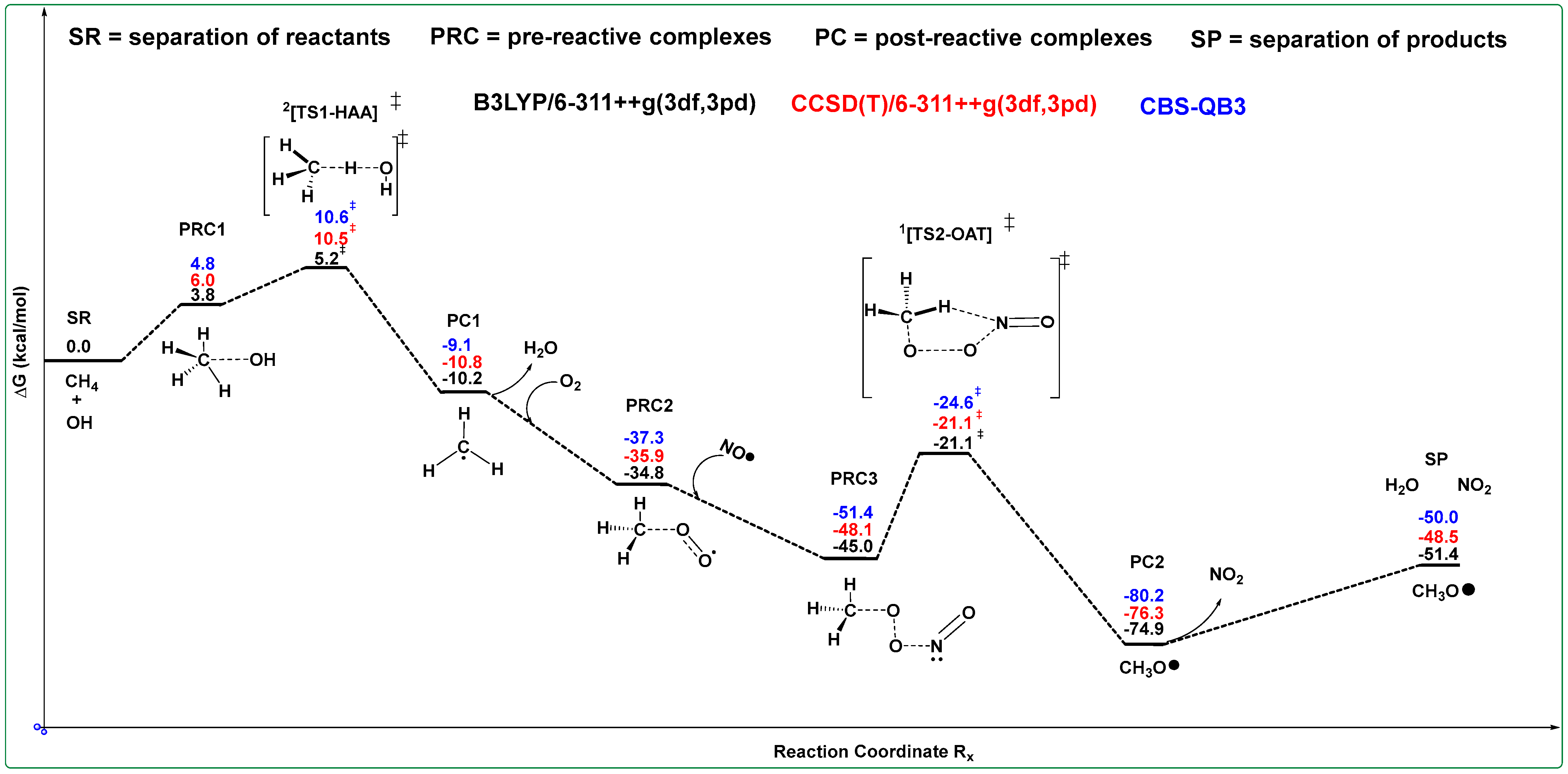
3. Results and Discussion
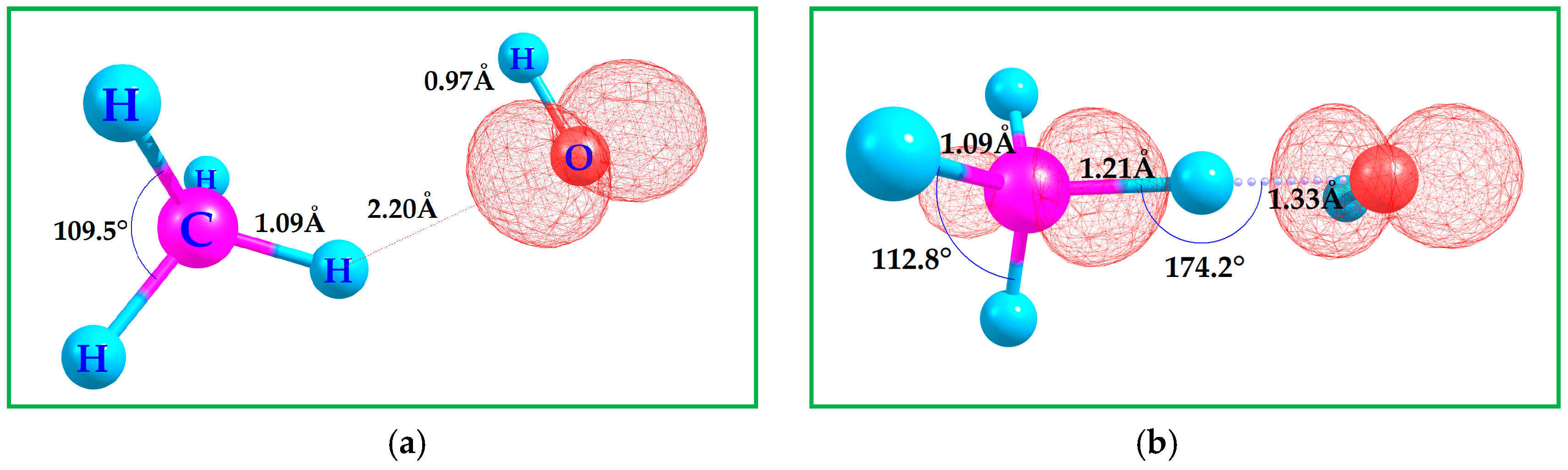
3.1. Binding CH4 with •OH
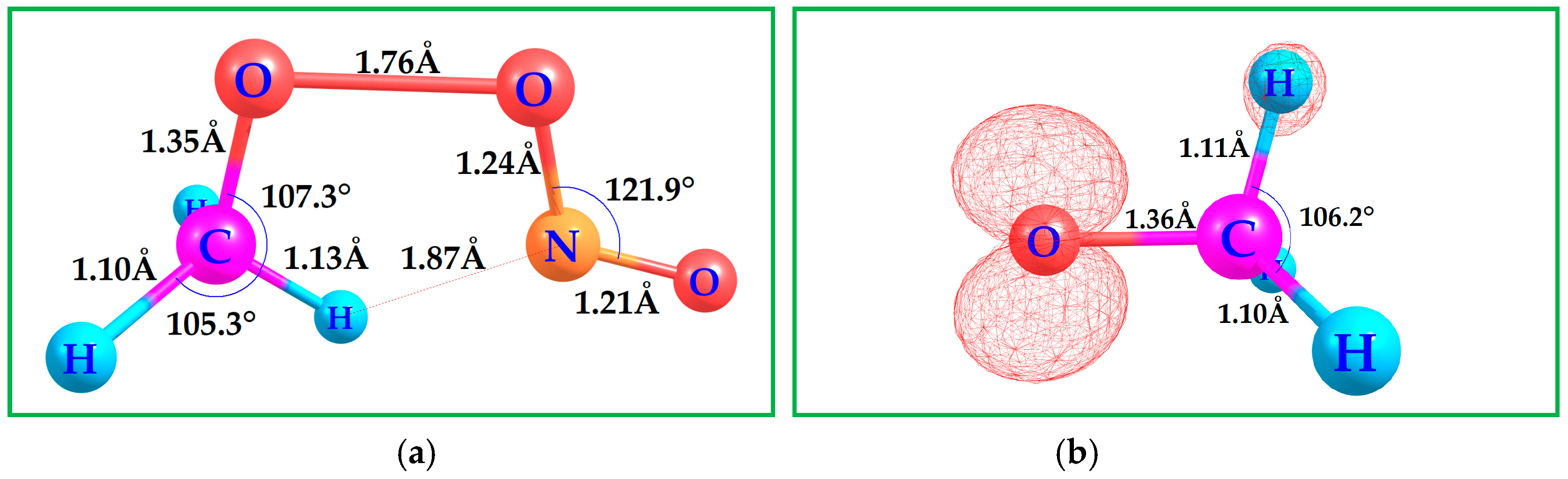
3.2. Binding DMS with CH3O•
4. Kinetics of DMS with CH3O•
Atmospheric Implications
5. Summary
6. Conclusions
7. Prospectus
- Experimental Validation: Laboratory investigations of the CH3O• with DMS reaction are essential to confirm the computational findings and quantify reaction products.
