Immune Responses to Filarial Nematodes: A Mechanistic Evaluation of Evasion and Modulation Strategies
Abstract
1. Introduction
2. Global Filarial Endemicity
3. Clinical Diagnosis
4. Filariasis: Causative Organisms
5. Pathophysiology
6. Clinical Stages
7. Immunomodulation via Parasite-Derived Molecules
8. Molecular Mimicry
9. Modulation of TLR and NF-κB Signalling Cascades Induced by Filarial Parasites
10. Effector T Cell Modulation
11. Role of Regulatory T Cells in Filarial Evasion and Pathogenesis
12. Apoptosis Induction in Host Immune Cells
13. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAMs | Alternatively Activated Macrophages |
| ADCC | Antibody-dependent cell-mediated toxicity |
| AhR | aryl hydrocarbon receptor |
| ALR | Absent in Melanoma 2-like receptor |
| APCs | Antigen Presenting Cells |
| Bm-ALT | Brugia malayi abundant larval transcript |
| BmHsp | Brugia malayi heat shock protein |
| BmK1 | Brugia malayi K1 |
| cAMP | Cyclic adenosine monophosphate |
| Cbl-b | Casitas B-lineage lymphoma proto-oncogene-b |
| c-Cbl | Casitas B-lineage lymphoma |
| CCR7 | C-C chemokine receptor 7 |
| CD 8 | Cluster of Differentiation 8 |
| CD16 | Cluster of Differentiation 16 |
| CD4 | Cluster of Differentiation 4 |
| CLR | C-type lectin receptor |
| CTLA-4 | Cytotoxic T- T-lymphocyte-associated protein 4 |
| DC | Dendritic cells |
| DEC | Diethylcarbamazine citrate |
| EMF | Endomyocardial Fibrosis |
| ES-62 | Excretory-secretory-62 |
| ESP | Excretory-secretory Product |
| FasL | Fas ligand |
| FcεRI | Fc epsilon receptor I |
| FOXP3 | Forkhead Box P3 |
| FTS | Filariasis Test Strip |
| GATA-3 | GATA binding protein 3 |
| gp | Glycoprotein |
| GSH | Glutathione |
| HIV-1 | Human Immundodeficiency virus-1 |
| ICOS | Inducible Co-stimulator |
| ICT | Immunochromatographic test |
| IDO | Indoleamine 2,3-dioxygenase |
| IFN-γ | Interferon-gamma |
| IgE | Immunoglobulin E |
| IgG1 | Immunoglobulin G1 |
| IgG4 | Immunoglobulin G4 |
| IKK | IκB kinase |
| IL | Interleukin |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| ILCs | Innate lymphoid cells |
| ITIM | Immunoreceptor Tyrosine-based Inhibition Motif |
| JAK | Janus kinases |
| LAG-3 | Lymphocyte Activation Gene |
| LAP | Leucyl aminopeptidase |
| LC3 | Microtubule-associated protein light-chain 3 |
| LPS | Lipopolysaccharides |
| MAPK | Mitogen-activated protein kinase |
| MDA | Mass Drug Administration |
| MF | Microfilariae |
| MHC | Major histocompatibility complex |
| MIF | Macrophage Migration Inhibitory Factor |
| mTOR | mammalian target of rapamycin |
| MyD88 | Myeloid differentiation primary response 88 |
| NEDD4 | Neural precursor cell-expressed developmentally downregulated protein 4 |
| NFκB | Nuclear factor kappa B |
| NK cells | Natural Killer cells |
| NLR | Nod-like receptor |
| NVBDCP | National Vector-borne Disease Control Program |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PC | Phosphorylcholine |
| PC-BSA | Phosphorylcholine-Bovine serum albumin |
| PCR | Polymerase Chain Reaction |
| PD-1 | Programmed cell death protein 1 |
| PD-L2 | Programmed cell death-ligand 2 |
| PGE2 | Prostaglandin E2 |
| PKC | Protein kinase C |
| RDT | Rapid Diagnostic Test |
| RELMα | Resistin-like molecule-α |
| RLR | RIG-I-like receptor |
| ROS | Reactive Oxygen Species |
| SAE | Severe adverse effects |
| SOCS | Suppressor of Cytokine Signalling |
| SOD | Superoxide Dismutase |
| TGF-β | Transforming Growth Factor-beta |
| Th1 | T helper 1 |
| Th2 | T helper 2 |
| TLR | Toll-like receptor |
| TNF-α | Tumour Necrosis Factor-α |
| TPE | Tropical Pulmonary Eosinophilia |
| Tr1 | Type 1 regulatory |
| TRAIL | Tumour necrosis factor-related apoptosis-inducing ligand |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| TrX | Thioredoxin |
| TSLP | Thymic stromal lymphopoietin |
| WHO | World Health Organisation |
References
- Taylor, M.J.; Hoerauf, A.; Bockarie, M. Lymphatic filariasis and onchocerciasis. Lancet 2010, 376, 1175–1185. [Google Scholar] [CrossRef]
- World Health Organization. Lymphatic Filariasis (Elephantiasis). Available online: https://www.who.int/health-topics/lymphatic-filariasis#tab=tab_1 (accessed on 27 October 2025).
- Rajamanickam, A.; Babu, S. Unraveling the dynamics of human filarial infections: Immunological responses, host manifestations, and pathogen biology. Pathogens 2025, 14, 223. [Google Scholar] [CrossRef]
- Babu, S.; Nutman, T.B. Immunology of lymphatic filariasis. Parasite Immunol. 2014, 36, 338–346. [Google Scholar] [CrossRef]
- World Health Organization. Lymphatic Filariasis Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis (accessed on 27 October 2025).
- National Center for Vector Borne Diseases Control. Magnitude of Disease. Available online: https://ncvbdc.mohfw.gov.in/index4.php?lang=1&level=0&linkid=455&lid=3732 (accessed on 27 October 2025).
- National Center for Vector Borne Diseases Control. List of 348 LF Endemic Districts. Available online: https://ncvbdc.mohfw.gov.in/WriteReadData/l892s/12145485381746781807.pdf (accessed on 27 October 2025).
- Juergens, A.L.; Goldin, J. Filariasis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556012/#:~:text=Diagnosis%20relies%20on%20identifying%20microfilariae,presence%20of%20living%20adult%20worms (accessed on 27 October 2025).
- World Health Organization. Diagnosis and Treatment Lymphatic Filariasis (Elephantiasis). Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/lymphatic-filariasis/diagnosis-and-treatment#:~:text=Circulating%20microfilariae%20can%20be%20detected,the%20periodicity%20of%20the%20microfilariae (accessed on 27 October 2025).
