Abstract
The clinical features and outcomes of adult acute leukemia (AL)-associated hemophagocytic lymphohistiocytosis (AL-HLH) remain insufficiently characterized. We retrospectively analyzed 45 adult patients diagnosed with AL-HLH between December 2019 and June 2023. Among 746 AL patients, 45 developed HLH, with 40 developing acute myeloid leukemia (AML), 4 developing acute lymphoblastic leukemia (ALL), and 1 developing mixed-phenotype acute leukemia (MPAL). According to the ELN 2022 criteria, 16 (35.6%) had favorable, 3 (6.7%) had interediate, and 26 (57.7%) had poor risk. At the time of HLH onset, seven (15.6%) patients were in composite complete remission (CCR), and 38 (84.4%) were in non-CCR states; 25 (55.6%) patients were newly diagnosed before induction chemotherapy. The HLH-94/04-based regimens (etoposide and dexamethasone) with or without ruxolitinib achieved an ORR (overall remission rate) of 82.2% and a CR rate of 66.7%. After anti-leukemic therapy, 60% (27/45) of patients achieved CCR for leukemia (including patients in CCR at HLH onset and those achieving CCR after treatment). Hematopoietic stem cell transplantation (HSCT) independently predicted sustained remission. The estimated overall rates at 6 and 12 months after HLH diagnosis were 73.1% and 59.2%, respectively. Multivariate Cox analysis identified failure to achieve CCR for leukemia as the only independent adverse prognostic factor. AL-HLH is an uncommon but severe complication that predominantly occurs in AML patients with poor-risk cytogenetics or active disease. Early recognition, effective HLH control, and achievement of CCR in AL are crucial for improving patient prognosis.
1. Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening syndrome that involves an immune disorder characterized by uncontrolled activation of lymphocytes, monocytes, and macrophages; overgrowth of inflammatory cytokines; and ultimately multiple organ failure (MOF) [1,2]. HLH can be classified into two types: primary (with gene mutations) and secondary (with sHLH). Secondary forms are most prevalent in adult patients and are mainly caused by infections, malignancies, or autoimmune diseases [3]. Lymphomas, particularly T-cell or natural killer (NK) cell lymphomas, are the major hematologic malignancies in adult HLH [4,5]. Moreover, malignancy-related HLH in adults is a progressive disease with a high mortality rate reportedly exceeding 65% [6,7,8].
The incidence of HLH in patients with acute leukemia (AL) varies considerably across cohorts. In adolescent populations, HLH has been reported in approximately 2.8–4.1% of acute leukemia cases [9]. However, published data on acute leukemia-related HLH (AL-HLH) in adults are scarce [10,11,12]. Owing to the lack of specific clinical features and laboratory findings in adult patients with HLH, early diagnosis and prompt treatment are challenging. Early diagnosis and treatment can improve patient prognosis. In acute leukemia, HLH is associated with refractory or unremitted leukemia, or is induced by bacterial, viral, or fungal infections. HLH has occasionally been described in case reports or in combination with other hematological malignancies in patients with acute leukemia. Patients with acute leukemia may be prone to developing HLH due to their disease- and/or treatment-related impaired immune system and high susceptibility to severe infections, which predisposes them to HLH [13].
Owing to the unmet need for early diagnosis and prompt treatment, and the poor prognosis of patients with HLH, we conducted a retrospective study of 45 adult patients with AL-HLH at our center. By systematically summarizing and analyzing the clinical characteristics, treatment regimens, therapeutic outcomes, and prognoses of these patients, this study aimed to provide a reference for the diagnosis and management of this rare disease.
2. Materials and Methods
2.1. Patients
Between December 2019 and June 2023, data from 746 adult patients with acute leukemia at our center were retrospectively reviewed. Informed consent was obtained from all the patients. The study was conducted in accordance with the Declaration of Helsinki and approved by the In-stitutional Review Board (or Ethics Committee) of Peking University People’s Hospital (protocol code, 2025PHB500-001and date of approval, 13 November 2025).In all patients, the diagnosis of HLH was based on the criteria proposed by the HLH Study Group of the Histiocyte Society in 1991 and updated in 2004 [14,15].
The diagnosis and evaluation of infections were based on the clinical manifestations of the patients; imaging such as computed tomography (CT); microbiological detection; culture of body fluid specimens, including blood, urine, or stool; and next-generation sequencing of pathogenic microorganisms.
2.2. Treatment of HLH
In patients with unrelieved leukemia, including patients with de novo acute leukemia and patients with refractory or recurrent acute leukemia, the treatment regimen was based on the HLH-94/04 protocol and was individualized, with or without etoposide, according to the patient’s leukemia status and identified triggers. Etoposide (VP-16) was administered at 150 mg/m2 twice weekly during weeks 1–2 and 150 mg/m2 once weekly during weeks 3–8 based on the regimen recommended by the 2004 Chinese Guidelines of HLH. Dexamethasone was administered at 10 mg/m2/day during weeks 1–2, 5 mg/m2/day during weeks 3–4, 2.5 mg/m2/day during weeks 5–6, and 1.25 mg/m2/day during weeks 7–8. The treatment plan was modified based on our experience with adult patients with AL-HLH. VP-16 was not included in the regimen for patients who achieved composite complete remission (CCR) and received consolidation therapy. Ruxolitinib (5 or 10 mg twice daily) was used in the HLH regimen to improve efficacy. A lower starting dose (5 mg BID) was preferred in patients with profound cytopenia or active severe infection, whereas 10 mg BID was preferred in patients with preserved marrow reserve and lower infection risk. Dose reduction or temporary interruption was performed in cases of progressive cytopenia (≥grade 3), invasive infection, or CMV reactivation. Salvage therapy for HLH was adopted. The protocol used was a combination of doxorubicin, etoposide, and methylprednisolone (DEP).