- Environmental Relevance: Field measurements in coastal urban boundary layers and other methane-rich environments could assess the real-world significance of CH3O• driven sulfur oxidation.
- Integration into Models: Incorporating CH3O• driven pathways into local and regional atmospheric models would refine predictions of aerosol-cloud interactions, improving our understanding of their impact on CCN and radiative forcing. By addressing these areas, the broader role of CH3O• in atmospheric chemistry can be elucidated, enabling more accurate predictions of methane and sulfur emissions’ contributions to climate dynamics. This work underscores the importance of integrating computational and experimental approaches to uncover the complexities of atmospheric processes.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMS | dimethyl sulfide |
| CCN | cloud condensation nuclei |
| HAA | hydrogen atom abstraction |
| OAT | oxygen atom transfer |
| MSM | methyl-thiomethylene |
| DFT | density functional theory |
| PES | potential energy surface |
References
- Letters to the Editor: Methane. Chemical & Engineering News. Available online: https://cen.acs.org/energy/hydrogen-power/Reactions/100/i2 (accessed on 21 January 2025).
- Methane Cuts Could Slow Extreme Climate Change. Chemical & Engineering News. Available online: https://cen.acs.org/environment/climate-change/Methane-cuts-slow-extreme-climate-change/99/i39 (accessed on 15 August 2023).
- Glass, R.S. Sulfur Radicals and Their Application. Top. Curr. Chem. 2018, 376, 22. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, Z.; Zhao, Y.; Sun, Y.; Zhu, Y. Mechanistic and dynamic investigations for multi-channel reaction of CH3O2 + NO. J. Mol. Struct. THEOCHEM 2005, 725, 103–109. [Google Scholar] [CrossRef]
- Lesar, A.; Hodošček, M.; Drougas, E.; Kosmas, A.M. Quantum Mechanical Investigation of the Atmospheric Reaction CH3O2 + NO. J. Phys. Chem. A 2006, 110, 7898–7903. [Google Scholar] [CrossRef]
- Charlson, R.J.; Lovelock, J.E.; Andreae, M.O.; Warren, S.G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 1987, 326, 655–661. [Google Scholar] [CrossRef]
- Lomans, B.P.; den Camp, H.J.M.; Pol, A.; Vogels, G.D. Anaerobic versus aerobic degradation of dimethyl sulfide and methanethiol in anoxic freshwater sediments. Appl. Environ. Microbiol. 1999, 65, 438–443. [Google Scholar] [CrossRef]
- Kiene, R.P. Production of methanethiol from dimethylsulfoniopropionate in marine surface waters. Mar. Chem. 1996, 54, 69–83. [Google Scholar] [CrossRef]
- Andreae, M.O.; Crutzen, P.J. Atmospheric Aerosols: Biogeochemical Sources and Role in Atmospheric Chemistry. Science 1997, 276, 1052–1058. [Google Scholar] [CrossRef]