- Mathison, B.A.; Couturier, M.R.; Pritt, B.S. Diagnostic identification and differentiation of microfilariae. J. Clin. Microbiol. 2019, 57, e00706-19. [Google Scholar] [CrossRef]
- Shaukat, A.; Aleem, M.; Kanwal, A.; Kalim, A.; Kalim, F.; Memoon, A.; Siddique, M.; Samad, M.; Shaukat, R.; Shaukat, I. Recent advances in diagnosis of filariasis. Zoonosis Unique Sci. Publ. Faisalabad Pak. 2023, 2, 45–57. [Google Scholar]
- Mendoza, N.; Li, A.; Gill, A.; Tyring, S. Filariasis: Diagnosis and treatment. Dermatol. Ther. 2009, 22, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Chandy, A.; Thakur, A.S.; Singh, M.P.; Manigauha, A. A review of neglected tropical diseases: Filariasis. Asian Pac. J. Trop. Med. 2011, 4, 581–586. [Google Scholar] [CrossRef]
- Weil, G.J.; Curtis, K.C.; Fakoli, L.; Fischer, K.; Gankpala, L.; Lammie, P.J.; Majewski, A.C.; Pelletreau, S.; Won, K.Y.; Bolay, F.K. Laboratory and field evaluation of a new rapid test for detecting Wuchereria bancrofti antigen in human blood. Am. J. Trop. Med. Hyg. 2013, 89, 11. [Google Scholar] [CrossRef]
- Mageto, S.K.; Waihenya, R.W.; Mwangi, A.W.; Rotich, P.K.; Munyao, M.M.; Teya, T.; Irekwa, R.M.; Yego, J.J.; Njoroge, C.W.; Kanyita, G.N.; et al. Expression and Evaluation of Wb-SXP-1 and Wb-123 Recombinant Antigens as Potential Diagnostic Biomarkers for Lymphatic Filariasis. Am. J. Mol. Biol. 2023, 13, 95–112. [Google Scholar] [CrossRef]
- Suresh, S.; Kumaraswami, V.; Suresh, I.; Rajesh, K.; Suguna, G.; Vijayasekaran, V.; Ruckmani, A.; Rajamanickam, M. Ultrasonographic diagnosis of subclinical filariasis. J. Ultrasound Med. 1997, 16, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Mahajan, A.; Shukla, S.; Pokar, N. Imaging and management of lymphedema in the era of precision oncology. Br. J. Radiol. 2025, 98, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Kuthi, L.; Zombori, T.; Tiszlavicz, L.; Hegedűs, F.; Almási, S.; Baráth, B.; Almakrami, M.; Ej, M.J.; Barta, N.; Ujfaludi, Z. Emerging human pulmonary dirofilariasis in Hungary: A single center experience. Diagn. Pathol. 2024, 19, 85. [Google Scholar] [CrossRef]
- Simonsen, P.; Fischer, P.; Hoerauf, A.; Weil, G. Manson’s Tropical Diseases, 23rd ed.; The Filariases; Elsevier: Amsterdam, The Netherlands, 2014; pp. 737–765. [Google Scholar]
- Liat, L.B.; Liliana, K.; Sudomo, M.; Joesoef, A. Status of brugian filariasis research in indonesia and future studies. Bul. Penelit. Kesehat. 1985, 13, 31–55. [Google Scholar]
- Ehrens, A.; Hoerauf, A.; Hübner, M.P. Eosinophils in filarial infections: Inducers of protection or pathology? Front. Immunol. 2022, 13, 983812. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, A.; Shriram, A.N.; Raju, K.H.K.; Kumar, A. Mosquitoes, lymphatic filariasis, and public health: A systematic review of Anopheles and Aedes surveillance strategies. Pathogens 2023, 12, 1406. [Google Scholar] [CrossRef]
- Nanduri, J.; Kazura, J.W. Clinical and laboratory aspects of filariasis. Clin. Microbiol. Rev. 1989, 2, 39–50. [Google Scholar] [CrossRef]
- Murdoch, M. Mapping the burden of onchocercal skin disease. Br. J. Dermatol. 2021, 184, 199–207. [Google Scholar] [CrossRef]
- Gyasi, M.E.; Okonkwo, O.N.; Tripathy, K. Onchocerciasis. In StatPearls; [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Elouardi, C.; Lefort, A.; Deconinck, L.; Peiffer-Smadja, N.; Houzé, S.; Argy, N. Imported loiasis: Diagnostic and therapeutic challenges. Infect. Dis. Now. 2025, 55, 105053. [Google Scholar] [CrossRef]
- Downes, B.; Jacobsen, K. A systematic review of the epidemiology of mansonelliasis. Afr. J. Infect. Dis. 2010, 4, 7–14. [Google Scholar] [CrossRef]
- Simonetti, O.; Zerbato, V.; Di Bella, S.; Luzzati, R.; Cavalli, F. Dracunculiasis over the centuries: The history of a parasite unfamiliar to the West. Le. Infez. Med. 2023, 31, 257. [Google Scholar]
- Risch, F.; Ritter, M.; Hoerauf, A.; Hübner, M.P. Human filariasis—Contributions of the Litomosoides sigmodontis and Acanthocheilonema viteae animal model. Parasitol. Res. 2021, 120, 4125–4143. [Google Scholar] [CrossRef] [PubMed]
- Bakowski, M.A.; McNamara, C.W. Advances in antiwolbachial drug discovery for treatment of parasitic filarial worm infections. Trop. Med. Infect. Dis. 2019, 4, 108. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Shinde, D.B.; Kulkarni, A.; Arote, R. Two decades of antifilarial drug discovery: A review. RSC Adv. 2017, 7, 20628–20666. [Google Scholar] [CrossRef]
- Nutman, T.B. Insights into the pathogenesis of disease in human lymphatic filariasis. Lymphat. Res. Biol. 2013, 11, 144–148. [Google Scholar] [CrossRef]
- Chulanetra, M.; Chaicumpa, W. Revisiting the mechanisms of immune evasion employed by human parasites. Front. Cell. Infect. Microbiol. 2021, 11, 702125. [Google Scholar] [CrossRef]
- Chakraborty, S.; Gurusamy, M.; Zawieja, D.C.; Muthuchamy, M. Lymphatic filariasis: Perspectives on lymphatic remodeling and contractile dysfunction in filarial disease pathogenesis. Microcirculation 2013, 20, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, Z.M.; Vieira, A.V.; Xavier, A.T.; Bezerra, G.S.; Lopes, M.d.F.C.; Bonfim, C.V.; Aguiar-Santos, A.M. Lymphatic filariasis: A systematic review on morbidity and its repercussions in countries in the Americas. Int. J. Environ. Res. Public. Health 2021, 19, 316. [Google Scholar] [CrossRef]