2.3. Assessment of HLH Treatment
The treatment assessment was previously described in a study on pediatric HLH [15], and the treatment was modified based on our experience with adult patients with HLH. The following quantifiable symptoms and laboratory markers were used to assess the treatment efficacy of the patients in this study every two weeks during the induction stage: levels of sCD25, ferritin, triglyceride, hemoglobin, neutrophil counts, platelet counts, and alanine aminotransferase (ALT). Patients in whom the above parameters returned to normal levels were defined as having a complete response (CR) [16]. A partial response (PR) was defined as at least a 25% improvement in two or more quantifiable symptoms and laboratory markers by two weeks following treatment: sCD25 response decreased by >1.5-fold; ferritin and triglyceride decreased at least 25%; for patients with an initial neutrophil count of 0.5 × 109/L; for patients with a neutrophil count of (0.5–2.0) × 109/L, an increase of at least 100% to >2.0 × 109/L was considered a response; and for patients with ALT > 400 U/L, response was defined as an ALT decrease of at least 50% [16]. Additionally, regardless of PR or CR, the body temperature of the patient should return to normal. Patients who did not achieve CR or PR were defined as having no response (NR) [17].
2.4. Definitions
2.4.1. Evaluation Criteria for Treatment Response in Acute Leukemia
CR was defined as bone marrow blasts ≤5%, absence of extramedullary disease, and recovery of peripheral blood counts (absolute neutrophil count [ANC] ≥ 1.0 × 109/L and platelets ≥ 100 × 109/L). CR with incomplete hematological recovery (CRi) was defined similarly, except for lack of recovery of either neutrophils or platelets, and morphologic leukemia-free state (MLFS) was defined as bone marrow blasts ≤5% without any blood count recovery. Composite CR (CCR) is a composite of CR, CRi, and MLFS [18]. Non-remission after leukemia treatment was defined as a proportion of blast cells in the bone marrow specimen being greater than or equal to 5% after chemotherapy.
2.4.2. Diagnostic Criteria for HLH
All patients underwent systematic evaluation for HLH-2004 diagnostic criteria. Fever was defined as a body temperature exceeding 38.5 °C for more than 7 days [16]. Hepatomegaly was defined as a longitudinal axis >15.5 cm at the hepatic midline or >16.0 cm at the midclavicular line under ultrasound examination. Splenomegaly was defined as a craniocaudal height of >13 cm. Hemocytopenia was defined as cytopenia affecting ≥2 of 3 lineages in the peripheral blood: hemoglobin <90 g/L (in infants <4 weeks: hemoglobin < 100 g/L), platelets < 100 × 109/L, neutrophils < 1.0 × 109/L. Hypertriglyceridemia was defined as fasting triglycerides ≥3.0 mmol/L. Hypofibrinogenemia was defined as fibrinogen ≤1.5 g/L. Infection was defined based on the symptoms associated with the infection (e.g., fever), laboratory test results, and etiological evidence. Hemophagocytosis in bone marrow was actively sought in all cases. NK-cell activity and sCD25 testing were performed when technically available; for patients lacking these data, diagnosis was made if ≥5 other criteria were fulfilled and confirmed by expert clinical adjudication.
2.4.3. Criteria for Infection
Criteria for infection included a definite requirement for microbiological confirmation from a sterile or clinically relevant site (e.g., positive blood culture/NGS, pathogen identified from BAL with compatible burden, tissue histology/culture/PCR), compatible clinical or radiologic findings, and compatible clinical and/or imaging findings in the absence of definitive microbiology but with strong supportive evidence (e.g., non-sterile site growth consistent with the disease focus, typical CT signs of invasive fungal disease, or rapid, sustained response to targeted antimicrobials). Possible clinical suspicion was considered when there was partial or nonspecific evidence, but no pathogens were identified.
2.5. Statistical Analysis
Continuous variables were expressed as medians and ranges, while categorical variables were expressed as frequencies and percentages. SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Age, sex, primary disease type, clinical manifestations, relevant laboratory tests, therapeutic regimens, and patient survival were analyzed. Patient survival was analyzed using the Kaplan–Meier survival curve. Potential prognostic factors for overall survival (OS) were evaluated by multivariate analysis using Cox proportional hazards regression with a forward stepwise model selection approach. Factors included in the regression model were AL treatment status (new vs. refractory/relapsed), risk category (favorable vs. intermediate and poor), AL status after treatment (CCR vs. non-CCR), AL status at HLH onset (CCR vs. non-CCR), HLH remission (CR vs. NR/PR), hematopoietic stem cell transplantation (HSCT) (no vs. yes), age (>50 vs. ≤50, years old), organ dysfunction (no vs. yes), serum ferritin level (lower than median vs. higher than median), sCD25 level (<10,000 vs. ≥10,000 pg/mL), infection status- microbiologically proven (no vs. yes), infection status-clinical proven (no vs. yes). Variables with a p value of less than 0.1 in the univariate analysis were subsequently entered into the multivariate logistic regression model using the forward: LR method, and the level of significance was set at p < 0.05 [19].
