Abstract
In the exploitation of ion-adsorption rare earth ores, the environmental effects of leaching agents are key constraints for green mining. Understanding the release behavior of typical heavy metals from soils under leaching conditions is of great significance. Laboratory column leaching experiments were conducted to systematically investigate the effects of three leaching agents—(NH4)2SO4, Al2(SO4)3, and MgSO4—as well as varying concentrations of Al2(SO4)3 on the release and speciation transformation of heavy metal Pb in mining-affected soils. The results revealed a three-stage pattern in Pb release—characterized by slow release, a sharp increase, and eventual stabilization—with environmental risks predominantly concentrated in the middle to late stages of leaching. Under 3% (NH4)2SO4 and 3% Al2(SO4)3 leaching conditions, Pb concentrations in soil increased significantly, with a higher proportion of labile fractions, indicating pronounced activation and risk accumulation. Due to its relatively weak ion-exchange capacity, MgSO4 exhibited a lower and more gradual Pb release profile, posing substantially lower pollution risks compared to (NH4)2SO4 and Al2(SO4)3. Pb release under varying Al2(SO4)3 concentrations showed a nonlinear response. At 3% Al2(SO4)3, both the proportion of bioavailable Pb and the Risk Assessment Code (RAC) peaked, while the residual fraction declined sharply, suggesting a threshold effect in risk induction. All three leaching agents promoted the transformation of Pb in soil from stable to more labile forms, including acid-soluble, reducible, and oxidizable fractions, thereby increasing the overall proportion of active Pb (F1 + F2 + F3). A combined analysis of RAC values and the proportion of active Pb provides a comprehensive framework for assessing Pb mobility and ecological risk under different leaching conditions. These findings offer a theoretical basis for the prevention and control of heavy metal risks in the green mining of ion-adsorption rare earth ores.
1. Introduction
Rare earth elements (REEs), as indispensable functional materials in modern industry, are widely used in electronics, advanced materials, energy conservation, environmental protection, aerospace, and national defense. They serve as key resources supporting high-tech development and hold significant strategic importance [1,2,3,4]. Ion-adsorption rare earth ores are particularly rich in medium and heavy REEs, which are mainly adsorbed onto weathered crust minerals in exchangeable ionic forms. These can be extracted through leaching with inorganic salt solutions such as (NH4)2SO4, Al2(SO4)3, and MgSO4 [5]. Although the in situ leaching method offers advantages such as high efficiency, simple operation, and low cost, long-term use of leaching agents can easily lead to soil structure damage, increased acidification, and an increased risk of heavy metal element migration [6,7]. Typical heavy metals such as Pb are widely present in rare earth mining areas [8,9]. However, the variation in Pb content and its speciation transformation under the influence of different leaching agents remain poorly understood, posing a major challenge for accurate environmental risk assessment and the development of effective remediation strategies in these mining areas [10,11]. Therefore, conducting leaching experiments using different types and concentrations of leaching agents, and analyzing the evolution of Pb content and speciation in both leachates and soils, is essential to provide a theoretical foundation for green mining practices and ecological protection in rare earth mining regions.
Leaching agents influence the release and migration of heavy metals by altering soil pH, disrupting cation exchange equilibrium, and facilitating complexation reactions [12]. For example, when (NH4)2SO4 is used as a leaching agent, the released ammonium ions (NH4+) chemically exchange with heavy metals such as Pb, Cu, Cd, and Zn, promoting their mobilization into acid-extractable forms [13,14]. Li et al. [15] reported that residual NH4+ in mining areas significantly alters the chemical speciation of heavy metals, resulting in combined contamination by ammonium nitrogen and heavy metals. Zhang Jun et al. [16] found that in bare mining areas subjected to (NH4)2SO4 leaching, soil pH dropped to 3.92–5.80, with severe ammonium nitrogen pollution and vegetation degradation, thereby enhancing heavy metal mobility. Through profile analysis, Chen et al. [17] demonstrated that Pb, Zn, and Cu were enriched in the upper layers of ore bodies, often exceeding background standards. Leaching activities facilitated the activation of surface heavy metals and impaired soil ecological functions [18,19]. Tan et al. [20] confirmed via leaching experiments that (NH4)2SO4 reduced the total contents of Cu, Zn, and Cr by 18.34%, 10.96%, and 26.00%, respectively, and facilitated the transformation of heavy metals from non-bioavailable to bioavailable forms. Guo et al. [21] found that MgSO4 concentration was negatively correlated with Cu, Pb, and Tl contents. Cu, Zn, and Pb exhibited significant vertical migration, while Tl showed minimal loss due to its chemical inertness. Although previous studies have examined the effects of leaching reagents on heavy metals in ion-adsorption rare earth mining areas, the overall research scope remains limited. Systematic investigations into the variations in Pb content and speciation under diverse leaching conditions are still lacking, and the understanding of the underlying mechanisms is fragmented. Therefore, experimental studies addressing the evolution of Pb content and speciation under different leaching reagent types and concentrations are urgently needed.
Existing studies have predominantly focused on the release of Pb under a single leaching reagent or a single concentration condition. Systematic investigations comparing the effects of different types of leaching reagents—such as (NH4)2SO4, Al2(SO4)3, and MgSO4—and their concentration variations on Pb speciation transformation remain limited [22,23]. In particular, the mechanistic pathways of novel alternative leaching reagents, such as Al2(SO4)3 [24], have not yet been clearly elucidated. Moreover, the transformation patterns of Pb in less labile forms—such as the reducible and oxidizable fractions—under different leaching conditions remain insufficiently quantified [25]. These knowledge gaps hinder the robust assessment of heavy metal migration risks in mining areas and the formulation of differentiated remediation strategies. In response, this study employed a self-designed indoor soil column setup to simulate leaching processes. We systematically compared the mechanisms through which different types and concentrations of leaching reagents influence Pb release and chemical speciation dynamics. The study elucidates the concentration-dependent patterns and ion-mediated regulatory mechanisms of Pb transformation, further clarifies the release mechanisms of Pb under varying leaching conditions, and aims to provide a theoretical basis for risk assessment, remediation strategy development, and safe agricultural utilization of contaminated soils in mining regions.
2. Materials and Methods
2.1. Soil Sample Collection
The soil samples used in this study were collected from a rare earth mining area in Fujian Province, China. The geographic location is shown in Figure 1. Sampling was conducted along the ridge line, including sites at the foot, mid-slope, and top of the mountain, as well as surrounding farmland. At each location, 3 to 5 samples were taken to reduce random error. Samples were collected from a depth of 3–18 m. After collection, the moisture content was measured, and the samples were air-dried by spreading them out in a well-ventilated indoor environment, with dead leaves and other debris manually removed. Once air-dried, larger soil clumps were crushed, and the samples were sealed in plastic bags and stored in a dry, ventilated location for future use. To avoid contamination from external metal sources, non-metallic tools were used throughout the sampling process.