- Azhar, S.; Abdullah, M.; Qamar, W.; Iqbal, M.; Asghar, S.; Ajaz, R.; Iqbal, H.; Sohail, M.; Tahir, S.; Assad, M. Basic insights into lymphatic filariasis. Zoonosis Unique Sci. Publ. Faisalabad Pak. 2023, 2, 73–88. [Google Scholar]
- Jungmann, P.; Figueredo-Silva, J.; Dreyer, G. Bancroftian lymphadenopathy: A histopathologic study of fifty-eight cases from northeastern Brazil. Am. J. Trop. Med. Hyg. 1991, 45, 325–331. [Google Scholar] [CrossRef]
- Kumaraswami, V. The clinical manifestations of lymphatic filariasis. Trop. Med. Sci. Pract. Lymphat. Filariasis 2000, 1, 103–125. [Google Scholar] [CrossRef]
- Bennuru, S.; Nutman, T.B. Lymphangiogenesis and lymphatic remodeling induced by filarial parasites: Implications for pathogenesis. PLoS Pathog. 2009, 5, e1000688. [Google Scholar] [CrossRef]
- Babu, S.; Nutman, T.B. Immunopathogenesis of lymphatic filarial disease. Semin. Immunopathol. 2012, 34, 847–861. [Google Scholar] [CrossRef]
- DeVries, C.R. Basic science of lymphatic filariasis. Indian. J. Urol. 2005, 21, 5–8. [Google Scholar] [CrossRef]
- Mues, K.E.; Lammie, P.J.; Klein, M.; Kleinbaum, D.G.; Addiss, D.; Fox, L.M. Changes in antibody levels during and following an episode of acute adenolymphangitis (ADL) among lymphedema patients in Léogâne, Haiti. PLoS ONE 2015, 10, e0141047. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Nutman, T.B. Vascular Responses in Human Lymphatic Filariasis. In Vascular Responses to Pathogens; Elsevier: Amsterdam, The Netherlands, 2016; pp. 209–220. [Google Scholar]
- Dierks, C.; Tober-Lau, P.; Veletzky, L.; Wang, Z.; Zühlke, B.; Ludwig, D.; Niewienda, A.; Freiwald, A.; Bardtke, L.; Kroneberg, P. Plasma Proteomics Reveals Distinct Signatures in Occult and Microfilaremic Loa loa Infections. J. Infect. Dis. 2025, 232, jiaf344. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Gomez-Escobar, N.; Gregory, W.F.; Murray, J.; Zang, X. Immune evasion genes from filarial nematodes. Int. J. Parasitol. 2001, 31, 889–898. [Google Scholar] [CrossRef]
- Allen, J.E.; Loke, P.N. Divergent roles for macrophages in lymphatic filariasis. Parasite Immunol. 2001, 23, 345–352. [Google Scholar] [CrossRef]
- Moreno, Y.; Geary, T.G. Stage-and gender-specific proteomic analysis of Brugia malayi excretory-secretory products. PLoS Neglected Trop. Dis. 2008, 2, e326. [Google Scholar] [CrossRef]
- Allen, J.E.; Maizels, R.M. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 2011, 11, 375–388. [Google Scholar] [CrossRef]
- Jafari, N.; Abediankenari, S. Role of microRNAs in immunoregulatory functions of epithelial cells. BMC Immunol. 2024, 25, 84. [Google Scholar] [CrossRef]
- Miller, J.F. The function of the thymus and its impact on modern medicine. Science 2020, 369, eaba2429. [Google Scholar] [CrossRef]
- Maizels, R.M.; McSorley, H.J. Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol. 2016, 138, 666–675. [Google Scholar] [CrossRef]
- Ajendra, J.; Allen, J.E. Neutrophils: Friend or foe in Filariasis? Parasite Immunol. 2022, 44, e12918. [Google Scholar] [CrossRef]
- Joardar, N.; Babu, S.P.S. A review on the druggability of a thiol-based enzymatic antioxidant thioredoxin reductase for treating filariasis and other parasitic infections. Int. J. Biol. Macromol. 2020, 142, 125–141. [Google Scholar] [CrossRef]
- Kwarteng, A.; Asiedu, E.; Koranteng, K.K.; Asiedu, S.O. Highlighting the relevance of CD8+ T cells in filarial infections. Front. Immunol. 2021, 12, 714052. [Google Scholar] [CrossRef]
- Lie Kian Joe, L.K.J. Occult filariasis: Its relationship with tropical pulmonary eosinophilia. Am. J. Trop. Med. Hyg. 1962, 11, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Beaver, P.C. Filariasis without microfilaremia. Am. J. Trop. Med. Hyg. 1970, 19, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Udwadia, F.E. Tropical eosinophilia—A correlation of clinical, histopathologic and lung function studies. Dis. Chest 1967, 52, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Nagaratnam, N. Arthritis, possibly due to filariasis. Trans. R Soc. Trop. Med. Hyg. 1973, 67, 405–409. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Gawdi, A.; Dey, S. Occult filarial infections. Natl. Med. J. India 1990, 3, 7–9. [Google Scholar]
- Chandrasoma, P.; Mendis, K.N. Filarial infection of the breast. Am. J. Trop. Med. Hyg. 1978, 27, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Yazdanbakhsh, M. Immune regulation by helminth parasites: Cellular and molecular mechanisms. Nat. Rev. Immunol. 2003, 3, 733–744. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Spickett, C.M. Chemistry of phospholipid oxidation. Biochim. Biophys. Acta (BBA)-Biomembr. 2012, 1818, 2374–2387. [Google Scholar] [CrossRef] [PubMed]
- Volinsky, R.; Kinnunen, P.K. Oxidized phosphatidylcholines in membrane-level cellular signaling: From biophysics to physiology and molecular pathology. FEBS J. 2013, 280, 2806–2816. [Google Scholar] [CrossRef]