3. Results
3.1. Study Population Patients
This study retrospectively analyzed 746 acute leukemia patients from December 2019 to June 2023 in our center, of whom 45 patients were diagnosed with HLH, accounting for 6.0% of patients overall. Of these, 28 were male (62%) and 17 were female (38%), with a median age of 49 (range: 18–75) years. Of the 45 patients recruited in the present study, 40 had acute myeloid leukemia, four had acute lymphoid leukemia, and one had mixed-phenotype acute leukemia. Forty-one (91%) patients were diagnosed with definite infections, and the most common sites of infection were lungs (66.7%), soft tissue (33.3%), and bacteremia (33.3%); nine (20%) patients had more than two sites of infection. Among the 45 patients included in the study, 7 (15.6%) had newly diagnosed acute leukemia who had achieved CCR after induction therapy and developed HLH during consolidation chemotherapy. Among the remaining 38 patients, 25 (55.6%) had newly diagnosed acute leukemia and developed HLH before achieving CCR following induction chemotherapy, and 13 (28.9%) had refractory or relapsed acute leukemia and developed HLH in a non-CCR state. The median follow-up time was 11.5 (0.4–57.7) months from HLH diagnosis to the last follow-up. The details are presented in Table 1.

Table 1.
Patient characteristics.
3.2. Analysis of Clinical Manifestations and Laboratory Findings
All patients had persistent fever at the time of AL-HLH diagnosis. The additional clinical manifestations included hepatomegaly and splenomegaly (36.4%, 12/33 evaluated). The common laboratory abnormalities were hemocytopenia (100%), hyperfer-ritinemia (100%), hypofibrinogenemia (14/45, 31.1%), hypertriglyceridemia (12/45, 26.7%), and hemophagocytosis in bone marrow (22/45, 48.9%), and most patients who underwent the tests showed low NK cell function (35/43 cases evaluated, 81.4%) and elevated soluble IL-2 receptor-α chain (sCD25, 36/44, 81.8%). Twenty-four (53.3%) of our patients had liver function damage (elevated transaminase). The serum ferritin concentration at the time of AL-HLH diagnosis was elevated in all patients, with a median ferritinemia level of 2631 ng/L (range, 1122–31,129 ng/L). Seventeen (68%) patients had hyperferritinemia ≥2000 ng/L, and four (16.0%) patients had hyperferritinemia ≥10,000 ng/L. All patients met at least five HLH-2004 criteria, even when NK or sCD25 testing was not available. Genetic screening for familial HLH–associated mutations (Table S1) was conducted in four patients (cases 16, 20, 35, and 36), and no pathogenic variants were detected (Table 2).

Table 2.
Clinical manifestations and laboratory examinations of patients.
3.3. Treatment Outcomes of HLH
Among the 45 patients with AL-HLH, HLH therapy was primarily based on the HLH-94/04 protocol and included glucocorticoids, etoposide (VP-16), and/or ruxolitinib. Specifically, 18 patients (40.0%) received glucocorticoids + VP-16 + ruxolitinib, 15 (33.3%) received glucocorticoids + ruxolitinib, 8 (17.8%) received glucocorticoids + VP-16, and 3 (6.7%) received glucocorticoids alone. One patient (2.2%) received a second-line DEP regimen (doxorubicin, etoposide, and methylprednisolone) after initial treatment failure. Thirty-seven (82.2%) patients responded to the regimen and achieved remission for HLH, including 30 patients (66.7%) with CR and seven patients (15.6%) with PR. In the univariate logistic regression analysis, multiple clinical and laboratory parameters were assessed for their association with CR achievement. However, none of these candidate variables remained statistically significant in multivariate analysis (p > 0.05), suggesting that no independent predictors of achieving HLH CR were identified in this cohort.
3.4. Treatment of AL
Among the 38 (84%) patients with unrelieved leukemia were diagnosed with HLH, the most frequent anti-leukemic regimen was venetoclax combined with azacitidine (Ven + AZA), accounting for approximately 40% of cases. Other induction or salvage regimens included CAG or D-CAG (low-dose cytarabine, aclarubicin, and granulocyte colony-stimulating factor, with or without decitabine; n = 4); IA or AE (idarubicin plus cytarabine or aclarubicin plus cytarabine; n = 4); arubicin plus cytarabine (n = 2); AEG or sorafenib combined with AEG (aclarubicin, etoposide, and granulocyte colony-stimulating factor; n = 2); and an APL-specific regimen consisting of dasatinib, all-trans retinoic acid (ATRA), and arsenic trioxide (n = 2).Additionally, several ALL-type protocols were administered, including VDP (vincristine, daunorubicin, and prednisone), VDLP (vincristine, daunorubicin, L-asparaginase, and prednisone), and R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone).Overall, 58% of patients achieved CCR after anti-leukemia therapy, while 42% remained non-responsive. In all the 45 patients, The CCR rate of acute leukemia (AL) after the antileukemia chemotherapy was 60%, including patients who were already in CCR at the onset of HLH and remained in CCR thereafter, as well as those who achieved CCR following treatment. Among the patients who achieved CCR, 13 underwent HSCT. Moreover, one patient with unremitting leukemia (NR) underwent HSCT. Notably, at the last follow-up, all the patients who underwent HSCT remained alive. Univariate and multivariate logistic regression analyses were conducted to identify factors associated with acute leukemia achieved complete remission after treatment (CRAL). In the univariate analysis, age ≥ 50 years (p = 0.062), HSCT (p = 0.021), HLH with refractory/relapsed AL (p = 0.021), and intermediate and poor AL (p = 0.051) were identified as potential factors associated with achieving CRAL (p < 0.1). These variables were subsequently included in a multivariate logistic regression model using the forward LR method. The results revealed that HSCT remained an independent factor associated with CRAL (OR = 0.083, 95% CI: 0.007–0.955, p = 0.046), indicating that patients who underwent HSCT were significantly more likely to keep CCR status of acute leukemia after treatment. Other variables were not statistically significant in the multivariate model.