- Jardine, K.; Yañez-Serrano, A.M.; Williams, J.; Kunert, N.; Jardine, A.; Taylor, T.; Abrell, L.; Artaxo, P.; Guenther, A.; Hewitt, C.N.; et al. Dimethyl sulfide in the Amazon rain forest. Glob. Biogeochem. Cycles 2015, 29, 19–32. [Google Scholar] [CrossRef]
- Ayers, G.P.; Cainey, J.M. The CLAW hypothesis: A review of the major developments. Environ. Chem. 2007, 4, 366–374. [Google Scholar] [CrossRef]
- Lucas, D.D.; Prinn, R.G. Mechanistic studies of dimethylsulfide oxidation products using an observationally constrained model. J. Geophys. Res. D Atmos. 2002, 107, ACH 12-1–ACH 12-26. [Google Scholar] [CrossRef]
- Linke, P.; Sommer, S.; Rovelli, L.; McGinnis, D.F. Physical limitations of dissolved methane fluxes: The role of bottom-boundary layer processes. Mar. Geol. 2010, 272, 209–222. [Google Scholar] [CrossRef]
- Alligood, B.W.; FitzPatrick, B.L.; Glassman, E.J.; Butler, L.J.; Lau, K. Dissociation dynamics of the methylsulfonyl radical and its photolytic precursor CH3SO2Cl. J. Chem. Phys. 2009, 131, 044305. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Blomquist, B.W.; Huebert, B.J. Constraining the concentration of the hydroxyl radical in a stratocumulus-topped marine boundary layer from sea-to-air eddy covariance flux measurements of dimethylsulfide. Atmos. Chem. Phys. 2009, 9, 9225–9236. [Google Scholar] [CrossRef]
- Novak, G.A.; Kilgour, D.B.; Jernigan, C.M.; Vermeuel, M.P.; Bertram, T.H. Oceanic emissions of dimethyl sulfide and methanethiol and their contribution to sulfur dioxide production in the marine atmosphere. Atmos. Chem. Phys. 2022, 22, 6309–6325. [Google Scholar] [CrossRef]
- Bourzac, K. The other important greenhouse gas. C&EN Glob. Enterp. 2021, 99, 28–33. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Lee, H.; Jin, P. Significance of anaerobic oxidation of methane (AOM) in mitigating methane emission from major natural and anthropogenic sources: A review of AOM rates in recent publications. Environ. Sci. Adv. 2022, 1, 401–425. [Google Scholar] [CrossRef]
- Prince, B.M.; Cundari, T.R. C-H Bond Activation of Methane by PtII-N-Heterocyclic Carbene Complexes. The Importance of Having the Ligands in the Right Place at the Right Time. Organometallics 2012, 31, 1042–1048. [Google Scholar] [CrossRef]
- Barnes, I.; Hjorth, J.; Mihalopoulos, N. Dimethyl Sulfide and Dimethyl Sulfoxide and Their Oxidation in the Atmosphere. Chem. Rev. 2006, 106, 940–975. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Long, B.; Bao, J.L.; Truhlar, D.G. Kinetics of the Strongly Correlated CH3O + O2 Reaction: The Importance of Quadruple Excitations in Atmospheric and Combustion Chemistry. J. Am. Chem. Soc. 2019, 141, 611–617. [Google Scholar] [CrossRef]