- Pal, B.; Kulkarni, S.; Bhandari, Y.; Ganesh, B.B.; Goswami, K.; Reddy, M. Lymphatic filariasis: Possible pathophysiological nexus with oxidative stress. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 650–655. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Yadav, S.; Gupta, S.; Selvaraj, C.; Doharey, P.K.; Verma, A.; Singh, S.K.; Saxena, J.K. In silico and in vitro studies on the protein-protein interactions between Brugia malayi immunomodulatory protein calreticulin and human C1q. PLoS ONE 2014, 9, e106413. [Google Scholar] [CrossRef]
- Joardar, N.; Bhattacharya, R.; Halder, S.; Sen, A.; Biswas, S.R.; Jana, K.; Babu, S.P.S. Filarial thioredoxin reductase exerts anti-inflammatory effects upon lipopolysaccharide induced inflammation in macrophages. Int. J. Biol. Macromol. 2021, 193, 1379–1390. [Google Scholar] [CrossRef]
- Desjardins, C.A.; Cerqueira, G.C.; Goldberg, J.M.; Hotopp, J.C.D.; Haas, B.J.; Zucker, J.; Ribeiro, J.M.; Saif, S.; Levin, J.Z.; Fan, L. Genomics of Loa loa, a Wolbachia-free filarial parasite of humans. Nat. Genet. 2013, 45, 495–500. [Google Scholar] [CrossRef]
- Gomez-Escobar, N.; Bennett, C.; Prieto-Lafuente, L.; Aebischer, T.; Blackburn, C.C.; Maizels, R.M. Heterologous expression of the filarial nematode alt gene products reveals their potential to inhibit immune function. BMC Biol. 2005, 3, 8. [Google Scholar] [CrossRef]
- Chhabra, S.; Chang, S.C.; Nguyen, H.M.; Huq, R.; Tanner, M.R.; Londono, L.M.; Estrada, R.; Dhawan, V.; Chauhan, S.; Upadhyay, S.K. Kv1. 3 channel-blocking immunomodulatory peptides from parasitic worms: Implications for autoimmune diseases. FASEB J. 2014, 28, 3952. [Google Scholar] [CrossRef] [PubMed]
- Harnett, W.; Harnett, M. Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory-secretory product. J. Immunol. 1993, 151, 4829–4837. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, J.P.; Harcus, Y.M.; Curwen, R.S.; Dowle, A.A.; Atmadja, A.K.; Ashton, P.D.; Wilson, A.; Maizels, R.M. The secretome of the filarial parasite, Brugia malayi: Proteomic profile of adult excretory–secretory products. Mol. Biochem. Parasitol. 2008, 160, 8–21. [Google Scholar] [CrossRef]
- Adjobimey, T.; Hoerauf, A. Induction of immunoglobulin G4 in human filariasis: An indicator of immunoregulation. Ann. Trop. Med. Parasitol. 2010, 104, 455–464. [Google Scholar] [CrossRef]
- Khatri, V.; Chauhan, N.; Kalyanasundaram, R. Parasite cystatin: Immunomodulatory molecule with therapeutic activity against immune mediated disorders. Pathogens 2020, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Manoury, B.; Gregory, W.F.; Maizels, R.M.; Watts, C. Bm-CPI-2, a cystatin homolog secreted by the filarial parasite Brugia malayi, inhibits class II MHC-restricted antigen processing. Curr. Biol. 2001, 11, 447–451. [Google Scholar] [CrossRef]
- Hewitson, J.P.; Grainger, J.R.; Maizels, R.M. Helminth immunoregulation: The role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 2009, 167, 1–11. [Google Scholar] [CrossRef]
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007, 317, 256–260. [Google Scholar] [CrossRef]
- Bunte, M.J.; Schots, A.; Kammenga, J.E.; Wilbers, R.H. Helminth glycans at the host-parasite interface and their potential for developing novel therapeutics. Front. Mol. Biosci. 2022, 8, 1358. [Google Scholar] [CrossRef]
- Melendez, A.J.; Harnett, M.M.; Pushparaj, P.N.; Wong, W.S.F.; Tay, H.K.; McSharry, C.P.; Harnett, W. Inhibition of FcεRI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat. Med. 2007, 13, 1375–1381. [Google Scholar] [CrossRef]
- Harnett, W.; McInnes, I.B.; Harnett, M.M. ES-62, a filarial nematode-derived immunomodulator with anti-inflammatory potential. Immunol. Lett. 2004, 94, 27–33. [Google Scholar] [CrossRef]
- Harnett, W.; Harnett, M.M. Helminth-derived immunomodulators: Can understanding the worm produce the pill? Nat. Rev. Immunol. 2010, 10, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Eason, R.J.; Bell, K.S.; Marshall, F.A.; Rodgers, D.T.; Pineda, M.A.; Steiger, C.N.; Al-Riyami, L.; Harnett, W.; Harnett, M.M. The helminth product, ES-62 modulates dendritic cell responses by inducing the selective autophagolysosomal degradation of TLR-transducers, as exemplified by PKCδ. Sci. Rep. 2016, 6, 37276. [Google Scholar] [CrossRef] [PubMed]
- Semnani, R.T.; Venugopal, P.G.; Leifer, C.A.; Mostböck, S.; Sabzevari, H.; Nutman, T.B. Inhibition of TLR3 and TLR4 function and expression in human dendritic cells by helminth parasites. Blood J. Am. Soc. Hematol. 2008, 112, 1290–1298. [Google Scholar] [CrossRef]
- Herbert, D.B.R.; Douglas, B.; Zullo, K. Group 2 innate lymphoid cells (ILC2): Type 2 immunity and helminth immunity. Int. J. Mol. Sci. 2019, 20, 2276. [Google Scholar] [CrossRef] [PubMed]
- Cotton, R.; McDonald-Fleming, R.; Boyd, A.; Spates, K.; Nutman, T.; Tolouei Semnani, R. Brugia malayi infective larvae fail to activate Langerhans cells and dermal dendritic cells in human skin. Parasite Immunol. 2015, 37, 79–91. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Tawill, S.; Le Goff, L.; Ali, F.; Blaxter, M.; Allen, J.E. Both free-living and parasitic nematodes induce a characteristic Th2 response that is dependent on the presence of intact glycans. Infect. Immun. 2004, 72, 398–407. [Google Scholar] [CrossRef]
- Van Die, I.; Cummings, R.D. Glycan gimmickry by parasitic helminths: A strategy for modulating the host immune response? Glycobiology 2010, 20, 2–12. [Google Scholar] [CrossRef]