3.5. Survival and Prognosis
The probabilities of OS at 6 and 12 months after HLH diagnosis were 73.1% (95% confidence interval [CI]: 60.1–86.1%) and 59.2% (95% CI: 44.7–73.8%) in all the patients, respectively (Figure 1a).
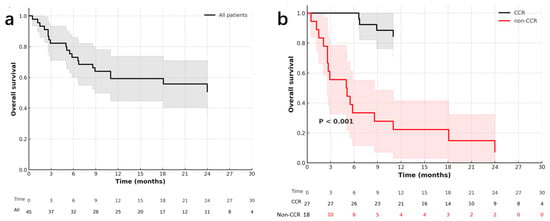
Figure 1.
Kaplan–Meier survival in acute leukemia-associated HLH. (a) Overall survival (OS) for all patients (n = 45). The estimated 6-month and 12-month OS were 73.1% (95% CI: 60.1–86.1%) and 59.2% (95% CI: 44.7–73.8%), respectively. Shaded areas denote 95% confidence intervals; number at risk is shown below the x-axis (0–30 months, 3-month intervals). (b) OS stratified by CCR status of acute leukemia. Patients who failed to achieve CCR (non-CCR, red curve) had significantly inferior survival compared with those who achieved CCR (black curve) (log-rank p < 0.001). Curves include 95% confidence bands and time-labeled number-at-risk tables.
Among 45 patients with AL-HLH, 20 died during follow-up. The leading cause of death was refractory leukemia (45%, 9/20), followed by leukemia relapse (35%, 7/20), and diffuse alveolar hemorrhage (DAH, 20%, 4/20). These findings indicate that the progression of leukemia accounted for the majority of deaths in this patient cohort, while severe complications such as DAH also contributed substantially. The causes of death among the patients are shown in Table 3. In the univariate Cox regression, several candidate variables yielded p < 0.10 and were entered into the multivariable model. After stepwise forward (LR) selection, failure to achieve CCR in acute leukemia emerged as the sole independent predictor of worse overall survival. Patients without CCR had a significantly elevated risk of death compared with those who achieved CCR (HR = 9.268, 95% CI: 2.999–28.641, p < 0.001). All other variables lost statistical significance in the adjusted model (Table 4).

Table 3.
Causes of death among the patients who died.

Table 4.
Multivariate analysis for overall survival.
Details of the clinical characteristics, treatments, efficacy, and survival of patients with AL-HLH are shown in Table S2.
4. Discussion
Hemophagocytic lymphohistiocytosis (HLH) represents a hyperinflammatory syndrome caused by excessive immune activation, which may occur secondary to infections, autoimmune disorders, or malignancies [1,2]. While lymphoma-associated HLH has been widely studied in adults, acute leukemia-associated HLH (AL-HLH) remains insufficiently characterized, with most prior reports limited to small case series or individual cases [3,10,11]. Our study, which retrospectively analyzed 45 adult patients with AL-HLH, represents a relatively large single-center study and provides novel insights into the clinical characteristics, treatment responses, and prognostic determinants of the disease.
We observed that the incidence of HLH among acute leukemia patients in our cohort was 6.0%, which is slightly higher than previously reported frequencies (approximately 2.8–4.1%) in other studies [6,9]. This difference may reflect the improved recognition and diagnostic vigilance at our institution. Clinical manifestations of persistent fever, cytopenia, hyperferritinemia, elevated sCD25 levels, and hypofibrinogenemiawere consistent with previous descriptions of malignancy-associated HLH [7,8]. Notably, serum ferritin levels are universal, and nearly 20% of patients exhibit ferritin levels exceeding 10,000 ng/mL, underscoring its diagnostic value as a marker of systemic hyperinflammation.
In adults, the secondary causes of HLH are mainly infections, malignancies, and autoimmune diseases [20,21]. Patients with acute leukemia are particularly vulnerable to HLH via multiple mechanisms. Leukemic cells may drive immune dysregulation, persistent antigenic stimulation, aberrant cytokine production, and interference with normal immunoregulation. Second, intensive chemotherapy causes profound bone marrow suppression and immunodeficiency, greatly increasing the susceptibility to severe infections (bacterial, fungal, and viral), which are well-known triggers of HLH [12]. In our cohort, >90% of patients had documented infections (mainly pulmonary or bloodstream), supporting the idea that infection acts synergistically with leukemia burden to precipitate HLH. Another distinctive feature of our study was that we stratified AL patients by disease phase and remission status. HLH occurred in 55.6% of cases at diagnosis (before induction), in 15.6% during consolidation after achieving CR, and 28.9% in refractory/relapsed disease, indicating that HLH may arise in both active leukemia and in periods of immunosuppression post-remission.