Figure 1.
Geographic location of soil sampling site.
2.2. Fundamental Physicochemical Properties of in Situ Ore Soil
- Moisture Content and Particle Size Distribution of In Situ Soil
The in situ soil samples from the mining area were analyzed for moisture content and particle size distribution. Moisture content was measured using the oven-drying method (at 105 °C until constant weight, with a mass variation rate < 0.1%). The results showed moisture content ranging from 14.13% to 16.35% (mean: 15.24% ± 0.72%), indicating relatively uniform spatial distribution of soil moisture. Particle size distribution was determined using a combined dry sieving–sedimentation method. Fine particles (<0.075 mm) accounted for 27.11% ± 1.2%, silt-sized particles (0.25–0.075 mm) accounted for 26.15%, and fine sand (0.5–0.25 mm) made up 14.49%. The particle gradation curve is shown in Figure 2. The calculated coefficient of uniformity (Cu) was 8.75, and the coefficient of curvature (Cc) was 1.18. According to relevant classification standards, the soil was identified as poorly graded. Its fine particle-dominated composition (characterized by low permeability and high specific surface area) significantly influences the adsorption–desorption behavior of heavy metal Pb.
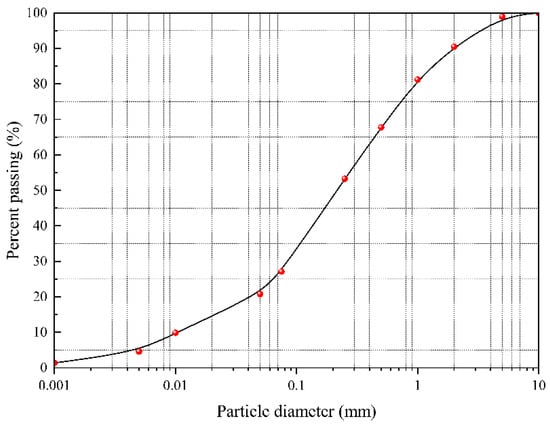
Figure 2.
Curve of particle size distribution of the soil samples.
- 2.
- Chemical and Mineralogical Composition of In Situ Soil
The chemical composition of the soil was analyzed using X-ray fluorescence spectroscopy (XRF), and the results are presented in Table 1. The soil was primarily composed of SiO2 and Al2O3, indicating that aluminosilicates are the dominant minerals in the clay fraction. Among the rare earth elements, Y2O3 was present in relatively high proportions, suggesting that the ore body exhibits characteristics of yttrium-rich heavy rare earth mineralization. The main associated heavy metals were Mn, Pb, and Zn. Pb is typically considered a potential environmental risk factor [26], and its content is closely related to the adsorption and enrichment capacity of clay minerals [27].

Table 1.
Composition analysis of soil samples [28].
The X-ray diffraction (XRD) pattern of the ore soil sample is shown in Figure 3. Phase identification was performed by comparing the diffraction peaks with standard reference patterns in the XRD database. Kaolinite (Al2O3·2SiO2·2H2O) and quartz (SiO2) were the dominant mineral phases in the soil, accounting for 72.6% ± 1.8% as corrected by the RIR method. This finding is consistent with the enrichment of Si–Al oxides revealed by XRF analysis, indicating the long-term influence of aluminosilicate weathering and pedogenesis [29]. The detection of secondary minerals such as goethite (α-FeOOH, 3.21%) and illite (KAl2(AlSi3O10)(OH)2, 1.97%) suggests that Fe oxidation–hydration processes and potassium feldspar alteration play roles in regulating the occurrence of heavy metals [30]. In particular, the specific adsorption of Pb2+ by goethite may significantly affect the geochemical mobility of Pb [31].
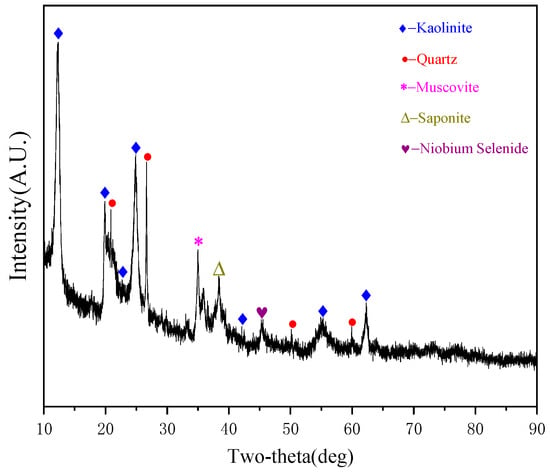
Figure 3.
X-ray diffraction (XRD) pattern of the soil sample.
2.3. Experimental Apparatus
A custom-designed soil column leaching device was used to simulate the in situ leaching process. The apparatus consisted of a plexiglass column, a peristaltic pump, infusion tubing, a reagent reservoir, a collection tank, and an iron support frame, as shown in Figure 4. The soil column was made of a plexiglass tube with a height of 100 cm, an outer diameter of 110 mm, and an inner diameter of 100 mm. Three sampling ports were positioned along the column at 15 cm intervals, with the first port located 35 cm from the top of the column (i.e., 15 cm below the top of the soil layer). The soil column was divided into six segments from top to bottom. The uppermost 0–10 cm served as a buffer zone for leaching solution application; the 10–15 cm section was filled with quartz sand (upper layer); the 15–20 cm section comprised surface soil (upper portion); the 20–90 cm section formed the main soil body; the 90–95 cm section consisted of surface soil (bottom portion); and the bottommost 95–100 cm section was filled with quartz sand (lower layer).
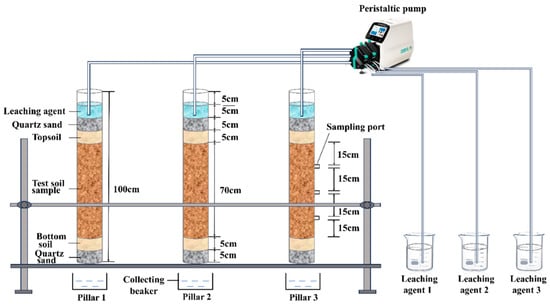
Figure 4.
Schematic diagram of the column leaching test apparatus.
The lead (Pb) content in the experimental samples was measured using an inductively coupled plasma optical emission spectrometer (ICP-OES, model 8800, Agilent Technologies, Santa Clara, CA, USA). Inductively coupled plasma mass spectrometry (ICP-MS) is widely applied for elemental and isotopic analysis across various sample types, including environmental, geological, metallic, and chemical materials. It enables qualitative, semi-quantitative, quantitative, and isotopic ratio analyses.