- Ludin, P.; Nilsson, D.; Mäser, P. Genome-wide identification of molecular mimicry candidates in parasites. PLoS ONE 2011, 6, e17546. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Escobar, N.; Gregory, W.F.; Maizels, R.M. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor β, expressed in microfilarial and adult stages of Brugia malayi. Infect. Immun. 2000, 68, 6402–6410. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Smits, H.H.; McSorley, H.J. Modulation of host immunity by helminths: The expanding repertoire of parasite effector molecules. Immunity 2018, 49, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Shiny, C.; Krushna, N.S.; Babu, S.; Elango, S.; Manokaran, G.; Narayanan, R.B. Recombinant Wolbachia heat shock protein 60 (HSP60) mediated immune responses in patients with lymphatic filariasis. Microbes Infect. 2011, 13, 1221–1231. [Google Scholar] [CrossRef]
- Dakshinamoorthy, G.; Samykutty, A.K.; Munirathinam, G.; Shinde, G.B.; Nutman, T.; Reddy, M.V.; Kalyanasundaram, R. Biochemical characterization and evaluation of a Brugia malayi small heat shock protein as a vaccine against lymphatic filariasis. PLoS ONE 2012, 7, e34077. [Google Scholar] [CrossRef]
- Liu, L.X.; Weller, P.F. Intravascular filarial parasites inhibit platelet aggregation. Role of parasite-derived prostanoids. J. Clin. Investig. 1992, 89, 1113–1120. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef]
- Oyesola, O.O.; Tait Wojno, E.D. Prostaglandin regulation of type 2 inflammation: From basic biology to therapeutic interventions. Eur. J. Immunol. 2021, 51, 2399–2416. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway Jr, C. The Toll receptor family and microbial recognition. Trends Microbiol. 2000, 8, 452–456. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Lien, E.; Ingalls, R.R. Toll-like receptors. Crit. Care Med. 2002, 30, S1–S11. [Google Scholar] [CrossRef]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Toll-like receptors. Curr. Protoc. Immunol. 2015, 109, 14.12.1–14.12.10. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef] [PubMed]
- Akira, S. Mammalian Toll-like receptors. Curr. Opin. Immunol. 2003, 15, 5–11. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol. 2005, 17, 338–344. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Riva, F.; Muzio, M. Updates on Toll-Like Receptor 10 Research. Eur. J. Immunol. 2025, 55, e202551840. [Google Scholar] [CrossRef]
- Barrat, F.J.; Coffman, R.L. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol. Rev. 2008, 223, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.H.; Fleuri, A.K.; Pellison, N.C.; Quirino, G.F.; Horta, C.V.; de Carvalho, R.V.; Oliveira, S.C.; Zamboni, D.S. Autophagy downstream of endosomal Toll-like receptor signaling in macrophages is a key mechanism for resistance to Leishmania major infection. J. Biol. Chem. 2017, 292, 13087–13096. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, Q.; Zhang, T.; Fan, J. C7ORF41 regulates inflammation by inhibiting NF-κB signaling pathway. BioMed Res. Int. 2021, 2021, 7413605. [Google Scholar] [CrossRef]
- Su, C.-M.; Wang, L.; Yoo, D. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci. Rep. 2021, 11, 13464. [Google Scholar] [CrossRef] [PubMed]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-κB: At the borders of autoimmunity and inflammation. Front. Immunol. 2021, 12, 716469. [Google Scholar] [CrossRef]
- Inoue, J.-i.; Kerr, L.D.; Kakizuka, A.; Verma, I.M. IκBγ, a 70 kd protein identical to the C-terminal half of p110 NF-κB: A new member of the IκB family. Cell 1992, 68, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Solan, N.J.; Miyoshi, H.; Carmona, E.M.; Bren, G.D.; Paya, C.V. RelB cellular regulation and transcriptional activity are regulated by p100. J. Biol. Chem. 2002, 277, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Perneel, J.; Vieri, M.K.; Colebunders, R.; Kumar-Singh, S. The secretome of filarial nematodes and its role in host-parasite interactions and pathogenicity in onchocerciasis-associated epilepsy. Front. Cell. Infect. Microbiol. 2021, 11, 662766. [Google Scholar] [CrossRef]
- Venugopal, P.G.; Nutman, T.B.; Semnani, R.T. Activation and regulation of toll-like receptors (TLRs) by helminth parasites. Immunol. Res. 2009, 43, 252–263. [Google Scholar] [CrossRef]
- Bhoj, P.; Togre, N.; Khatri, V.; Goswami, K. Harnessing immune evasion strategy of lymphatic filariae: A therapeutic approach against inflammatory and infective pathology. Vaccines 2022, 10, 1235. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Anuradha, R.; Bethunaickan, R. TLR specific immune responses against helminth infections. J. Parasitol. Res. 2017, 2017, 6865789. [Google Scholar] [CrossRef]
- Bąska, P.; Norbury, L.J. The role of nuclear factor kappa B (NF-κB) in the immune response against parasites. Pathogens 2022, 11, 310. [Google Scholar] [CrossRef]
- McManus, C.M.; Maizels, R.M. Regulatory T cells in parasite infections: Susceptibility, specificity and specialisation. Trends Parasitol. 2023, 39, 547–562. [Google Scholar] [CrossRef]
- Taylor, M.D.; van der Werf, N.; Maizels, R.M. T cells in helminth infection: The regulators and the regulated. Trends Immunol. 2012, 33, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Steel, C.; Nutman, T.B. CTLA-4 in filarial infections: Implications for a role in diminished T cell reactivity. J. Immunol. 2003, 170, 1930–1938. [Google Scholar] [CrossRef]