AL-HLH and severe infection/sepsis both share overlapping manifestations, such as fever, cytopenia, and elevated inflammatory markers. Thus, it can be challenging to differentiate the two. HLH involves profound immune dysregulation with uncontrolled macrophage activation and cytokine storm, whereas sepsis is primarily driven by pathogen-induced inflammation [22,23]. Accurate and early recognition of HLH in patients with leukemia is vital because a delayed diagnosis is directly correlated with poorer outcomes. Ferritin, sCD25, and NK-cell function assays—alongside bone marrow evaluation—are indispensable diagnostic tools used to distinguish HLH from infectious or treatment-related complications [8,21]. Therefore, increased awareness and systematic application of the HLH-2004 criteria in hematology settings are essential for timely diagnosis and intervention.
Most patients received the HLH-94/04 protocol (etoposide and dexamethasone) with or without ruxolitinib. The overall remission rate (CR + PR) of 82.2% and a CR rate of 66.7% were encouraging compared with earlier reports in adult HLH [15,23]. The present analysis highlights the critical prognostic impact of the CCR status in patients with acute leukemia–associated HLH. Persistent leukemia burden was associated with inferior survival outcomes and increased inflammatory activity, thereby worsening HLH control; these findings were consistent with those in the previous literature [1,24,25]. In acute myeloid leukemia (AML), achieving deep remission, particularly minimal residual disease negativity, has been shown to strongly correlate with improved long-term outcomes [19,26,27,28]. These findings underscore the fact that CCR should be regarded as a key clinical stratification factor and therapeutic goal in this setting. Future prospective studies are warranted to validate the prognostic value and optimize treatment strategies for durable CCR. HSCT provided significant survival benefits in our cohort. All patients who underwent HSCT were alive at the last follow-up, supporting the notion that HSCT can eradicate leukemic clones and restore immune homeostasis [28]. Therefore, achieving CCR before HSCT should be regarded as a key therapeutic goal for improving the long-term survival of patients with AL-HLH.
According to the Chinese HLH Diagnosis and Treatment Guidelines, the second-line regimen for pediatric HLH involves ruxolitinib (5 or 10 mg twice daily) to improve efficacy [16]. As an inhibitor of the JAK-STAT signaling pathway, ruxolitinib plays an anti-inflammatory role in the treatment of HLH [29]. HLH is an extremely hyper-inflammatory condition, and inflammatory factors can activate the JAK pathway during its development. It also plays an important role in the treatment of rheumatoid arthritis and myeloproliferative diseases. Experimental studies in mouse HLH models have shown that ruxolitinib inhibits the activation of CD8+ T lymphocytes and the production of interferon gamma, interleukin (IL) -6, IL-12, and other cytokines, thereby preventing HLH progression [30,31]. Although ruxolitinib may suppress hyperinflammation through JAK/STAT inhibition and has shown clinical activity in secondary HLH, its efficacy in AL-HLH remains to be fully established. Our observations are retrospective and based on a limited number of patients, without randomized comparisons. Therefore, the potential benefits of ruxolitinib should be interpreted with caution, and prospective controlled studies are required to validate these preliminary findings before it can be recommended as standard therapy in this population.
In our cohort, HLH-directed therapy and leukemia-directed therapy were frequently administered concurrently. Given that both regimens involve potent immunosuppressive and cytotoxic agents, such simultaneous administration may lead to enhanced hematologic toxicity, increased susceptibility to infections, and overlapping immunosuppression. This complex interplay likely contributes to the high incidence of severe infections and related complications observed in our patients. To mitigate these risks, careful clinical monitoring and individualized dose modifications—such as dose reduction or temporary interruption of ruxolitinib in the setting of profound cytopenia or active infection—were implemented. These observations highlight the need for future prospective studies to better delineate the extent and nature of these interactions, and to optimize the timing, dosing, and sequencing of HLH and leukemia-directed therapies. Establishing evidence-based strategies to balance efficacy with safety will be essential to improving outcomes in this high-risk population.
Previous studies of HLH with hematological malignancies have mainly focused on lymphomas. International series of adult lymphoma-associated HLH (LA-HLH), especially those enriched for T/NK-cell lymphomas, consistently report aggressive clinical courses and high early mortality, reflecting the underlying lymphoma biology and multiorgan involvement. In contrast, our adult AL-HLH cohort was predominantly AML, with >90% having microbiologically or clinically documented infections at HLH onset, and overall survival of 73.1% and 59.2% at 6 and 12 months, respectively. Crucially, multivariable analysis identified failure to achieve CCR of leukemia as the only independent adverse prognostic factor, whereas infection status did not retain significance; leukemia progression accounted for most deaths. Together, these data suggest that while infections commonly precipitate hyperinflammation in AL-HLH, long-term outcomes are primarily determined by leukemia control. These contrast with LA-HLH underscore disease-specific management priorities: rapid HLH suppression must be integrated with effective anti-leukemia therapy and timely HSCT when feasible. We found that HLH can also occur in patients with acute leukemia; however, there have been few previous reports on this topic. Due to the low incidence and recognition of AL-HLH, the majority of published research findings to date mainly consist of case reports. Consequently, there is a paucity of large-scale studies on this topic. We inferred that this could be due to insufficient awareness of AL combined with HLH in clinical practice, which has not yet been fully recognized. To our knowledge, this is the first report of acute leukemia complicated by hemophagocytic syndrome in a relatively large number of cases. Furthermore, the similarity of the clinical features and laboratory data of acute leukemia with infections and HLH is also a challenge for the early and rapid diagnosis of AL-HLH [18,19]. However, early diagnosis and treatment are important for improving patient prognosis.
