Abstract
The article describes the paleobiogeographic history of the modern subfamilies so-called “crown deer” of the family Cervidae (Artiodactyla, Mammalia) in the world from the late Miocene to the late Pleistocene. The study overviews the taxonomic diversity and evolutionary radiation of Cervidae from all zoogeographic realms where this systematic group is present in the paleontological record. The evolutionary diversification of the fossil Cervidae is based on the estimations of species body masses that are regarded here as a proxy of occupied ecological niches. The study reveals two important evolutionary radiations of Cervidae during the late Miocene of Eurasia that gave the origin of the modern subfamilies Cervinae and Capreolinae. The evolutionary radiation of Capreolinae during the Pleistocene in South America shows a range of diversity comparable to the late Miocene radiations of Old World deer and provides multiple examples of evolutionary convergences with Eurasian Pleistocene cervids. The article discusses factors that shaped the modern biogeographic distribution of representatives of the subfamilies Cervinae and Capreolinae.
1. Introduction
The family Cervidae is the second most diversified systematic group of modern ruminants (after bovids) with a vast area of distribution ranging from Northwest Africa and Southeast Europe to the Southern Cone of South America [1]. Unlike most herbivores, cervids have evolved a particular eco-evolutionary strategy that made them flexible colonisers of new ecosystems. Such a particular ecological and evolutionary strategy is driven by the needs of cervid males for high quality and rich nutrients forage required for the high energetic expenses for early growth and antler shedding [1,2]. This is unique in the animal world, with the development of the fast-growing and regularly antler shedding representing one of the most characteristic features of the family Cervidae that attracts the attention of researchers from various domains of biology. These large annually growing and shed appendages are resource-demanding and are regarded as an evolutionary luxurious specialisation defining the complex mechanisms of social biology [1,2,3]. Therefore, the remarkable ecological flexibility of cervids is rather a consequence of physiological and biological constraints. This ecological opportunism, according to Geist [1], enabled cervids to have quick access to new ecological resources that are particularly rich in nutrients forage and allowed them to avoid direct competition with other systematic groups of herbivores with higher evolutionary specialisation and more conservative evolutionary strategy [1]. The rather uniform and, as a rule, little specialised craniodental morphology of cervids across the entire family is a consequence of their opportunistic ecological and evolutionary strategy. In most cases, the difference in body size is the only eco-physiological factor enabling the partitioning of ecological resources among sympatric deer species [4], since the larger body size increases the physiological tolerance of ruminants to the higher fibre content in forage and thus broadens the range of food resources [5]. In turn, the evolutionary diversity of cervid antlers is extremely high, reflecting the fine adaptations to diverse environmental conditions—from Arctic tundra to tropical forests—under the pressure of several factors defining the antler development: function of weapons in intra-specific combats or social interactions; defence against predators; thermoregulatory function during their period of growth; species-specific or even subspecies-specific visual communication [1,2,3,6]. A general Bauplan of antlers and their morphological details are traditionally regarded as the main taxonomic criterion for cervid systematics [7,8,9]. Such an approach to deer taxonomy is methodologically justified, since deer antlers are species and even subspecies-specific organs of communication during reproduction, thus ensuring the genetic isolation of cervid species in natural conditions [1,2]. However, the classification of cervids at a higher rank is based on the type of reduction in lateral metacarpals that reflects two different directions of adaptation to cursoriality and represents one of the most essential characteristics splitting the modern cervids into two subfamilies: Cervinae and Capreolinae. Representatives of the subfamily Cervinae have completely lost distal parts of their lateral (second and fifth) metacarpals but retain proximal vestiges of those bones (the plesiometacarpal condition). Deer of the subfamily Capreolinae are characterised by a different type of lateral metacarpals reduction, retaining only their distal portions (the telemetacarpal condition) [8,9]. The first detailed study of the relationship of the systematics of modern cervids with their zoogeography was published by Brooke [10] who recognised two different ways of lateral metacarpal reduction in modern deer and described their geographical distribution in the world. The work of Brooke [10] was thoroughly discussed and even criticised in subsequent publications [11], but it remains one of the most important systematic studies of the family Cervidae confirmed by modern systematic approaches [11,12,13,14].
The diversity and geographical distribution of modern cervids represent an important characteristic of modern zoogeographical realms. The systematic diversity and the degree of evolutionary specialisation of modern deer reflect faunal transformations in different biogeographic areas during the Plio–Pleistocene glacial–interglacial cycles [1]. Cervids are absent in the old ecosystems of the Afrotropical realm but are important in biogeographic areas affected by the glacial and interglacial cycles in the past, such as the Palearctic, Nearctic, Oriental, and Neotropical realms. The most complete information on the evolutionary diversity of cervids is provided by the paleontological record that contains information on dispersals, regional radiations, extinctions, and interactions with environmental conditions and other systematic groups. The views on global paleobiogeography of crown deer remain contradictory and do not provide an adequate interpretation of the fossil record. The old broadly accepted hypothesis assumes a link between the presumed primitive forerunners of modern plesiometacarpal deer (the subfamily Cervinae) and the late Miocene holometacarpal deer from Eastern Europe that maintain the ancestral type of limb morphology with complete lateral metacarpals and in turn are regarded as a transitional evolutionary link between primitive muntjacs and “true deer” [9,15,16]. This hypothesis does not explain however the disparity between the continent-scale distance between the supposed transitional evolutionary link and the high modern and past taxonomic diversity of the subfamily Cervinae in Southeast Asia. The origin of telemetacarpal deer (subfamily Capreolinae) remains obscure. The subfamily Capreolinae was hypothetically related by some authors to North American Dremotheriinae, thus attempting to explain the missing paleontological record of the early evolutionary radiation of the telemetacarpal deer in Eurasia [9,17]. The recently published taxonomical revision of the so-called pliocenvine deer redefined them as representatives of the archaic evolutionary radiation of the subfamily Capreolinae [18]. The new taxonomical and systematic model of the family Cervidae that revealed the holometacarpal stages of evolution of Cervinae and Capreolinae [18] requires a reconsideration of the paleobiogeography of this ruminant family, which has an almost global zoogeographic distribution: only sub-Saharan Africa and Australia remain outside the natural area of distribution of cervids [1].