2.4. Experimental Methods
The total digestion of heavy metals was performed using a multi-stage acid dissolution and thermal decomposition system [32]. Hydrofluoric acid (10 mL), perchloric acid (4 mL), and concentrated nitric acid (10 mL × 2) were sequentially added into a PTFE digestion vessel. The sample was digested stepwise at elevated temperatures of 235–240 °C, 265–270 °C, and 235 °C, utilizing oxidation and complexation reactions to break down silicate mineral lattices and decompose organic matter. Temperature was strictly controlled during digestion, maintaining the solution volume at ≥3 mL to prevent drying. In the final stage, ultrapure water (15 mL) and hydrogen peroxide (1 mL) were added to drive off acids until the white fumes dissipated and the solution became clear and viscous. After cooling and rinsing, the digestate was diluted to 50 mL and filtered through a 0.45 μm membrane. Digestion efficiency and data reliability were validated by dynamic temperature monitoring, solution clarity, and replicate experiments with relative standard deviation (RSD) below 5%. The chemical speciation of heavy metals was extracted using the BCR sequential extraction procedure [33,34] applied to soil samples under varying leaching durations and conditions. Detailed extraction parameters are provided in Table 2. Lead (Pb) concentrations in both the total digested samples and the BCR sequentially extracted fractions (acid-exchangeable, reducible, oxidizable, and residual) were measured using inductively coupled plasma mass spectrometry (ICP-MS) [35]. The measurements were validated using characteristic spectral lines and an RSD < 3.5% [36]. The Pb spike recovery rates ranged from 95.2% to 103.8%, meeting analytical quality requirements.

Table 2.
Extraction conditions for the chemical speciation of soil samples.
3. Variation in Pb Concentration in Leachate
3.1. Effects of Different Types of Leaching Agents
The effects of three leaching agents—(NH4)2SO4, Al2(SO4)3, and MgSO4—on Pb concentrations in leachate are shown in Figure 5. At a concentration of 3%, both (NH4)2SO4 and Al2(SO4)3 exhibited similar Pb concentration evolution patterns, characterized by three stages: an initial slow-release phase, a mid-term surge, and a late stabilization phase. The concentration values in each stage were highly consistent, indicating that the two agents share a common Pb release mechanism [37]. MgSO4 exhibited a distinct three-phase pattern of “fluctuation–slow release–surge,” with peak Pb concentrations reduced by 90.7% and 90.3% compared to (NH4)2SO4 and Al2(SO4)3, respectively. This suggests a more environmentally friendly profile; however, improvements in leaching efficiency are required to meet industrial application demands.
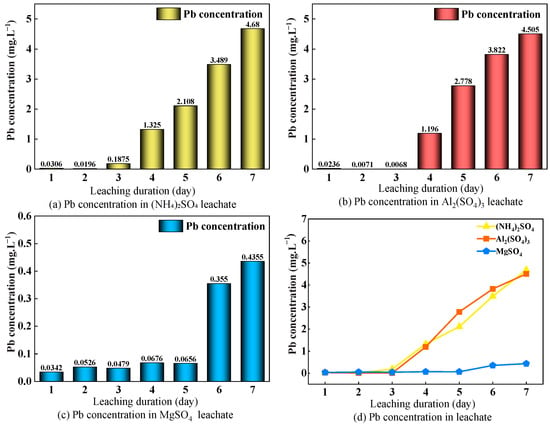
Figure 5.
Changes in Pb concentration in the leachate with different leaching agents applied.
Among the three leaching agents, the magnitude of Pb concentration variation followed the order (NH4)2SO4 > Al2(SO4)3 > MgSO4. This is attributed to the stronger ion-exchange capacity of Al3+ and NH4+ compared to Mg2+, enabling more effective displacement of Pb2+ from the soil. Additionally, the hydrolysis of Al2(SO4)3 produces stronger acidity than that of (NH4)2SO4, significantly enhancing the dissolution of poorly soluble Pb compounds, whereas the hydrolysis of MgSO4 is weak and exerts minimal influence on pH [38]. Therefore, the trend of Pb concentration change under Al2(SO4)3 leaching is similar to that of (NH4)2SO4, with significantly higher concentrations in the mid to late stages compared to MgSO4 leaching.
The evolution of Pb concentration in the leachate indicates that the high-risk period for Pb pollution under 3% (NH4)2SO4 and Al2(SO4)3 leaching is concentrated in the later stage of leaching. At this stage, peak Pb concentrations exceeded the limit specified by the Environmental Quality Standards for Surface Water (0.01 mg/L) by more than 400 times, posing significant risks of contaminating nearby water bodies via infiltration or runoff. In comparison, the peak Pb concentration under 3% MgSO4 leaching was only 0.4355 mg/L (43 times the standard limit). Although Pb activation efficiency under MgSO4 was lower and the environmental risk relatively controllable, long-term accumulation effects should still be considered. This leaching agent is more suitable for low-intensity mining in ecologically sensitive areas, and the resulting leachate must not be directly discharged into domestic water sources to prevent water pollution.
3.2. Effects of Different Concentrations of Leaching Agent
Under varying concentrations of Al2(SO4)3 (1–7%), the Pb release process exhibited a characteristic three-stage pattern, as illustrated in Figure 6. The initial inhibition phase involves Al3+ hydrolysis producing Al(OH)3 colloids that adsorb Pb2+ and PbSO4 micro-precipitates that suppress Pb release, followed by a mid-term surge characterized by peak dissolution of residual Pb, and finally a late stabilization phase where dissolution–precipitation equilibrium is established. This evolution pattern was consistently observed across all concentration gradients, indicating a concentration-independent mechanism of Al2(SO4)3 on Pb release.
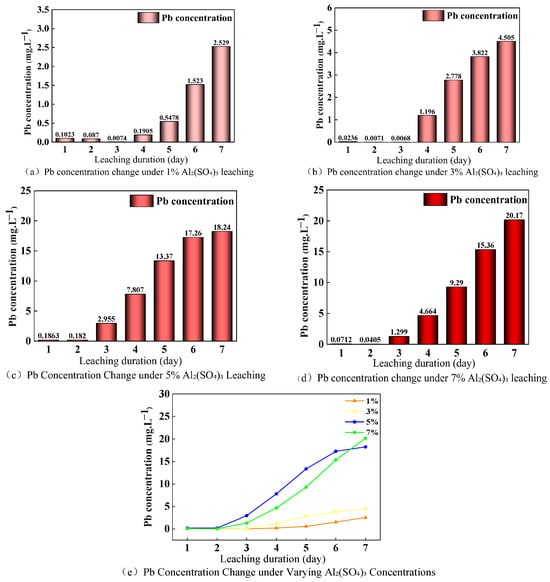
Figure 6.
Changes in Pb concentration in the leachate during leaching with varying concentrations of Al2(SO4)3.