- Orlando, J.J.; Tyndall, G.S.; Wallington, T.J. The Atmospheric Chemistry of Alkoxy Radicals. Chem. Rev. 2003, 103, 4657–4690. [Google Scholar] [CrossRef]
- Atkinson, R. Rate constants for the atmospheric reactions of alkoxy radicals: An updated estimation method. Atmos. Environ. 2007, 41, 8468–8485. [Google Scholar] [CrossRef]
- Mallick, S.; Kumar, A.; Kumar, P. Revisiting the reaction energetics of the CH3O• + O2 (3Σ−) reaction: The crucial role of post-CCSD(T) corrections. Phys. Chem. Chem. Phys. PCCP 2019, 21, 6559–6565. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. 3.01—Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 1–64. [Google Scholar]
- Dibble, T.S.; Chai, J. Critical Review of Atmospheric Chemistry of Alkoxy Radicals. In Advances in Atmospheric Chemistry; Critical Review of Atmospheric Chemistry of Alkoxy Radicals; World Scientific: Singapore, 2016; pp. 185–269. [Google Scholar]
- Hu, H.; Dibble, T.S. Quantum Chemistry, Reaction Kinetics, and Tunneling Effects in the Reaction of Methoxy Radicals with O2. J. Phys. Chem. A 2013, 117, 14230–14242. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.E.; Cicerone, R.J. Future global warming from atmospheric trace gases. Nature 1986, 319, 109–115. [Google Scholar] [CrossRef]
- Ueyama, M.; Fujimoto, A.; Ito, A.; Takahashi, Y.; Ide, R. Constraining models for methane oxidation based on long-term continuous chamber measurements in a temperate forest soil. Agric. For. Meteorol. 2021, 310, 108654. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Jackels, C.F. A potential-energy surface study of the 2A1 and low-lying dissociative states of the methoxy radical. J. Chem. Phys. 1985, 82, 311–322. [Google Scholar] [CrossRef]
- Becke, A.D. Correlation energy of an inhomogeneous electron gas: A coordinate-space model. J. Chem. Phys. 1988, 88, 1053. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Attard, P. Statistical mechanical theory for steady state systems. II. Reciprocal relations and the second entropy. J. Chem. Phys. 2005, 122, 154101. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Crittenden, D.L. A Systematic CCSD(T) Study of Long-Range and Noncovalent Interactions between Benzene and a Series of First- and Second-Row Hydrides and Rare Gas Atoms. J. Phys. Chem. A 2009, 113, 1663. [Google Scholar] [CrossRef] [PubMed]