This study had several limitations. First, this was a retrospective study with a relatively small sample size. Accordingly, further prospective studies are required to confirm this hypothesis. Considering the high mortality rate, poor prognosis, and limited number of previous reports, there is an imminent need to improve our clinical understanding and awareness of the disease in patients with acute leukemia in order to improve patient prognosis. Through this report, we hope to improve our understanding of acute leukemia complicated by hemophagocytic syndrome. Another limitation of our study is the absence of genetic screening for HLH-associated mutations in patients with newly diagnosed AL. Adult-onset secondary HLH can harbor pathogenic variants in PRF1, UNC13D, STXBP2, and RAB27A, which predispose to exaggerated immune activation [32,33]. Incorporating HLH gene panels in patients presenting with hyperinflammation may refine the diagnosis and identify genetically susceptible subgroups. Because NK-cell activity and sCD25 testing were not routinely available during the study period, some patients did not undergo complete diagnostic evaluation according to HLH-2004 criteria. Although this may raise concerns regarding potential underdiagnosis or misclassification, all patients included in this study met at least five diagnostic criteria and were carefully reviewed by experienced hematologists. This reduces, though does not eliminate, the risk of diagnostic inaccuracy. Future studies with more comprehensive diagnostic work-up are warranted to further validate these findings. Furthermore, although some patients received ruxolitinib in addition to standard HLH-directed therapy, ruxolitinib was preferentially used in those with more severe or refractory disease, leading to substantial baseline heterogeneity. Because of this selection bias and the limited sample size, we did not conduct a formal comparative analysis between patients with and without ruxolitinib. Future prospective studies with standardized treatment indications and larger cohorts are needed to better define its therapeutic impact.
5. Conclusions
In conclusion, AL-HLH is a rare but severe hyper-inflammatory complication with a high mortality rate. Early recognition, prompt initiation of both anti-HLH and anti-leukemia therapies, and achievement of CCR are crucial for improving outcomes. Greater clinical awareness, the accumulation of multicenter data, and the integration of genetic testing are warranted to enhance the understanding and management of this challenging condition.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/immuno5040058/s1, Table S1: The gene profile of familial HLH tested in our study. Table S2. Details of the clinical characteristics, treatments, efficacy, and survival of patients with AL-HLH.
Author Contributions
Conceptualization, W.-J.Y., X.-J.H. and H.J.; Data curation, W.-J.Y., Y.W., W.-B.D., Q.C., X.-Y.P. and X.-L.Z.; Formal analysis, W.-J.Y., Y.W., W.-B.D., Q.C., X.-Y.P., J.-S.J., J.W. and X.-L.Z.; Funding acquisition, W.-J.Y., X.-J.H. and H.J.; Investigation, W.-J.Y., Y.W. and J.W.; Methodology, W.-J.Y., Y.W., W.-B.D., Q.C., X.-Y.P., J.-S.J., J.W., X.-L.Z. and X.-S.Z.; Project administration, W.-J.Y. and J.-S.J.; Resources, W.-J.Y., Y.W., W.-B.D., Q.C., X.-Y.P. and X.-L.Z.; Software, W.-J.Y. and X.-S.Z.; Supervision, X.-J.H. and H.J.; Validation, W.-J.Y., Y.W., W.-B.D., Q.C., X.-Y.P., J.-S.J., J.W., X.-S.Z. and X.-J.H.; Visualization, W.-J.Y., Y.W. and W.-B.D.; Writing—original draft, W.-J.Y. and Y.W.; Writing—review and editing, W.-J.Y., Y.W., W.-B.D., Q.C., X.-Y.P., J.-S.J., J.W., X.-L.Z., X.-S.Z., X.-J.H. and H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2021YFC2500300), Beijing Research Ward Excellence Program, BRWEP (BRWEP2024W134080100), Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0502400), Being Nova Program (No. 20220484076).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Peking University People’s Hospital (protocol code, 2025PHB500-001and date of approval, 13 November 2025).
Informed Consent Statement
This study was conducted as a retrospective review of existing clinical data. The requirement for obtaining written informed consent was waived by the Ethics Committee of Peking University People’s Hospital (Approval No. 2025PHB500-001) because the research involved no direct contact with patients, posed no additional risk to the participants, and used fully de-identified data. The waiver did not adversely affect the rights or welfare of the patients.