The high-risk period for each leaching concentration consistently occurred during the mid-phase of the leaching process, during which the Pb release rate peaked, with an average daily increase exceeding 70%, warranting intensified monitoring and control efforts [39]. The peak concentration of Pb significantly exceeds the Chinese national Class III standard limit of 0.01 mg/L, and the associated environmental risk index increased exponentially with rising leaching agent concentrations. The peak Pb concentrations and their exceedance multiples are summarized in Table 3.

Table 3.
Peak Pb concentration in the leachate and corresponding exceedance multiple.
Experimental results revealed a nonlinear relationship between Al2(SO4)3 concentration and Pb activation efficiency: when the concentration increased from 5% to 7%, the peak Pb concentration rose by only 1.93%, indicating that Pb activation efficiency under Al2(SO4)3 leaching conditions approached saturation. In the low-concentration group (1–3%), Pb was primarily released from oxide-bound forms, and mineral dissociation was limited, resulting in a “high” environmental risk level (252–450 times above the standard), necessitating interception and stabilization measures. In contrast, the high-concentration group (5–7%) dissolved weak acid-extractable Pb, breaching the mineral stability barrier, significantly enhancing activation efficiency and escalating the environmental risk to an “extremely high” level (1824–2017 times above the standard), posing a potential risk of regional ecological imbalance. Notably, in the later stages, the Pb concentration in the low-concentration group declined sharply, suggesting that the chemical inertness of residual Pb became a critical limiting factor. Meanwhile, the high-concentration group maintained a relatively high activation efficiency, indicating that part of the activation threshold of weak acid-extractable Pb had already been exceeded.
4. Variation in Pb Content in Soil
4.1. Types of Leaching Agents
The dynamic variations in soil Pb content under the application of three leaching agents—(NH4)2SO4, Al2(SO4)3, and MgSO4—are shown in Figure 7. All treatments exhibited a typical three-stage pattern: rapid increase, oscillatory adjustment, and final stabilization.
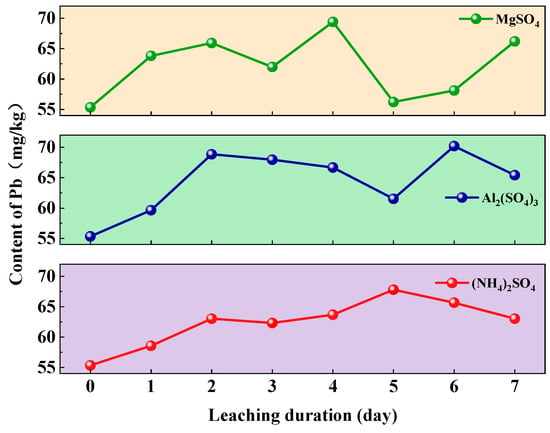
Figure 7.
Changes in soil Pb content under different types of leaching agents.
However, soil Pb pollution was most severe under Al2(SO4)3 leaching. The trends observed under (NH4)2SO4 and Al2(SO4)3 were highly similar, which can be attributed to their shared mechanisms of hydrolytic acidification, enhanced ion-exchange capacity, and mineral interactions. Specifically, hydrolysis led to soil acidification, increased ionic strength, efficient ion exchange involving NH4+ and Al3+, and chemical interactions between hydrolysis products and soil minerals. In contrast, MgSO4 exhibited the weakest driving effect on Pb release due to its limited hydrolysis and low ion-exchange capacity. The temporal profile of Pb release under MgSO4 leaching differed markedly from those of (NH4)2SO4 and Al2(SO4)3, due to chemical differences among the leaching agents and heterogeneous interaction mechanisms with soil [40]. Hydrolysis of (NH4)2SO4 and Al2(SO4)3 released H+, substantially lowering pH and enhancing Pb solubilization. MgSO4 exhibited minimal acidification and was less effective in dissolving Pb-bearing minerals, resulting in a significantly slower release rate.
A comparative analysis of the activation efficiency of three leaching agents—(NH4)2SO4, Al2(SO4)3, and MgSO4—on soil Pb was conducted, as shown in Table 4, revealing significant differences in dynamic behavior and activation intensity among the leaching agents.

Table 4.
Activation efficiency and dynamic variation characteristics.
Analysis reveals significant differences in the Pb activation mechanisms of the three sulfates: Al2(SO4)3 exhibits the highest initial activation efficiency, as its strong acidity rapidly dissolves carbonate- and oxide-bound Pb; however, in the later stages, the formation of Al(OH)3 colloids inhibits Pb release, causing notable fluctuations in concentration. (NH4)2SO4 achieves moderate activation efficiency via NH4+ ion exchange with controllable environmental risk [41]. MgSO4 gradually releases residual Pb through Mg2+ ion exchange and weak acid effects, with secondary activation occurring in later stages due to mineral structure degradation. Comparative analysis of the effects of the three leaching agents on Pb concentrations in leachate and soil indicates significant differences in their environmental risk impact and ecological compatibility, as summarized in Table 5.

Table 5.
Environmental risk and ecological compatibility.
The assessment indicates that Al2(SO4)3 exhibits the highest leaching efficiency but also poses the greatest environmental risk, necessitating the implementation of interception and passivation measures; MgSO4 causes minimal environmental disturbance but requires the establishment of a long-term dynamic monitoring system to mitigate secondary activation risks; comparatively, (NH4)2SO4 offers the best balance between overall effectiveness and risk controllability, resulting in a lower risk associated with Pb release.
4.2. Effects of Different Concentrations of Leaching Agents
The three-stage dynamic pattern of soil Pb content under leaching with varying concentrations of Al2(SO4)3 (1–7%) is illustrated in Figure 8. The stages include initial activation (release of reducible Pb via Al3+ ion exchange), mid-term fluctuations (competition between colloidal adsorption–desorption and mineral dissolution), and late-stage desorption (Pb release driven by colloid aging and common ion effects). This pattern is universally observed across all concentration gradients, indicating that variations in Al2(SO4)3 concentration regulate only the reaction rate without altering the fundamental pathway of Pb release.
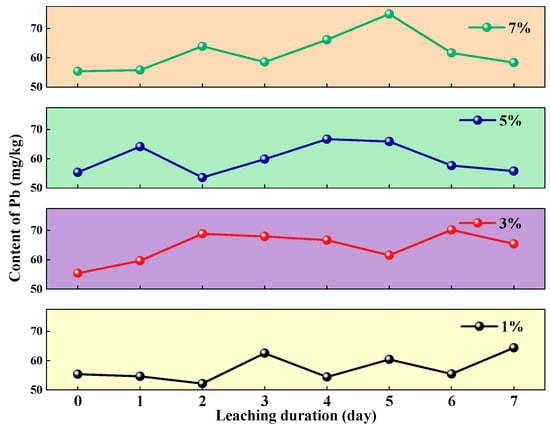
Figure 8.