- Jurecka, P.; Sponer, J.; Cerny, J.; Hobza, P. Benchmark database of accurate (MP2 and CCSD(T) complete basis set limit) interaction energies of small model complexes. Phys. Chem. Chem. Phys. 2006, 8, 1985. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J.Chem.Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Montgomery, J.A., Jr.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J.Chem.Phys. 1999, 110, 2822–2827. [Google Scholar] [CrossRef]
- Bosch, E.; Moreno, M.; Lluch, J.M.; Bertrán, J. Intrinsic reaction coordinate calculations for reaction paths possessing branching points. Chem. Phys. Lett. 1989, 160, 543–548. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Gordon, M.S.; Dupuis, M. The intrinsic reaction coordinate and the rotational barrier in silaethylene. J.Am.Chem.Soc. 1985, 107, 2585–2589. [Google Scholar] [CrossRef]
- Engel, T.; Reid, P. Thermodynamics, Statistical Thermodynamics and Kinetics, 4th ed.; Pearson: London, UK, 2019; pp. 526–530. [Google Scholar]
- Montgomery, J.A., Jr.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VII. Use of the minimum population localization method. J.Chem.Phys. 2000, 112, 6532–6542. [Google Scholar] [CrossRef]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Hampson, R.F.; Kerr, J.A.; Rossi, M.J.; Troe, J. Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Supplement VI. IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry. J. Phys. Chem. Ref. Data 1997, 26, 1329–1499. [Google Scholar] [CrossRef]
- Walch, S.P.; Dunning, T.H., Jr. Calculated barrier to hydrogen atom abstraction from CH4 by O(3P). J.Chem.Phys. 1980, 72, 3221–3227. [Google Scholar] [CrossRef]
- Suleimanov, Y.V.; Espinosa-Garcia, J. Recrossing and Tunneling in the Kinetics Study of the OH + CH4 → H2O + CH3 Reaction. J. Phys. Chem. B 2016, 120, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Azbar, N.; Yonar, T.; Kestioglu, K. Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 2004, 55, 35–43. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L. Kinetic study of hydroxyl radical formation in a continuous hydroxyl generation system. RSC advances 2018, 8, 4632–4638. [Google Scholar] [CrossRef]
- Burdge, J. Chapter 19 Electrochemistry. In Chemistry; McGraw Hill, 2023; p. 970. [Google Scholar]
- Morrison, A.M.; Agarwal, J.; Schaefer, H.F.; Douberly, G.E. Infrared laser spectroscopy of the CH3OO radical formed from the reaction of CH3 and O2 within a helium nanodroplet. J. Phys. Chem. A 2012, 116, 5299–5304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, C.; Xie, B.; Wu, X. Revisiting the chemical kinetics of CH3 + O2 and its impact on methane ignition. Combust. Flame 2019, 200, 125–134. [Google Scholar] [CrossRef]