Data Availability Statement
Raw datasets of this work are not publicly archived due to institutional restrictions and capacity reasons but can be obtained after request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AE | Aclarubicin + Etoposide |
| AEG | Aclarubicin, Etoposide and G-CSF |
| AL | Acute Leukemia |
| ALL | Acute Lymphoblastic Leukemia |
| ALT | Alanine Aminotransferase |
| AML | Acute Myeloid Leukemia |
| ANC | Absolute Neutrophil Count |
| APL | Acute Promyelocytic Leukemia |
| ATRA | All-trans Retinoic Acid |
| AZA | Azacitidine |
| BAL | Bronchoalveolar Lavage |
| CCR | Composite Complete Remission |
| CI | Confidence Interval |
| CMV | Cytomegalovirus |
| CR | Complete Response |
| CRi | Complete Remission with Incomplete Hematologic Recovery |
| CRAL | Complete Remission after Leukemia treatment |
| CT | Computed Tomography |
| DAH | Diffuse Alveolar Hemorrhage |
| DEP | Doxorubicin, Etoposide, and Methylprednisolone |
| EBV | Epstein–Barr Virus |
| ELN | European LeukemiaNet |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| HLH | Hemophagocytic Lymphohistiocytosis |
| HR | Hazard Ratio |
| HSCT | Hematopoietic Stem Cell Transplantation |
| IA | Idarubicin + Cytarabine |
| IDH | Isocitrate Dehydrogenase |
| IL | Interleukin |
| MLFS | Morphologic Leukemia-Free State |
| MOF | Multiple Organ Failure |
| MPAL | Mixed-Phenotype Acute Leukemia |
| NGS | Next-Generation Sequencing |
| NK | Natural Killer |
| NR | No Response |
| OR | Odds Ratio |
| ORR | Overall Remission Rate |
| OS | Overall Survival |
| PCR | Polymerase Chain Reaction |
| PR | Partial Response |
| sCD25 | Soluble Interleukin-2 Receptor α chain |
| sHLH | Secondary Hemophagocytic Lymphohistiocytosis |
| VP-16 | Etoposide |
| VDP | Vincristine, Daunorubicin, Prednisone |
| VDLP | Vincristine, Daunorubicin, L-asparaginase, Prednisone |
References
- Jordan, M.B.; Allen, C.E.; Weitzman, S.; Filipovich, A.H.; McClain, K.L. How I treat hemophagocytic lymphohistiocytosis. Blood 2011, 118, 4041–4052. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Berliner, N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood 2015, 125, 2908–2914. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zeron, P.; Lopez-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult haemophagocytic syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, M.; Wu, W.C.; Zhou, H.J.; Zou, L.Q. Lymphoma-associated hemophagocytic syndrome: A retrospective study from a single center. Hematology 2022, 27, 909–916. [Google Scholar] [CrossRef]
- Takahashi, N.; Miura, I.; Chubachi, A.; Miura, A.B.; Nakamura, S. A clinicopathological study of 20 patients with T/natural killer (NK)-cell lymphoma-associated hemophagocytic syndrome with special reference to nasal and nasal-type NK/T-cell lymphoma. Int. J. Hematol. 2001, 74, 303–308. [Google Scholar] [CrossRef]
- Brito-Zeron, P.; Kostov, B.; Moral-Moral, P.; Martinez-Zapico, A.; Diaz-Pedroche, C.; Fraile, G.; Pérez-Guerrero, P.; Fonseca, E.; Robles, A.; Vaquero-Herrero, M.P.; et al. Prognostic factors of death in 151 adults with hemophagocytic syndrome: Etiopathogenically driven analysis. Mayo. Clin. Proc. Innov. Qual. Outcomes 2018, 2, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Kobayashi, R.; Tanaka, J.; Hashino, S.; Ota, S.; Torimoto, Y.; Kakinoki, Y.; Yamamoto, S.; Kurosawa, M.; Hatakeyama, N.; et al. Risk factor analysis of non-Hodgkin lymphoma-associated haemophagocytic syndromes: A multicentre study. Br. J. Haematol. 2014, 165, 786–792. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y.; Jin, F.; Dehoedt, C.; Rao, J.; Zhou, Y.; Li, P.; Yang, G.; Wang, M.; Zhang, R.; et al. Clinical characteristics and prognostic factors of adult hemophagocytic syndrome patients: A retrospective study of increasing awareness of a disease from a single-center in China. Orphanet. J. Rare. Dis. 2015, 10, 20. [Google Scholar] [CrossRef]
- Hannah, A.Y.; Jenny, O.N.; Andrew, J.W.; Satyen, G.; Jessica, J.M.; Elspeth, M.P. Safety and efficacy of anakinra in hemophagocytic lymphohistiocytosis associated with acute leukemia. Haematologica 2024, 109, 1947–1950. [Google Scholar] [CrossRef]
- Roe, C.; Bennett, J.; Zhang, L.; Chavez, J.; Shah, B.; Sokol, L.; Komrokji, R. Hemophagocytic lymphohistiocytosis in malignant hematology: Uncommon but should not be forgotten? Clin. Lymphoma Myeloma Leuk. 2015, 15, S147–S150. [Google Scholar] [CrossRef]
- Jamy, O.; Nunnery, S.; Giri, S.; Wiedower, E.; Johnson, B.; Yaghmour, G.; Martin, M.G. Under-recognition of hemophagocytic syndrome in United States’ rural, non-teaching hospitals. Leuk. Lymphoma 2016, 57, 2911–2913. [Google Scholar] [CrossRef]
- Delavigne, K.; Berard, E.; Bertoli, S.; Corre, J.; Duchayne, E.; Demur, C.; Mas, V.M.-D.; Borel, C.; Picard, M.; Alvarez, M.; et al. Hemophagocytic syndrome in patients with acute myeloid leukemia undergoing intensive chemotherapy. Haematologica 2014, 99, 474–480. [Google Scholar] [CrossRef]