Changes in soil heavy metal Pb content under different concentrations of Al2(SO4)3 leaching.
The high-risk periods for Pb release at different leaching concentrations are concentrated in the mid-to-late stages, during which the Pb release rate peaks (daily increase > 70%) accompanied by the maximum accumulation, necessitating focused control measures. Soil Pb accumulation increases with the concentration gradient of the leaching agent, reaching or exceeding the risk-screening value for agricultural land (70 mg/kg) demonstrating a significant dose–response relationship; the peak Pb content and corresponding risk levels in the soil are summarized in Table 6.

Table 6.
Peak concentration of soil heavy metal Pb and corresponding risk levels.
Studies indicate a dual effect of Al2(SO4)3 concentration gradients on Pb activation mechanisms: the low-concentration group (1–3%) activates Pb in oxide-bound forms via Al3+ ion exchange, yet initial release is significantly suppressed by colloidal adsorption, concentrating risks in the later stage (peak contents near or exceeding risk-screening values). This necessitates combined interception and passivation measures, with ecological risks remaining manageable, making it suitable for short-term or sensitive-area mining, albeit requiring dynamic monitoring. In contrast, the high concentration group (5–7%) induces direct dissolution of silicate minerals through strong acid hydrolysis, releasing residual Pb and surpassing Pb stability barriers. Concurrently, SO42− complexation enhances Pb mobility, resulting in a high-risk profile throughout the entire cycle (peak content reaching risk-screening values), with irreversible risks especially during the later desorption phase, potentially triggering regional ecological imbalance.
5. Analysis of Pb Chemical Speciation
5.1. Different Types of Leaching Agents
During ionic rare earth mining, cation exchange reactions induced by leaching solutions alter the chemical speciation of heavy metals, thereby driving their migration and release. Based on the BCR sequential extraction method, the environmental risk levels of heavy metal chemical forms are classified as follows:
- Acid-extractable fraction (F1): water-soluble/exchangeable form, exhibiting high mobility and bioavailability, capable of plant uptake and food chain contamination (high risk);
- Reducible fraction (F2): bound to iron and manganese oxides, stable under oxidizing conditions but released under anoxic conditions (conditional risk);
- Oxidizable fraction (F3): associated with organic matter and sulfides, released upon oxidative decomposition (potential secondary risk);
- Residual fraction (F4): fixed within mineral lattices, chemically inert, with low bioavailability and minimal environmental risk.
The mobility, bioavailability, environmental impact range, and impact intensity of heavy metals in each chemical fraction are summarized in Table 7. Analysis indicates that fractions F1, F2, and F3 exhibit significantly higher mobility and bioavailability compared to F4 (mobility coefficient > 1.8, bioavailability index ≥ 0.65). Their combined environmental effects (diffusion radius > 50 m, pollution equivalent ×3.2) are markedly greater than the residual fraction, thus they are defined as active components.

Table 7.
Characteristics of different chemical forms of heavy metals.
The Risk Assessment Code (RAC) is a morphology-based method for evaluating the environmental risk of heavy metals. It is founded on the principle that a higher proportion of acid-extractable fraction (F1) indicates greater environmental risk. The method uses the percentage of F1 in the total concentration as the risk index, with the classification as follows: <1% (no risk), 1–10% (low risk), 10–30% (moderate risk), 30–50% (high risk), and >50% (very high risk). Based on this framework, the effects of different leaching agents on the chemical speciation of Pb and associated environmental risks were compared. The distribution of Pb chemical species under different leaching agents is shown in Figure 9.
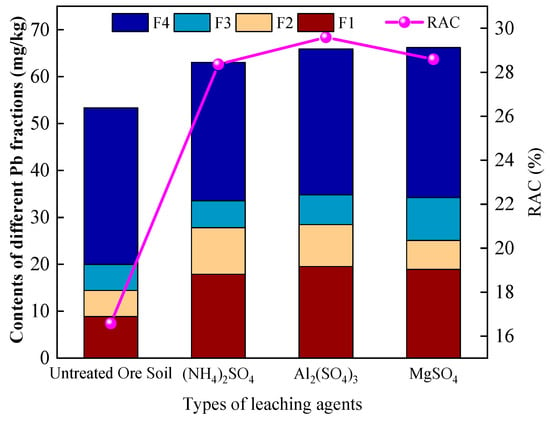
Figure 9.
Distribution of different chemical forms of Pb under various leaching agents.
Analysis indicated that Pb in the raw ore soil was primarily present in the residual fraction (F4), accounting for 62.6%, while the labile fractions (F1 + F2 + F3) made up 37.4%, with a corresponding RAC value of 16.6%, indicating moderate environmental risk. After leaching with (NH4)2SO4, the proportion of labile Pb increased to 53.2%, and the RAC value rose to 28.4%, approaching the threshold of high risk. Following Al2(SO4)3 leaching, the labile fraction reached 52.8%, slightly lower than that under (NH4)2SO4 treatment, while the RAC increased to 29.6%, indicating a stronger promotion of short-term Pb mobility [42]. In the MgSO4 leaching group, the labile fraction was 51.7%, with an RAC value of 28.6%, slightly lower than that observed with Al2(SO4)3. The significant activation of Pb under Al2(SO4)3 treatment was attributed to the strong acidification induced by Al3+ hydrolysis, resulting in the most pronounced increase in Pb mobility risk. MgSO4 facilitated Pb desorption through ionic competition with Mg2+, leading to a moderate increase in risk. In contrast, the limited complexation capacity of NH4+ in (NH4)2SO4 resulted in a comparatively lower disruption of stable Pb forms. These results suggest that the cationic characteristics of the leaching agents (Al3+/Mg2+/NH4+) are critical determinants in regulating the environmental behavior of heavy metals.
Leaching with all three extractants significantly increased the proportion of labile Pb, but differences in the Risk Assessment Code (RAC) values were observed. This discrepancy arises because the RAC is based solely on the proportion of the acid-extractable fraction (F1), reflecting immediate bioavailability, whereas the labile fraction also includes reducible (F2) and oxidizable (F3) forms, which indicate potential release under reductive or oxidative conditions. In the MgSO4 leaching treatment, the contents of F2 and F3 were relatively high, leading to an increase in the overall labile Pb proportion; however, the low F1 content resulted in a limited increase in RAC. By contrast, Al2(SO4)3 leaching produced the highest F1 proportion and the highest RAC value, indicating its greater capacity to activate rapid Pb mobilization. RAC and the labile fraction index reflect Pb environmental behavior from distinct perspectives; their combined application provides a more comprehensive evaluation of Pb mobility and ecological risk under different leaching conditions.