- Stimac, P.J.; Barker, J.R. Non-RRKM Dynamics in the CH3O2 + NO Reaction System. J. Phys. Chem. A 2008, 112, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.B.; Campuzano-Jost, P.; Hynes, A.J.; Pounds, A.J. Experimental and Theoretical Studies of the Reaction of the OH Radical with Alkyl Sulfides: 3. Kinetics and Mechanism of the OH Initiated Oxidation of Dimethyl, Dipropyl, and Dibutyl Sulfides: Reactivity Trends in the Alkyl Sulfides and Development of a Predictive Expression for the Reaction of OH with DMS. J. Phys. Chem. A 2009, 113, 6697–6709. [Google Scholar] [CrossRef]
- Salta, Z.; Lupi, J.; Barone, V.; Ventura, O.N. H-Abstraction from Dimethyl Sulfide in the Presence of an Excess of Hydroxyl Radicals. A Quantum Chemical Evaluation of Thermochemical and Kinetic Parameters Unveils an Alternative Pathway to Dimethyl Sulfoxide. ACS Earth Space Chem. 2020, 4, 403–419. [Google Scholar] [CrossRef]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Crowley, J.N.; Hampson, R.F.; Hynes, R.G.; Jenkin, M.E.; Rossi, M.J.; Troe, J. Evaluated kinetic and photochemical data for atmospheric chemistry: Volume I—Gas phase reactions of Ox, HOx, NOx and SOx species. Atmos. Chem. Phys. 2004, 4, 1461–1738. [Google Scholar] [CrossRef]
- Atkinson, R. Gas-Phase Tropospheric Chemistry of Volatile Organic Compounds: 1. Alkanes and Alkenes. J. Phys. Chem. Ref. Data 1997, 26, 215–290. [Google Scholar] [CrossRef]
- Michoud, V.; Kukui, A.; Camredon, M.; Colomb, A.; Borbon, A.; Miet, K.; Aumont, B.; Beekmann, M.; Durand-Jolibois, R.; Perrier, S.; et al. Radical budget analysis in a suburban European site during the MEGAPOLI summer field campaign. Atmos. Chem. Phys. 2012, 12, 11951–11974. [Google Scholar] [CrossRef]
- Hynes, A.J.; Wine, P.H.; Semmes, D.H. Kinetics and mechanism of hydroxyl reactions with organic sulfides. J. Phys. Chem. 1986, 90, 4148–4156. [Google Scholar] [CrossRef]
- Mallick, S.; Kumar, A.; Mishra, B.K.; Kumar, P. Influence of water on the CH3O• + O2 → CH2O + HO2· reaction. Phys. Chem. Chem. Phys. PCCP 2019, 21, 15734–15741. [Google Scholar] [CrossRef] [PubMed]
- Mardyukov, A.; Schreiner, P.R. Atmospherically Relevant Radicals Derived from the Oxidation of Dimethyl Sulfide. Acc. Chem. Res. 2018, 51, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Truhlar, D.G.; Garrett, B.C.; Klippenstein, S.J. Current Status of Transition-State Theory. J. Phys. Chem. 1996, 100, 12771–12800. [Google Scholar] [CrossRef]
- Nicovich, J.M.; Parthasarathy, S.; Pope, F.D.; Pegus, A.T.; McKee, M.L.; Wine, P.H. Kinetics, Mechanism, and Thermochemistry of the Gas Phase Reaction of Atomic Chlorine with Dimethyl Sulfoxide. J. Phys. Chem. A 2006, 110, 6874–6885. [Google Scholar] [CrossRef]