- Bertozzi, A.I.; Suc, A.; Rubie, H.; Duchayne, E.; Demur, C.; Robert, A. Hemophagocytic syndrome associated with neutropenia after chemotherapy. Arch. Pediatr. 2002, 9, 125–129. [Google Scholar] [CrossRef]
- Henter, J.I.; Elinder, G.; Soder, O.; Ost, A. Incidence in Sweden and clinical features of familial hemophagocytic lymphohistio-cytosis. Acta. Paediatr. Scand. 1991, 80, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Samuelsson-Horne, A.; Arico, M.; Egeler, R.M.; Elinder, G.; Filipovich, A.H.; Gadner, H.; Imashuku, S.; Komp, D.; Ladisch, S.; et al. Treatment of hemophagocytic lym-phohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood 2002, 100, 2367–2373. [Google Scholar] [CrossRef]
- Chinese Society of Hematology, Chinese Society of Pediatrics, Chinese Expert Alliance on Hemophagocytic Syndrome. Guidelines for the diagnosis and treatment of hemophagocytic syndrome in China (2022 edition). Chin. Med. J. 2022, 102, 1492–1499. [Google Scholar]
- Lai, W.; Wang, Y.; Wang, J.; Wu, L.; Jin, Z.; Wang, Z. Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in adults and adolescents: A life-threatening disease: Analysis of 133 cases from a single center. Hematology 2018, 23, 810–816. [Google Scholar] [CrossRef]
- Yu, W.J.; Kong, J.; Zheng, F.M.; Mo, X.D.; Zhang, X.H.; Xu, L.P.; Zhang, Y.-Y.; Sun, Y.-Q.; Jin, J.; Huang, X.-J.; et al. Treatment of minimal residual disease in myeloid malignancies after allo-HSCT with venetoclax-based regimens in patients ineligible for or failed in the immunotherapy. Hematology 2024, 29, 2418653. [Google Scholar] [CrossRef]
- Yu, W.J.; Mo, X.D.; Zhang, X.H.; Xu, L.P.; Wang, Y.; Yan, C.H.; Chen, H.; Chen, Y.-H.; Han, W.; Wang, F.-R.; et al. Occurrence and severity of donor lymphocyte infusion-associated chronic graft-versus-host disease influence the clinical outcomes in relapsed acute leukemia after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2019, 25, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Melissa, R.G. Hemophagocytic lymphohistiocytosis: Review of etiologies and management. J. Blood Med. 2014, 5, 69–86. [Google Scholar] [CrossRef]
- La Rosée, P.; Horne, A.; Hines, M.; von Bahr Greenwood, T.; Machowicz, R.; Berliner, N.; Birndt, S.; Gil-Herrera, J.; Girschikofsky, M.; Jordan, M.B.; et al. Recommendations for the man-agement of hemophagocytic lymphohistiocytosis in adults. Blood 2019, 133, 2465–2477. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Machowicz, R.; Janka, G.; Wiktor-Jedrzejczak, W. Similar but not the same: Differential diagnosis of HLH and sepsis. Crit. Rev. Oncol. Hematol. 2017, 114, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rivière, S.; Galicier, L.; Coppo, P.; Marzac, C.; Aumont, C.; Lambotte, O.; Fardet, L. Reactive hemophagocytic syndrome in adults: A retrospective analysis of 162 patients. Am. J. Med. 2014, 127, 1118–1125. [Google Scholar] [CrossRef]
- Henter, A.; Park, S.; Gatt, D.; Yu, A.Y.L.; Chan, L.Y.C. Hemophagocytic syndromes (HPSs) including hemophagocytic lympho-histiocytosis (HLH) in adults: A systematic scoping review. Blood Rev. 2016, 30, 411–420. [Google Scholar]
- Buckley, S.A.; Wood, B.L.; Othus, M.; Hourigan, C.S.; Ustun, C.; Linden, M.A.; DeFor, T.E.; Malagola, M.; Anthias, C.; Valkova, V.; et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: A meta-analysis. Haematologica 2017, 102, 865–873. [Google Scholar] [CrossRef]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.-C.; et al. 2021 update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef] [PubMed]
- Keenan, C.; Nichols, K.E.; Albeituni, S. Use of the JAK inhibitor ruxolitinib in the treatment of hemophagocytic lymphohistio-cytosis. Front. Immunol. 2021, 12, 614704. [Google Scholar] [CrossRef]
- Wei, W.; Li, N.; Li, Z.; Yan, L. The notch pathway promotes NF-κB activation through Asb2 in T cell acute lymphoblastic leukemia cells. Cell Mol. Biol. Lett. 2018, 23, 37. [Google Scholar] [CrossRef]
- Sulis, M.L.; Aiello, A.I.; Qian, Y.; Hsieh, D.; Bhatia, M.; Garg, T.; Paietta, E.; Tallman, M.S.; Rowe, J.M.; De Keersmaecker, K.; et al. Synergistic antileukemic therapies in NOTCH1-induced T-ALL. Proc. Natl. Acad. Sci. USA 2017, 114, 2006–2011. [Google Scholar]
- Zhang, K.; Jordan, M.B.; Marsh, R.A.; Johnson, J.A.; Kissell, D.; Meller, J.; Villanueva, J.; Risma, K.A.; Wei, Q.; Klein, P.S.; et al. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood 2011, 118, 5794–5798. [Google Scholar] [CrossRef] [PubMed]
- Borel, C.; Jeannin, J.P.; Gendron, M.; Benamara, M.; Legrand, Y.; Boucher, B.; Mahlaoui, N.; Garcelon, N.; Lambotte, O.; Launay, D.; et al. Severe adult hemophagocytic lymphohistiocytosis correlates with HLH-related gene variants. J. Allergy Clin. Immunol. 2023, 153, 256–264. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).