- Babu, S.; Blauvelt, C.P.; Kumaraswami, V.; Nutman, T.B. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: Implications for parasite persistence. J. Immunol. 2006, 176, 3248–3256. [Google Scholar] [CrossRef]
- Semnani, R.T.; Liu, A.Y.; Sabzevari, H.; Kubofcik, J.; Zhou, J.; Gilden, J.K.; Nutman, T.B. Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL-12 and IL-10, and reduce their capacity to activate CD4+ T cells. J. Immunol. 2003, 171, 1950–1960. [Google Scholar] [CrossRef]
- Semnani, R.T.; Law, M.; Kubofcik, J.; Nutman, T.B. Filaria-induced immune evasion: Suppression by the infective stage of Brugia malayi at the earliest host-parasite interface. J. Immunol. 2004, 172, 6229–6238. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, I.; Nair, M.G.; Zang, X.; Brombacher, F.; Mohrs, M.; Allison, J.P.; Allen, J.E. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J. Immunol. 2007, 179, 3926–3936. [Google Scholar]
- Georgiev, P.; Charbonnier, L.-M.; Chatila, T.A. Regulatory T cells: The many faces of Foxp3. J. Clin. Immunol. 2019, 39, 623–640. [Google Scholar] [CrossRef]
- Grover, P.; Goel, P.N.; Greene, M.I. Regulatory T cells: Regulation of identity and function. Front. Immunol. 2021, 12, 750542. [Google Scholar] [CrossRef]
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; Cross, R.; Sehy, D.; Blumberg, R.S.; Vignali, D.A. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007, 450, 566–569. [Google Scholar] [CrossRef]
- Abbas, A.K.; Benoist, C.; Bluestone, J.A.; Campbell, D.J.; Ghosh, S.; Hori, S.; Jiang, S.; Kuchroo, V.K.; Mathis, D.; Roncarolo, M.G. Regulatory T cells: Recommendations to simplify the nomenclature. Nat. Immunol. 2013, 14, 307–308. [Google Scholar] [CrossRef]
- Grazia Roncarolo, M.; Gregori, S.; Battaglia, M.; Bacchetta, R.; Fleischhauer, K.; Levings, M.K. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006, 212, 28–50. [Google Scholar] [CrossRef]
- Mak, T.W.; Saunders, M.E.; Jett, B.D. Primer to the Immune Response; Newnes; Academic Cell: Cambridge, MA, USA, 2013. [Google Scholar]
- Xing, Y.; Hogquist, K.A. T-cell tolerance: Central and peripheral. Cold Spring Harb. Perspect. Biol. 2012, 4, a006957. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Schwartz, J.A.; Sandrock, C.; Bellemore, S.M.; Nikoopour, E. Modulation of autoimmune diseases by interleukin (IL)-17 producing regulatory T helper (Th17) cells. Indian. J. Med. Res. 2013, 138, 591–594. [Google Scholar] [PubMed]
- Pellerin, L.; Jenks, J.A.; Bégin, P.; Bacchetta, R.; Nadeau, K.C. Regulatory T cells and their roles in immune dysregulation and allergy. Immunol. Res. 2014, 58, 358–368. [Google Scholar] [CrossRef]
- Bellemore, S.; Nikoopour, E.; Schwartz, J.; Krougly, O.; Lee-Chan, E.; Singh, B. Preventative role of interleukin-17 producing regulatory T helper type 17 (Treg17) cells in type 1 diabetes in non-obese diabetic mice. Clin. Exp. Immunol. 2015, 182, 261–269. [Google Scholar] [CrossRef]
- Sharma, R.; Kinsey, G.R. Regulatory T cells in acute and chronic kidney diseases. Am. J. Physiol.-Ren. Physiol. 2018, 314, F679–F698. [Google Scholar] [CrossRef] [PubMed]
- McSorley, H.J.; Harcus, Y.M.; Murray, J.; Taylor, M.D.; Maizels, R.M. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. J. Immunol. 2008, 181, 6456–6466. [Google Scholar] [CrossRef]
- Yurchenko, E.; Tritt, M.; Hay, V.; Shevach, E.M.; Belkaid, Y.; Piccirillo, C.A. CCR5-dependent homing of naturally occurring CD4+ regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J. Exp. Med. 2006, 203, 2451–2460. [Google Scholar] [CrossRef]
- Belkaid, Y. Regulatory T cells and infection: A dangerous necessity. Nat. Rev. Immunol. 2007, 7, 875–888. [Google Scholar] [CrossRef]
- Belkaid, Y.; Blank, R.B.; Suffia, I. Natural regulatory T cells and parasites: A common quest for host homeostasis. Immunol. Rev. 2006, 212, 287–300. [Google Scholar] [CrossRef]
- Maizels, R.; Yazdanbakhsh, M. Regulation of the immune response by helminth parasites: Cellular and molecular mechanisms. Nat. Rev. Immunol. 2006, 3, 733. [Google Scholar] [CrossRef] [PubMed]
- Ouaissi, A. Regulatory cells and immunosuppressive cytokines: Parasite-derived factors induce immune polarization. BioMed Res. Int. 2007, 2007, 094971. [Google Scholar] [CrossRef] [PubMed]
- Metenou, S.; Nutman, T.B. Regulatory T cell subsets in filarial infection and their function. Front. Immunol. 2013, 4, 305. [Google Scholar] [CrossRef]
- Song, Y.; Wang, N.; Chen, L.; Fang, L. Tr1 cells as a key regulator for maintaining immune homeostasis in transplantation. Front. Immunol. 2021, 12, 671579. [Google Scholar] [CrossRef]
- Satoguina, J.S.; Weyand, E.; Larbi, J.; Hoerauf, A. T regulatory-1 cells induce IgG4 production by B cells: Role of IL-10. J. Immunol. 2005, 174, 4718–4726. [Google Scholar] [CrossRef]
- King, C.L.; Mahanty, S.; Kumaraswami, V.; Abrams, J.S.; Regunathan, J.; Jayaraman, K.; Ottesen, E.A.; Nutman, T.B. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J. Clin. Investig. 1993, 92, 1667–1673. [Google Scholar] [CrossRef]