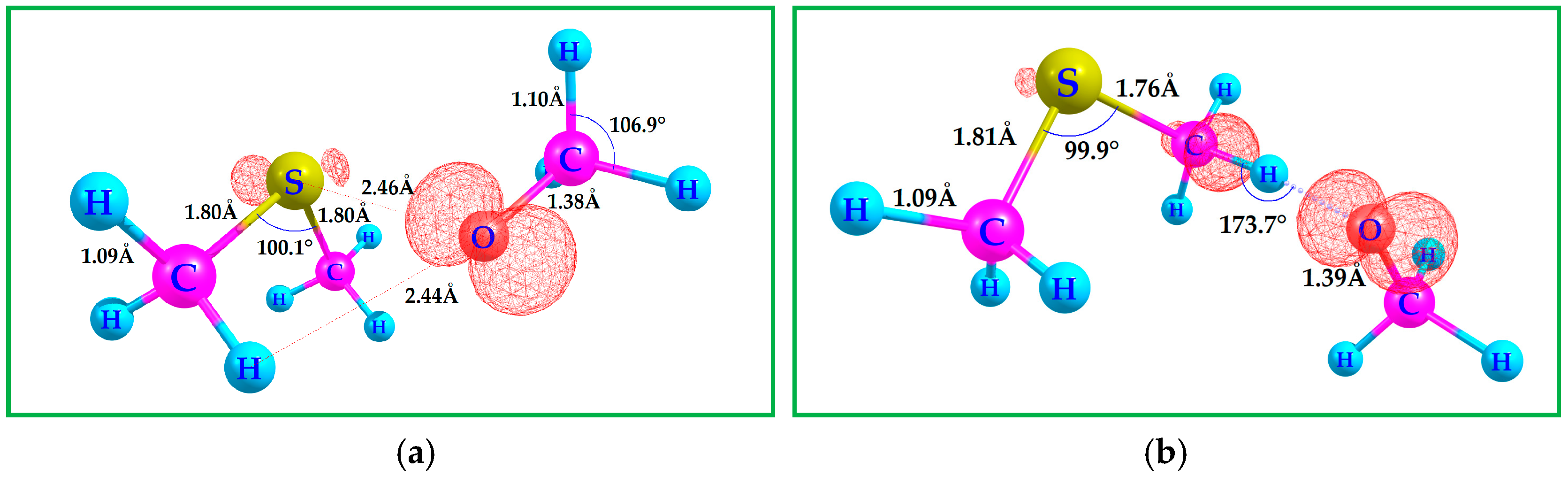
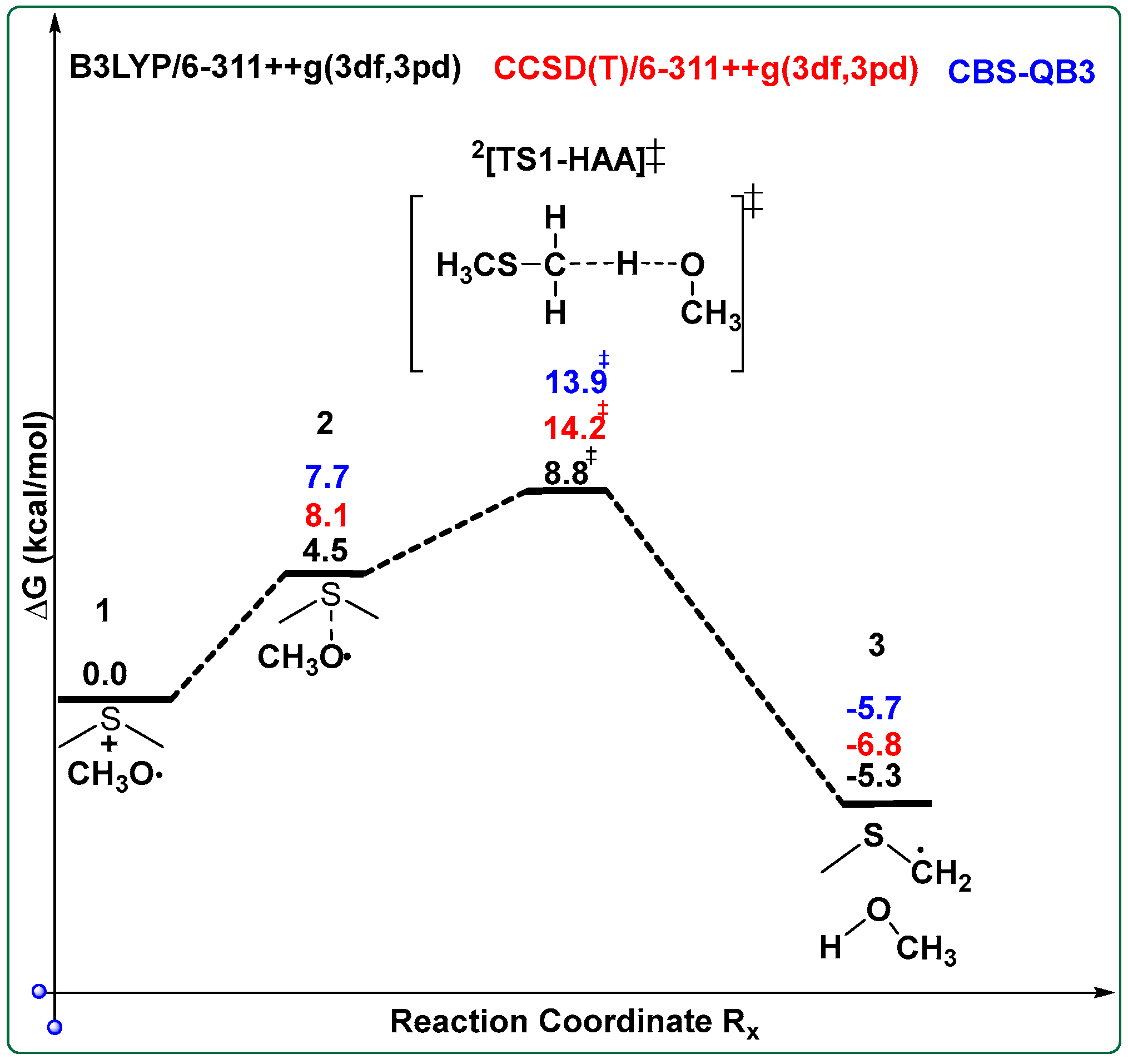
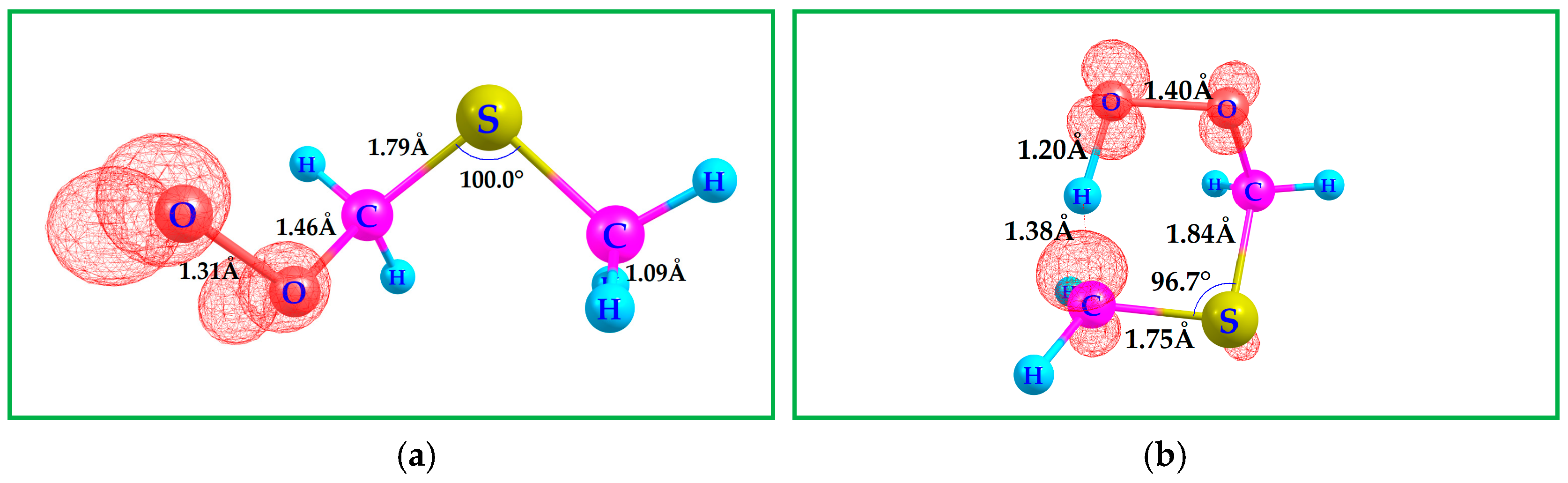
| Species/Reaction | Type of Calculation | Level of Theory | Purpose/Notes |
|---|---|---|---|
| All reactants, intermediates, and transition states | Geometry optimization, TS search, vibrational frequencies | B3LYP-D3(BJ)/6-311++G(3df,3pd) | Location and characterization of stationary points |
| Selected stationary points | Single-point electronic energies | CCSD(T)/6-311++G(3df,3pd) | High-level electronic energy refinement |
| Selected stationary points | Composite thermochemistry | CBS-QB3 | Internally consistent composite energies and thermochemistry |
| CH4 + •OH → CH3• + H2O | PES mapping, ΔE‡, ΔG‡ | B3LYP-D3(BJ); CCSD(T)//B3LYP-D3(BJ); CBS-QB3 | Benchmark methane oxidation pathway |
| CH3OO• + NO• → CH3O• + NO2 | TS search, IRC | B3LYP-D3(BJ) | Formation of methoxy radical |
| CH3O• + DMS → CH3SCH2• + CH3OH | TS search, energetics | B3LYP-D3(BJ); CCSD(T)//B3LYP-D3(BJ); CBS-QB3 | Primary HAA pathway |
| DMS + •OH → CH3SCH2• + H2O | TS search, kinetics | B3LYP-D3(BJ); CCSD(T)//B3LYP-D3(BJ); CBS-QB3 | Reference oxidation pathway |
| Reaction | ΔE0‡ (kcal/mol) | Rate Constant (cm3/molecule/s) | Relative Importance |
|---|---|---|---|
| DMS + •OH | 0.3 | 4.34 × 10−12 | Dominant globally |
| DMS + CH3O• | 5.0 | 3.05 × 10−16 | Locally significant |
| CH3O• + O2 ⟶ CH2O + HO2• | 4.9 | 2.38 × 10−17 | Rapid removal of CH3O• |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Prince, B.M.; Vrinceanu, D.; Harvey, M.C.; Jensen, M.P.; Zawadowicz, M.; Kuang, C. Computational Investigation of Methoxy Radical-Driven Oxidation of Dimethyl Sulfide: A Pathway Linked to Methane Oxidation. Gases 2026, 6, 2. https://doi.org/10.3390/gases6010002
Prince BM, Vrinceanu D, Harvey MC, Jensen MP, Zawadowicz M, Kuang C. Computational Investigation of Methoxy Radical-Driven Oxidation of Dimethyl Sulfide: A Pathway Linked to Methane Oxidation. Gases. 2026; 6(1):2. https://doi.org/10.3390/gases6010002
Chicago/Turabian StylePrince, Bruce M., Daniel Vrinceanu, Mark C. Harvey, Michael P. Jensen, Maria Zawadowicz, and Chongai Kuang. 2026. "Computational Investigation of Methoxy Radical-Driven Oxidation of Dimethyl Sulfide: A Pathway Linked to Methane Oxidation" Gases 6, no. 1: 2. https://doi.org/10.3390/gases6010002
APA StylePrince, B. M., Vrinceanu, D., Harvey, M. C., Jensen, M. P., Zawadowicz, M., & Kuang, C. (2026). Computational Investigation of Methoxy Radical-Driven Oxidation of Dimethyl Sulfide: A Pathway Linked to Methane Oxidation. Gases, 6(1), 2. https://doi.org/10.3390/gases6010002