5.2. Effect of Leaching Agent Concentration
As shown in Figure 10, leaching with varying concentrations of Al2(SO4)3 markedly affected the speciation of Pb in soil. Under 1% Al2(SO4)3 treatment, the residual fraction (F4) decreased to 51.2%, and the RAC value reached 26.8%, indicating that Al2(SO4)3 promotes the transformation of stable Pb into more labile forms, thereby enhancing its mobility and bioavailability. When the concentration increased to 3%, the total proportion of labile Pb reached 52.8%, the residual fraction dropped to 47.2%, and the RAC value rose to 29.6%, the highest among all treatments, suggesting the strongest activation effect and a significant disruption of speciation equilibrium, posing the greatest environmental risk. Further increasing Al2(SO4)3 concentration to 5% and 7% led to a slight decrease in F1, fluctuations in F2 and F3, and a slower reduction in the residual fraction. The RAC values were 26.6% and 27.3%, respectively, slightly lower than that of the 3% treatment, indicating a stabilization of the activation effect and an approach toward dynamic equilibrium. Notably, under 7% Al2(SO4)3 treatment, neither the labile Pb proportion nor the RAC value showed further increases, suggesting that higher concentrations do not necessarily result in a linear enhancement of Pb activation.
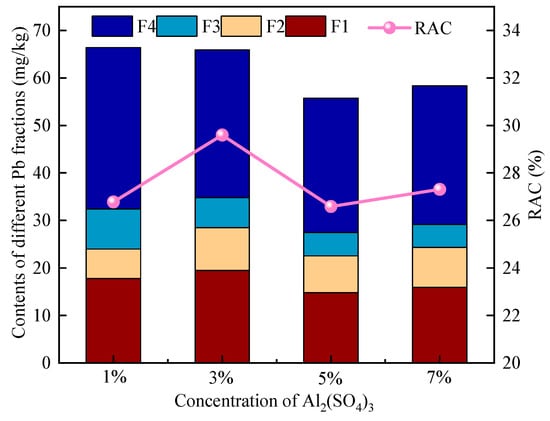
Figure 10.
Distribution of different chemical forms of Pb under different concentrations of Al2(SO4)3.
As shown in the figure, the F1 content follows the order: 3% > 1% > 7% > 5% > raw soil, further confirming that 3% is the optimal activation concentration, potentially due to enhanced Al3+ reactivity, improved Pb release kinetics, and synergistic interactions with soil components. Differences among other concentration groups are primarily attributed to changes in ionic strength affecting reaction equilibrium. The variation in residual Pb proportion follows the order: 3% > 7% > 5% > 1%, indicating that 3% leaching induces the most pronounced alteration in Pb speciation, with the highest RAC value and greatest environmental risk. The raw soil exhibited the highest proportion of residual Pb, followed by 1% > 5% > 7% > 3%, further suggesting that the 1% concentration had minimal impact, while 5% and 7% treatments demonstrated a gradual increase in risk. The concentration of Al2(SO4)3 exhibited a nonlinear response in terms of Pb speciation transformation and RAC values. The 3% treatment significantly increased both the proportion of labile Pb and the RAC value, indicating the highest ecological risk at this concentration.
6. Conclusions
(1) (NH4)2SO4, Al2(SO4)3, and MgSO4 all enhanced Pb release and mobilization. Pb under (NH4)2SO4 and Al2(SO4)3 exhibited comparable dynamics—gradual early release, mid-phase intensification, and late stabilization—concentrating risk in the final stage. In contrast, MgSO4 induced a “fluctuation–gradual–increase” trajectory with lower peaks, indicating relatively contained environmental risk.
(2) The release of Pb under different concentrations of Al2(SO4)3 exhibited a nonlinear response. Increasing concentrations markedly elevated Pb peak levels. At low concentrations (1% and 3%), Al2(SO4) primarily mobilized oxide-bound Pb, posing considerable drainage risks. At higher concentrations (5% and 7%), it further activated weak acid–extractable Pb, raising drainage risks to a critical level and potentially disrupting ecological balance.
(3) (NH4)2SO4, Al2(SO4)3, and MgSO4 showed distinct differences in Pb mobilization efficiency and environmental risks. Al2(SO4)3 was the most efficient, rapidly dissolving carbonate- and oxide-bound Pb, but carried the highest risk. MgSO4 was the least effective, inducing minimal disturbance and suitable for sensitive ecosystems, while (NH4)2SO4 demonstrated moderate efficiency and risk with favorable ecological compatibility.
(4) Under varying Al2(SO4)3 concentrations, Pb accumulation increased substantially with higher concentrations, in some cases exceeding agricultural risk-screening thresholds. At low concentrations (1% and 3%), Al2(SO4)3 mobilized oxide-bound Pb via Al3+ exchange with manageable ecological risks, whereas at higher concentrations (5% and 7%), it activated residual Pb through strong acid dissolution and complexation, markedly elevating risks and potentially causing irreversible ecological imbalance.
(5) (NH4)2SO4, Al2(SO4)3, and MgSO4 all facilitated the transformation of Pb from residual to more labile forms, thereby increasing its ecological mobility. Al2(SO4)3 exerted the strongest effect through acidification caused by Al3+ hydrolysis, resulting in the highest F1 fraction and RAC values. MgSO4 was less effective due to weaker ion-exchange capacity, whereas (NH4)2SO4 showed moderate activation. Although RAC captures the immediate risk associated with F1, integrating F2 and F3 as potential release fractions yields a more comprehensive evaluation of Pb environmental risk.
(6) Al2(SO4)3 concentration had a nonlinear effect on Pb speciation and RAC. The 3% treatment exhibited the strongest activation and mobility, with higher F1 and RAC values and a pronounced decline in the residual fraction, resulting in the greatest ecological risk. In contrast, although the 5% and 7% groups showed increased labile fractions, their RAC gains were limited and the system approached stabilization, suggesting that increasing concentration does not necessarily translate into proportionally greater risk.
Author Contributions
Conceptualization, Z.G.; methodology, Z.G.; software, S.X.; validation, S.X., F.L. and Q.L.; formal analysis, S.X.; investigation, F.L.; writing—original draft preparation, S.X., F.L. and Q.L.; writing—review and editing, Z.G., S.X. and J.Z.; visualization, F.L.; supervision, J.Z.; project administration, Z.G.; funding acquisition, Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (52364012), the Natural Science Foundation of Jiangxi Province, China (20224BAB214035), Key Laboratory of Ionic Rare Earth Resources and Environment, Ministry of Natural Resources of the People’s Re-public of China (2023IRERE403).