- Ritter, M.; Osei-Mensah, J.; Debrah, L.B.; Kwarteng, A.; Mubarik, Y.; Debrah, A.Y.; Pfarr, K.; Hoerauf, A.; Layland, L.E. Wuchereria bancrofti-infected individuals harbor distinct IL-10-producing regulatory B and T cell subsets which are affected by anti-filarial treatment. PLoS Neglected Trop. Dis. 2019, 13, e0007436. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Karnam, A.; Das, M.; Babu, S.P.S.; Bayry, J. Wuchereria bancrofti filaria activates human dendritic cells and polarizes T helper 1 and regulatory T cells via toll-like receptor 4. Commun. Biol. 2019, 2, 169. [Google Scholar] [CrossRef]
- Babu, S.; Kumaraswami, V.; Nutman, T.B. Transcriptional control of impaired Th1 responses in patent lymphatic filariasis by T-box expressed in T cells and suppressor of cytokine signaling genes. Infect. Immun. 2005, 73, 3394–3401. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; LeGoff, L.; Harris, A.; Malone, E.; Allen, J.E.; Maizels, R.M. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J. Immunol. 2005, 174, 4924–4933. [Google Scholar] [CrossRef]
- Taylor, M.D.; Harris, A.; Babayan, S.A.; Bain, O.; Culshaw, A.; Allen, J.E.; Maizels, R.M. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J. Immunol. 2007, 179, 4626–4634. [Google Scholar] [CrossRef]
- Mariano, F.S.; Gutierrez, F.R.; Pavanelli, W.R.; Milanezi, C.M.; Cavassani, K.A.; Moreira, A.P.; Ferreira, B.R.; Cunha, F.Q.; Cardoso, C.R.; Silva, J.S. The involvement of CD4+ CD25+ T cells in the acute phase of Trypanosoma cruzi infection. Microbes Infect. 2008, 10, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Satoguina, J.; Mempel, M.; Larbi, J.; Badusche, M.; Löliger, C.; Adjei, O.; Gachelin, G.; Fleischer, B.; Hoerauf, A. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes Infect. 2002, 4, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- White, M.P.; McManus, C.M.; Maizels, R.M. Regulatory T-cells in helminth infection: Induction, function and therapeutic potential. Immunology 2020, 160, 248–260. [Google Scholar] [CrossRef]
- Babu, S.; Blauvelt, C.P.; Nutman, T.B. Filarial parasites induce NK cell activation, type 1 and type 2 cytokine secretion, and subsequent apoptotic cell death. J. Immunol. 2007, 179, 2445–2456. [Google Scholar] [CrossRef]
- Narasimhan, P.B.; Bennuru, S.; Meng, Z.; Cotton, R.N.; Elliott, K.R.; Ganesan, S.; McDonald-Fleming, R.; Veenstra, T.D.; Nutman, T.B.; Tolouei Semnani, R. Microfilariae of Brugia malayi inhibit the mTOR pathway and induce autophagy in human dendritic cells. Infect. Immun. 2016, 84, 2463–2472. [Google Scholar] [CrossRef]
- Ricciardi, A.; Bennuru, S.; Tariq, S.; Kaur, S.; Wu, W.; Elkahloun, A.G.; Arakelyan, A.; Shaik, J.; Dorward, D.W.; Nutman, T.B. Extracellular vesicles released from the filarial parasite Brugia malayi downregulate the host mTOR pathway. PLoS Neglected Trop. Dis. 2021, 15, e0008884. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, S.K.; Sahoo, P.K.; Bal, M.; Satapathy, A. Increased Fas ligand expression of peripheral B-1 cells correlated with CD4+ T-cell apoptosis in filarial-infected patients. Parasite Immunol. 2017, 39, e12421. [Google Scholar] [CrossRef]
- Kalyanasundaram, R.; Khatri, V.; Chauhan, N. Advances in vaccine development for human lymphatic filariasis. Trends Parasitol. 2020, 36, 195–205. [Google Scholar] [CrossRef]
- Won, K.Y.; Gass, K.; Biamonte, M.; Dagne, D.A.; Ducker, C.; Hanna, C.; Hoerauf, A.; Lammie, P.J.; Njenga, S.M.; Noordin, R. Diagnostics to support elimination of lymphatic filariasis—Development of two target product profiles. PLoS Neglected Trop. Dis. 2021, 15, e0009968. [Google Scholar] [CrossRef]
- Kumar, V.; Mishra, A.; Singh, A. Identification of promising nutraceuticals against filarial immune-modulatory proteins: Insights from in silico and ex vivo studies. RSC Adv. 2022, 12, 22542–22554. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kumar, S.; Singh, A. Biosynthesis and characterization of Ocimum sanctum green silver nanoparticles and unravelling their enhanced anti-filarial activity through a HRAMS proteomics approach. RSC Adv. 2024, 14, 5893–5906. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kumar, V.; Kumar, S.; Singh, H.; Singh, A. HRAMS Proteomics Insights on the Anti-Filarial Effect of Ocimum sanctum: Implications in Phytochemical-Based Drug-Targeting and Designing. Proteomes 2024, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mishra, A.; Singh, S.P.; Singh, A. Anti-filarial efficacy of Centratherum anthelminticum: Unravelling the underlying mechanisms through biochemical, HRAMS proteomics and MD simulation approaches. RSC Adv. 2024, 14, 25198–25220. [Google Scholar] [CrossRef]
- Kumar, S.; Mishra, A.; Kumar, V.; Singh, T.; Singh, A.K.; Singh, A. Designing and comparative analysis of anti-oxidant and heat shock proteins based multi-epitopic filarial vaccines. BMC Infect. Dis. 2024, 24, 1436. [Google Scholar] [CrossRef]
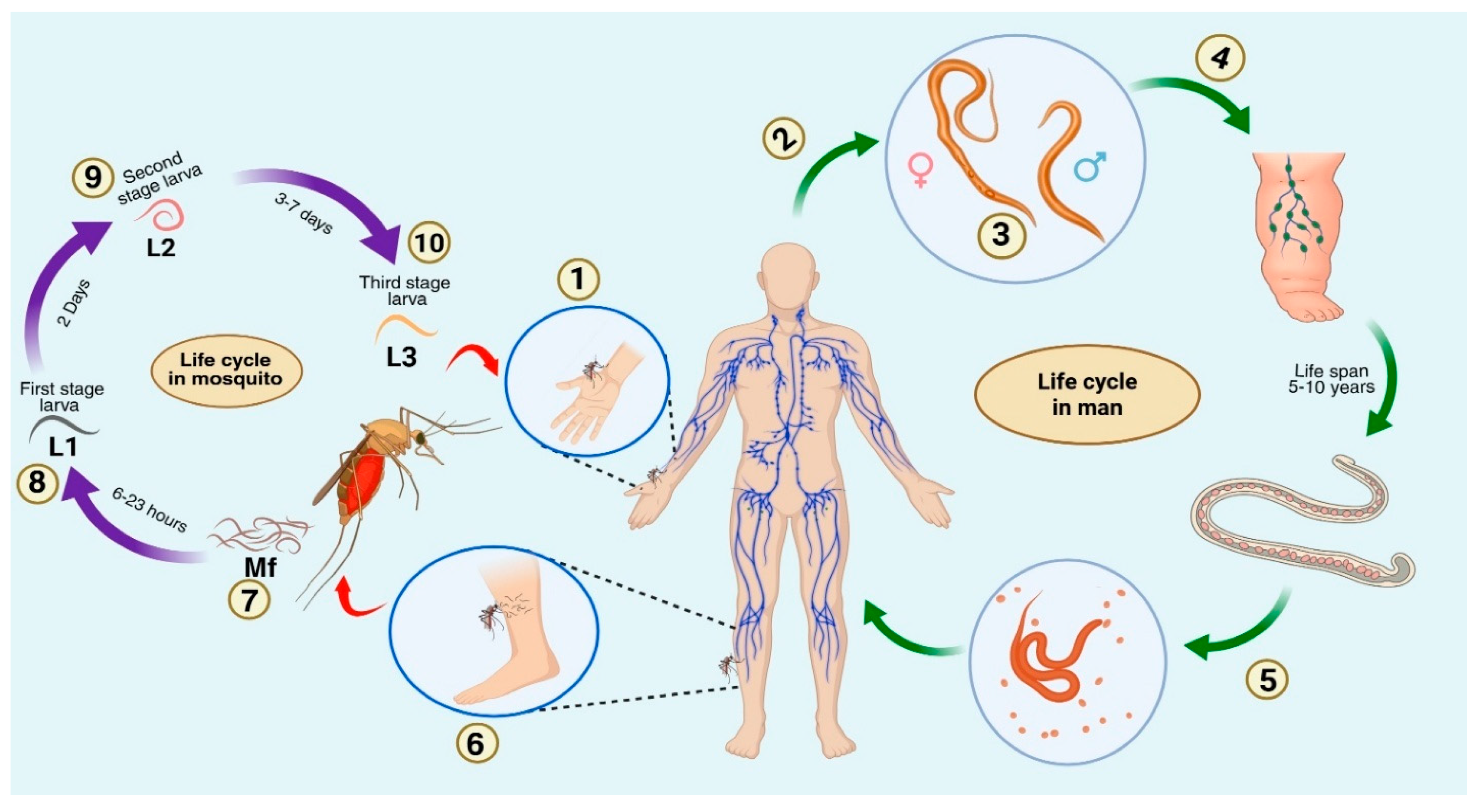
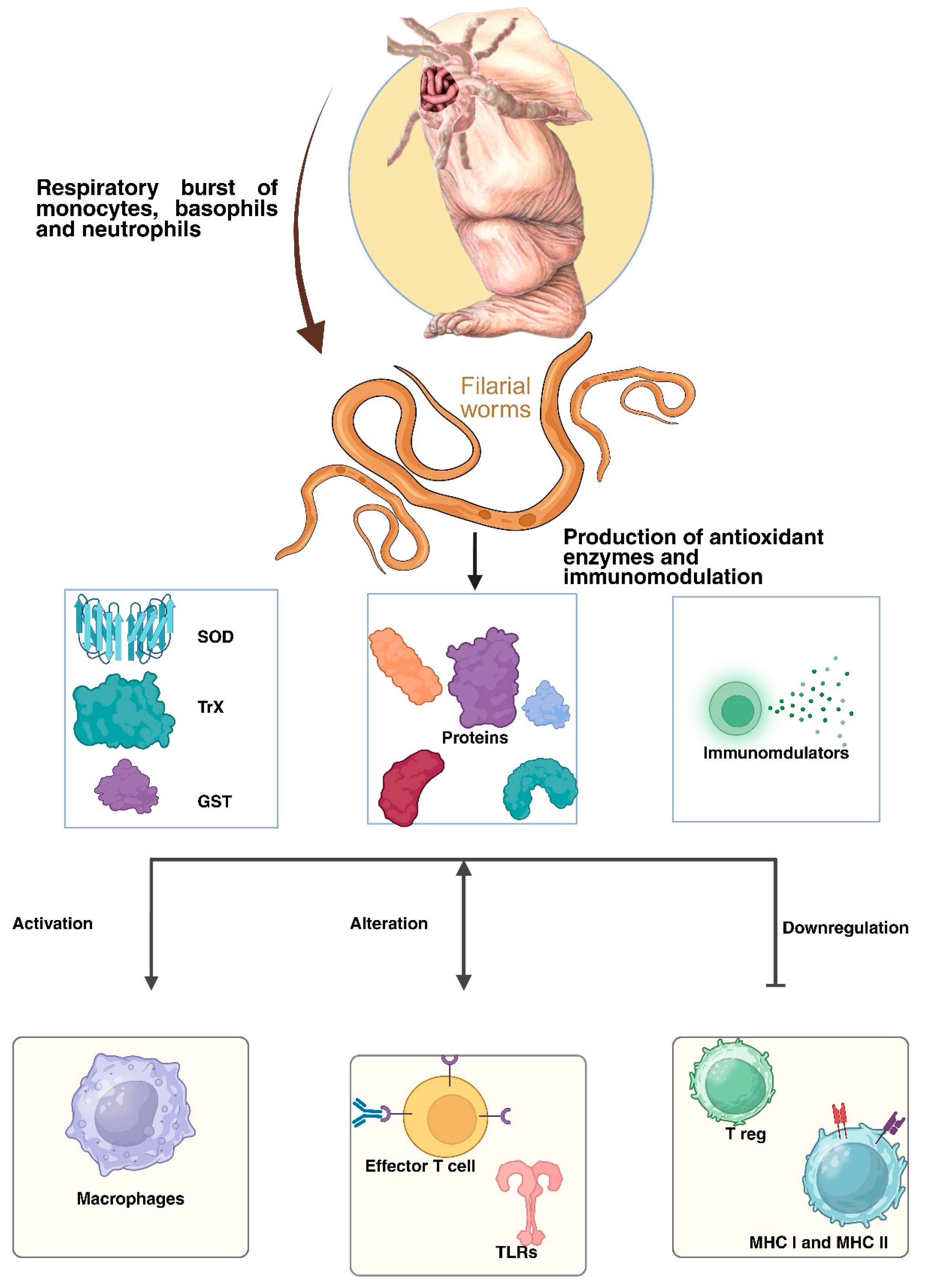
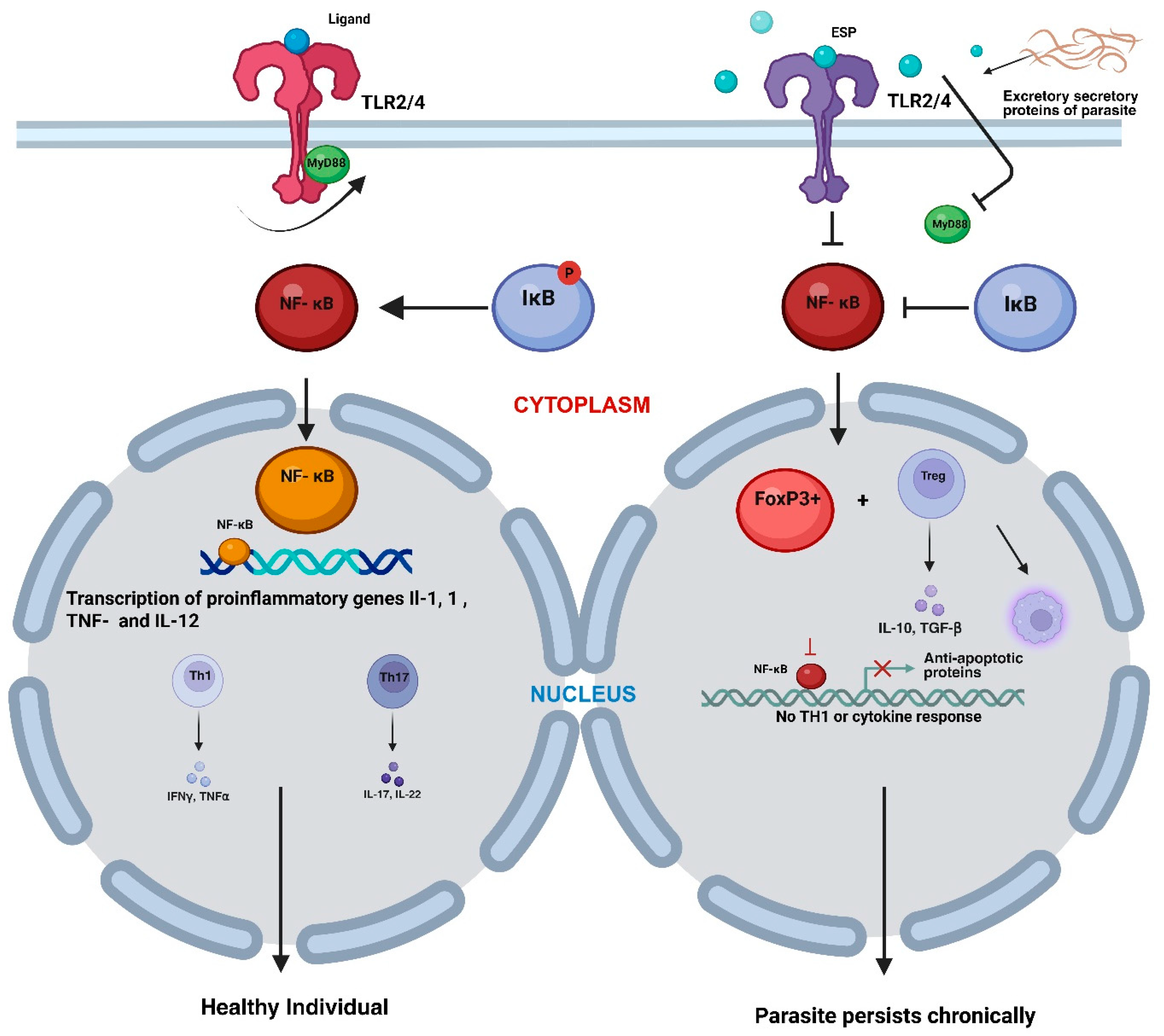
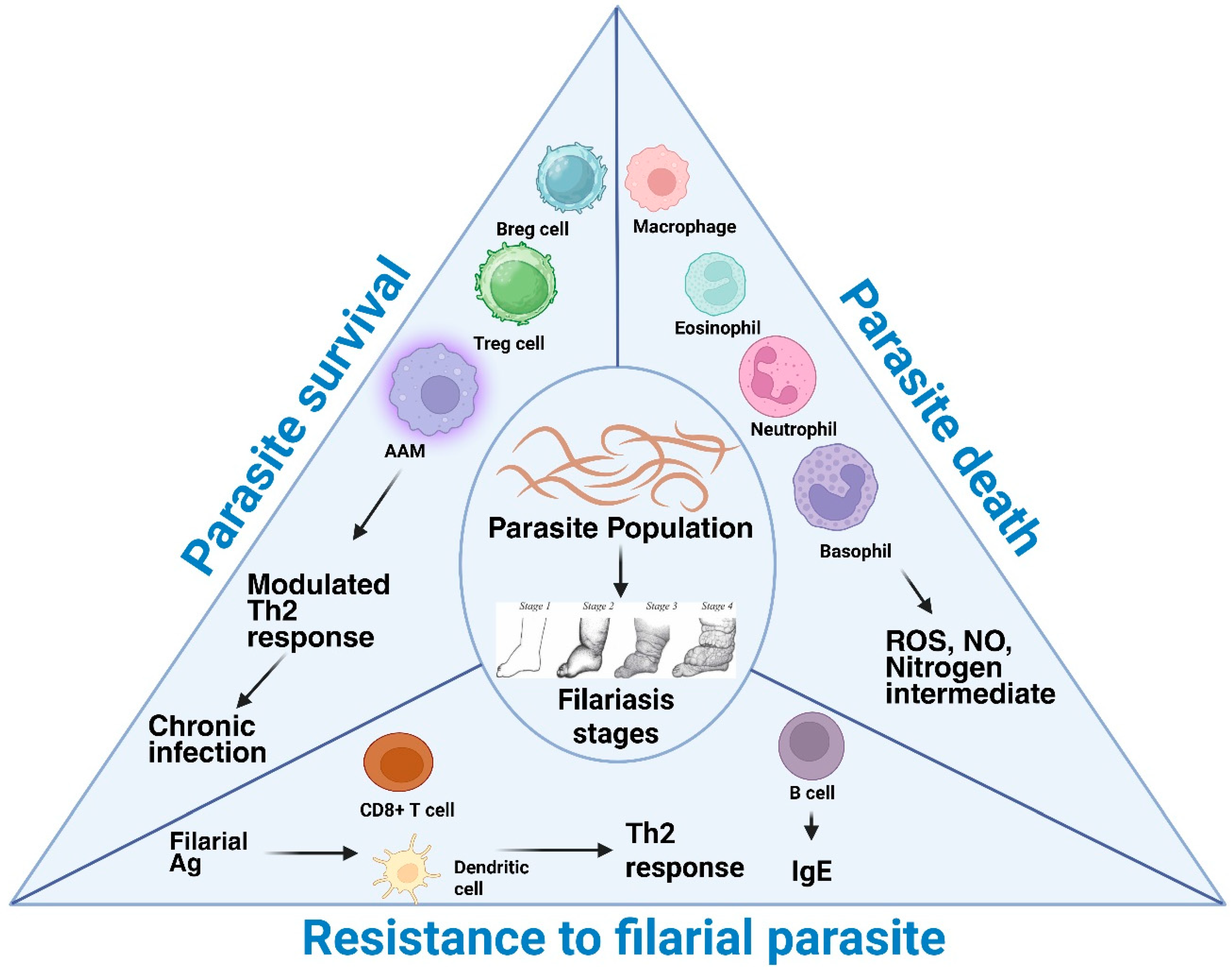
| S.No. | Filarial Parasite | Associated Disease | Vector Involved | Cause of Symptoms | Recommended Treatment | Affected Regions | Experimental Model | References |
|---|---|---|---|---|---|---|---|---|