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, G.F.; Xu, J.; Ran, L.Y.; Zhu, R.L.; Ling, B.W.; Liang, X.L.; Kang, S.C.; Wang, Y.Y.; Wei, J.M.; Ma, L.Y.; et al. A green and efficient technology to recover rare earth elements from weathering crusts. Nat. Sustain. 2022, 6, 81–92. [Google Scholar] [CrossRef]
- Borst, M.A.; Smith, P.M.; Finch, A.A.; Mk, S.; Dv, S.A. Adsorption of rare earth elements in regolith-hosted clay deposits. Nat. Commun. 2020, 11, 4386. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.W.; Dong, J.S.; Wang, L.S.; Feng, Z.Y.; Xue, Q.N.; Meng, X.L. Selective recovery of rare earth elements from ion-adsorption rare earth element ores by stepwise extraction with HEH(EHP) and HDEHP. Green Chem. 2017, 19, 1345–1352. [Google Scholar] [CrossRef]
- Wang, G.F.; Zhu, J.X.; Liang, X.L.; Ling, B.W.; Xu, J.; Yang, Y.Q.; Kang, S.C.; Tan, W.; Xu, Y.J.; Zou, X.S.; et al. Industrial-scale sustainable rare earth mining enabled by electrokinetics. Nat. Sustain. 2025, 8, 182–189. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Zhou, J.R.; Zhou, K.F.; Jin, J.F.; Wang, X.J.; Zhao, K. Soil-water characteristics of weathered crust elution-deposited rare earth ores. Trans. Nonferr. Met. Soc. China 2021, 31, 1452–1464. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Lai, Y.M.; Jin, J.F.; Zhou, J.-R.; Zhao, K.; Sun, Z. Effect of particle size and grain composition on two-dimensional infiltration process of weathered crust elution-deposited rare earth ores. Trans. Nonferr. Met. Soc. China 2020, 30, 1647–1661. [Google Scholar] [CrossRef]
- He, Q.; Qiu, J.; Chen, J.F.; Zan, M.M.; Xiao, Y.F. Progress in green and efficient enrichment of rare earth from leaching liquor of ion adsorption type rare earth ores. J. Rare Earths 2022, 40, 353–364. [Google Scholar] [CrossRef]
- Luo, X.P.; Zhang, Y.B.; Zhou, H.P.; He, K.Z.; Luo, C.G.; Liu, Z.S.; Tang, X.K. Review on the Development and Utilization of Ionic Rare Earth Ore. Minerals 2022, 12, 554. [Google Scholar] [CrossRef]
- Tang, J.; Qiao, J.Y.; Xue, Q.; Liu, F.; Fan, X.; Liu, S.W.; Huang, Y.Y. Behavior and mechanism of different fraction lead leach with several typical sulfate lixiviants in the weathered crust elution-deposited rare earth ore. Environ. Sci. Pollut. Res. 2021, 28, 31885–31894. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.F.; Li, J.Q.; Huang, L.; Xu, J.F.; Xiao, Y.F. Strengthening rare earth and inhibiting aluminum leaching in magnesium salt-acetic acid compound system from ion-adsorption type rare earth ore. Sep. Purif. Technol. 2024, 334, 126070. [Google Scholar]
- Guo, Q.; Li, Z.; Pan, J.X.; Li, B.; Zhao, L.S.; Liu, D.P.; Zheng, X.D.; Wang, C.M. Leaching Mechanism of Aluminum during Column Leaching of Ion-Adsorption Rare Earth Ore Using Magnesium Sulfate. Minerals 2023, 13, 401. [Google Scholar] [CrossRef]
- Liu, Z.W.; Lu, C.B.; Yang, S.; Zeng, J.F.; Yin, S.Y. Release Characteristics of Manganese in Soil under Ion-absorbed Rare Earth Mining Conditions. Soil Sediment Contam. 2020, 29, 703–720. [Google Scholar]
- Zhang, Q.Y.; Ren, F.T.; Li, F.D.; Chen, G.L.; Yang, G.; Wang, J.Q.; Du, K.; Liu, S.B.; Li, Z. Ammonia Nitrogen Sources and Pollution along Soil Profiles in an In-Situ Leaching Rare Earth Ore. Environ. Pollut. 2020, 267, 115449. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Chen, L.K.; Liu, C.Y.; Qiu, L.R.; He, S. Pb speciation in rare earth minerals and use of entropy and fuzzy clustering methods to assess the migration capacity of Pb during mining activities. Ecotoxicol. Environ. Saf. 2018, 165, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Zhang, L.; Zhou, X.M. Resource and Environmentally Protective Mining Model of Ionic Rare Earth in Southern China. Rare Earths 2010, 31, 80–85. [Google Scholar]
- Zhang, J.; Hu, F.J.; Liu, Z.W.; Xu, C.Y.; Yang, X.Y.; Lin, Y.Q. Migration Patterns of Ammonium Nitrogen in Soil of Ionic Rare Earth Mining Areas. Rare Earths 2018, 39, 108–116. [Google Scholar]
- Chen, L.K.; Chen, H.X.; Jin, X.W.; Zhang, L.; Liu, J.H.; Liu, C.Y.; Xu, S.; Wu, K.X.; He, S.; Sun, T.; et al. Study and Prospect on Particle Size, Clay Minerals, Base Ion Migration and Heavy Metal Release in Ionic Rare Earth Deposits. Chin. J. Rare Earths 2022, 40, 194–215. [Google Scholar]
- Xu, Z.G.; Li, G.; Yang, H.F.; Sha, A.Y.; He, Z.Y.; Tang, Y.C.; Wu, M.; Qu, J. Development Review on Leaching Technology and Leaching Agents of Weathered Crust Elution-Deposited Rare Earth Ores. Minerals 2023, 13, 1223. [Google Scholar] [CrossRef]
- Hu, M.B.; Shao, Y.J.; Chen, G.L. Kinetics of Ion Exchange in Magnesium Sulfate Leaching of Rare Earths and Aluminum from Ionic Rare Earth Ores. Minerals 2025, 15, 290. [Google Scholar] [CrossRef]
- Tan, Q.H.; Zhao, Y.H.; Huang, L.; Wan, C.; Yang, Z.; Zhou, D. Influence of Ammonium Sulfate on the Release and Speciation Transformation of Heavy Metals in Ion-Adsorption Rare Earth Mining Area Soil. Rare Met. Sci. Eng. 2022, 13, 134–144. [Google Scholar]
- Guo, Z.Q.; Tang, T.; Luo, F.Y.; Liu, Q.Q.; Wang, H.X.; Feng, X.J. Study on Pollutant Release and Migration Induced by In-Situ Leaching Mining of Ion-Adsorption Rare Earth and Its Influencing Factors. Rare Met. Sci. Eng. 2025, 16, 306–315. [Google Scholar]
- Zerrari, N.; Rais, N.; Ijjaali, M. Distribution, Source and Contamination Level of REEs and Heavy Metals in Agricultural Soils of Fez-Upstream, Morocco. Soil Sediment Contam. 2023, 32, 1066–1094. [Google Scholar] [CrossRef]