| 1. | Oncocerca volvulus, Oncocerca ochengi | Onchocerciasis | Blackflies (Simulium spp.) | Microfilariae (MF)-related immune response | Ivermectin (generally in co-endemic areas), not advised in case of areas co-endemic with loiasis | Sub-Saharan Africa, Yemen, Uganda, Cameroon, small foci in South America | Mice in the case of Oncocerca ochengi | [21] |
| 2. | Wuchereria bancrofti, Brugia malayi, Brugia timori | Lymphatic filariasis (Elephantiasis) | Mosquito species (Aedes, Anopheles, Culex, Mansonia, etc.) | Adult worm-specific immune responses TPE: lung mf trapping | Diethylcarbamazine citrate (DEC), Ivermectin, Albendazole (Different combinations of these drugs are given in areas with co-endemicity) | Tropical regions of Asia, America, the Pacific, Africa, and countries like Indonesia, Malaysia, Thailand | Ferrets, Mice, and Jirds | [30] |
| 3. | Loa loa | Loiasis | Chrysops flies | Due to the migration of adult worms, Calabar swelling, severe reactions to DEC treatment, and hypereosinophilia | DEC or Albendazole (Due to the possibility of SAEs, treatment is not always advised) | West and Central Africa | Primates (Baboons) and rodents | [31] |
| 4. | Mansonella perstans, Mansonella ozzardi, Mansonella streptocerca | Mansonellosis | Midges of the genus Culicoides | Due to adult worm migration, ocular symptoms brought on by MF migration | Doxycycline | Eastern, Western, and Central Africa, parts of South and Central America, Caribbean islands | No known animal model till date | [31] |
| S.No. | Clinical Stages in Individuals | Healthy/Diseased | Parasitic Stages | Circulating Filarial Antigens | Symptoms | References |
|---|---|---|---|---|---|---|
| 1. | Normal | Healthy | None | Absent | Nil | [40] |
| 2. | Endemic Normals | Health status is questionable as they are living in endemic areas, but have not been tested for filarial infections | [4] | |||
| 3. | Microfilaraemic/Asymptomatic | Diseased | Microfilariae in blood, live adult worms in lymphatics | Present | Clinically asymptomatic | [41] |
| 4. | Acute Clinical Disease | Diseased | Adult worms in lymphatics | Present | Episodes of lymphangitis, filarial fever, lymph nodes, localised inflammation | [42] |
| 5. | Chronic Pathology | Diseased | Usually, dead adult worms are present in the lymphatics | Present | Lymphedema, elephantiasis, and hydrocele | [43] |
| 6. | Occult | Diseased | Adult worms present, but no circulating microfilariae | May or may not be present | Symptoms include TPE, restrictive pulmonary changes, filarial arthritis, glomerulonephritis, and breast abscesses, among others. | [44] |
| S.No. | Cell Type | Location | Functions and Roles | References |
|---|---|---|---|---|
| 1. | Regulatory T cells | Thymus and Periphery | Maintains tolerance and prevents pathologies. Present in high levels in the asymptomatic stage and low levels in the chronic stage | [50,51] |
| 2. | Regulatory B cells | Blood circulation and inflammation site | Perform Immune regulatory mechanisms by secreting immunosuppressive cytokines. Induce Treg cells, suppressing CD4+, CD8+ T cells and Natural Killer (NK) cells | [33] |
| 3. | Eosinophils | Derived from bone marrow, later migrates to tissue | Contributes both to the protection and development of filarial pathology | [21] |
| 4. | Neutrophils | Circulates in blood later migrate to tissue | Release toxins to eliminate parasites. Involved in protective immune responses and pathological aggravation of disease. | [52] |
| 5. | Alternatively Activated Macrophages | Reside in Blood and Tissue | Blood-derived AAMs perform immune regulatory roles whereas tissue-resident AAMs responsible for fibrosis seen during chronic infections | [4] |
| 6. | Dendritic cells | Present in epithelial tissues | Parasite-derived products interact with DCs to initiate profound changes in immune responses leading to suppressed inflammation suitable for prolonged survival of parasites characteristic of chronic infections. | [53] |
| 7. | CD4+ T cells | Thymus and peripheral blood circulation | Involved in parasite clearance along with type-2 cytokines. Presence of Treg balances Th1/Th2 responses. | [54] |
| 8. | CD8+ T cells | Thymus and peripheral blood circulation | Involved in the cytotoxic killing of filarial parasites with the help of type-2 cytokines, persistence of filaria antigens contributes to chronic pathology | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, T.; Sharma, S.; Tripathi, A.; Kumar, S.; Singh, A. Immune Responses to Filarial Nematodes: A Mechanistic Evaluation of Evasion and Modulation Strategies. Immuno 2025, 5, 57. https://doi.org/10.3390/immuno5040057
Singh T, Sharma S, Tripathi A, Kumar S, Singh A. Immune Responses to Filarial Nematodes: A Mechanistic Evaluation of Evasion and Modulation Strategies. Immuno. 2025; 5(4):57. https://doi.org/10.3390/immuno5040057
Chicago/Turabian StyleSingh, Tripti, Shivani Sharma, Animesh Tripathi, Sunil Kumar, and Anchal Singh. 2025. "Immune Responses to Filarial Nematodes: A Mechanistic Evaluation of Evasion and Modulation Strategies" Immuno 5, no. 4: 57. https://doi.org/10.3390/immuno5040057
APA StyleSingh, T., Sharma, S., Tripathi, A., Kumar, S., & Singh, A. (2025). Immune Responses to Filarial Nematodes: A Mechanistic Evaluation of Evasion and Modulation Strategies. Immuno, 5(4), 57. https://doi.org/10.3390/immuno5040057