- Liu, Z.W.; Tian, S.; Zhang, L.N.; Zhu, Y.C.; Zhang, J.; Zeng, J.F.; Mao, Q.; Ye, H.M. Risk Assessment and Source Appointment of Heavy Metal and Metalloid Pollution in Soil from a Typical Ion-Adsorption Rare Earth Tailing Abandoned for 15 Years. Eurasian Soil Sci. 2023, 57, 349–361. [Google Scholar] [CrossRef]
- Ma, Y.X.; Wen, M.L.; Liu, P.F.; Jiang, Y.X.; Zhang, X.H. Speciation Characteristics and Risk Assessment of Heavy Metals in Cultivated Soil in Pingshui Village, Zhaoping County, Hezhou City, Guangxi. Appl. Sci. 2024, 14, 11361. [Google Scholar] [CrossRef]
- Qiao, J.Y.; Tang, J.; Xue, Q. Study on Pb Release by Several New Lixiviants in Weathered Crust Elution-Deposited Rare Earth Ore Leaching Process: Behavior and Mechanism. Ecotoxicol. Environ. Saf. 2020, 190, 110138. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Liu, Z.W.; Mao, Q.; Ye, H.M.; Tian, C.S.; Zhu, Y.C.; Zhang, L.N. Leaching Characteristics and Environmental Impact of Heavy Metals in Tailings under Rainfall Conditions: A Case Study of an Ion-Adsorption Rare Earth Mining Area. Ecotoxicol. Environ. Saf. 2024, 281, 116642. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.G.; Yan, Z.L.; Shang, L.N.; Chen, J. Environmental Risk of Ion-Absorbed Rare Earth Ores: Concentration of Leaching Agent and Fractionation of Pb. Environ. Sci. Pollut. Res. 2024, 31, 6425–6436. [Google Scholar] [CrossRef]
- Cai, W.Q.; Zhou, D.; Qin, L.; Zhao, Y.H. Adsorption Behavior and Speciation Distribution of Magnesium Sulfate in Ionic Rare Earth Ores. Nonferr. Met. Sci. Eng. 2024, 15, 941–951. [Google Scholar]
- Yan, R.H.; Cui, H.M.; Shi, J.S.; Yan, N.F.; Weng, Y.Q.; Cheng, T.; Wang, W. A Novel Extraction Process for Efficient Recovery of Rare Earth Elements from the Leaching Liquor of Ion-Adsorption Type Rare Earth Ore Using Unsaponified N,N-Di(2-Ethylhexyl)-Diglycolamic Acid. Chem. Eng. Sci. 2025, 302, 120820. [Google Scholar]
- Zhang, J.; Zeng, J.F.; Tian, S.; Liu, Z.W. Ageing of Pb in Farmland Soil near an Ionic Rare Earth Mine. Eurasian Soil Sci. 2023, 56, 1172–1177. [Google Scholar] [CrossRef]
- Wang, H.Y.; Meng, S.J.; Zhou, W.Z.; Wang, G.F.; Chen, Z.B.; Chen, Z.L. Impact of Leaching Process for Ion-Adsorption Rare Earth Ore on the Characteristics of Topsoil and the Absorption of Rare Earth by Dicranopteris Pedata. Biogeochemistry 2025, 168, 1–16. [Google Scholar] [CrossRef]
- Tang, X.D.; Liu, C.P.L.; Liu, D.K.; Li, B.; Ma, C.Y.; Wei, Q.Q.; Zhao, Y. Sample Digestion Methods for Determining Heavy Metal Elements in Soil. Chem. Anal. Meterol. 2019, 28, 13–18. [Google Scholar]
- Forsyth, K.; Dia, A.; Marques, R.; Prudêncio, M.I.; Obregón Castro, C.; Diamantino, C.; Carvalho, E.; Pattier, M.; Davranche, M.; Pédrot, M. Relationship between the Distribution of Rare Earth Elements in Soil Pools with Plant Uptake: A Sequential Extraction Study. Plant Soil. 2024, 512, 1137–1152. [Google Scholar] [CrossRef]
- Mittermüller, M.; Saatz, J.; Daus, B. A Sequential Extraction Procedure to Evaluate the Mobilization Behavior of Rare Earth Elements in Soils and Tailings Materials. Chemosphere 2016, 147, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Guagliardi, I.; Ricca, N.; Cicchella, D. Comparative Evaluation of Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and X-Ray Fluorescence (XRF) Analysis Techniques for Screening Potentially Toxic Elements in Soil. Toxics 2025, 13, 314. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.M.; Islam, A.; Islam, A.R.M.T.; Pal, S.C.; Mahammad, S.; Alam, E. Assessment of Soil Heavy Metal Pollution and Associated Ecological Risk of Agriculture Dominated Mid-Channel Bars in a Subtropical River Basin. Sci. Rep. 2023, 13, 11104. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.S.; Liang, T.M.; Liu, Q.S.; Qiu, T.S.; Ai, G.H. Compound Leaching Behavior and Regularity of Ionic Rare Earth Ore. Powder Technol. 2018, 333, 106–114. [Google Scholar] [CrossRef]
- Liu, D.P.; Yin, W.Q.; Li, Z.; Pan, J.X.; Zhao, L.S.; Wang, C.M. Leaching of Rare Earths and Aluminum in Weathered Crust Elution-Deposited Rare Earth Ore Using Magnesium Sulfate: Effect of Aluminum Content in Leaching Agent Solution. J. Rare Earths 2025, 43, 191–198. [Google Scholar] [CrossRef]
- Long, Q.B.; Yan, H.S.; Wu, H.; Qiu, S.; Zhou, X.W.; Qiu, T.S. Influence Mechanism of Leaching Agent Anions on the Leaching of Aluminium Impurities in Ionic-Type Rare Earth Ores: A DFT Simulation Combined with Experimental Verification. Sep. Purif. Technol. 2025, 354, 128768. [Google Scholar] [CrossRef]
- He, Q.; Qiu, J.; Rao, M.; Xiao, Y.F. Leaching Behaviors of Calcium and Aluminum from an Ionic Type Rare Earth Ore Using MgSO4 as Leaching Agent. Minerals 2021, 11, 716. [Google Scholar] [CrossRef]
- Huang, S.X.; Li, Z.H.; Yu, J.X.; Feng, J.; Hou, H.B.; Ruan, C. Vertical Distribution and Occurrence State of the Residual Leaching Agent (Ammonium Sulfate) in the Weathered Crust Elution-Deposited Rare Earth Ore. J. Environ. Manag. 2021, 299, 113642. [Google Scholar] [CrossRef]
- He, Q.; Chen, J.F.; Gan, L.M.; Gao, M.L.; Zan, M.M.; Xiao, Y.F. Insight into Leaching of Rare Earth and Aluminum from Ion Adsorption Type Rare Earth Ore: Adsorption and Desorption. J. Rare Earths 2023, 41, 1398–1407. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).