The present study proposes an overview of the evolutionary radiations of the crown subfamilies of Cervidae (Cervinae and Capreolinae), their taxonomic and ecological diversity, dispersals, and possible factors that influenced their evolution from the late Miocene to the late Pleistocene and their modern zoogeographic distribution. The ultimate aim of this study is to reveal the factors that influenced cervid biodiversity and define the biogeographic distribution of modern representatives of the family Cervidae.
2. Materials and Methods
The concept of the study is largely based on the phylogenetic approach to biogeography proposed by Holt et al. [19]. The present paper proposes an adapted biogeographic division that reflects the evolutionary radiations and dispersals of cervids in the past: the western Palearctic, the Eastern Palearctic, the Sino-Malayan zoogeographic unit that corresponds to the Sino-Japanese realm and the eastern part of Oriental realm [19], and the western part of Oriental realm. The Nearctic and Neotropical realms are considered according to Holt et al. [19] (Figure 1). The present study includes only continental cervid species since insular endemic deer represents a particular case of cervid evolution. The exception is made only for some Pleistocene species of the Greater Sunda Islands as they were part of continental ecosystems during the Pleistocene sea-level lowstands. The list of species, their body mass estimations [20], and biogeographic and geochronological distributions are presented in Table S1 of the Supplementary Materials. The list of recognised fossil deer species and their biostratigraphic distribution is largely based on the regional studies of cervid faunas [16,17,21,22,23,24,25,26,27,28,29] (the complete bibliographic list is available in the Table S2).
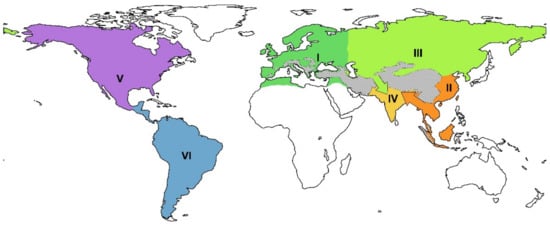
Figure 1.
Zoogeographic areas based on the evolutionary history of the crown deer: I, western Palearctic (early evolutionary radiation of the subfamily Capreolinae); II, Sino-Malayan area (early evolutionary radiation of the subfamily Cervinae); III, eastern Palearctic (dispersals of Capreolinae and Cervinae); IV, western Oriental (dispersals of the subfamily Cervinae); V, Nearctic (dispersals and local evolution of Capreolinae); VI, Neotropic (second evolutionary radiation of Capreolinae). The dashed area represents the Alpine-Himalayan Mountain Belt. The green and orange arrows mark the Siva-Bactrian and Siva-Malayan dispersal paths.
The predicted body mass is used as a proxy for evolutionary differentiation and the range of occupied ecological niches. This approach is based on the observation that sympatric deer species are always clearly distinguished by their body size. Body size difference often is the only morphological criterion that allows to establish the number of deer species in fossil faunas when antler remains are unavailable [7,8,15,21,25]. The body mass prediction is based on craniodental linear variables according to Janis [20]. Dental remains are most suitable for body size estimations as they show a weak sexual size dimorphism and usually are well-presented in the paleontological record. The most predicted body masses used in the present study are based on crown lengths of M2, M2, P4, M3, lower premolar and molar series [20]. The estimation of body masses of cervid species known only from antler remains and parts of frontals bones is based on pedicle and antler base diameters of fully grown antlers that allow to assign a cervid to a body size class of species with known body mass (Table S1). The data are treated in Jupyter Notebook (Anaconda3) (Table S3). The term “crown systematic group” is applied according to the definition proposed by Cirilli et al. [30]: the modern subfamilies of deer and their direct extinct forerunners are regarded in this study as crown cervids.
3. Description
3.1. Late Miocene Evolutionary Radiations of Deer
3.1.1. Early Evolutionary Radiation of Capreolinae
The compilation of the available taxonomic data reveals that the subfamily Capreolinae provided one of the most diversified evolutionary radiation of cervids during the late Miocene in western Eurasia (Table 1). In most cases, the paleontological record does not include evidence on the type of reduction in the lateral metacarpal bones. However, the abundant fossil material of Metadicrocerus variabilis (Cervavitus variabilis) from the late Miocene of Ukraine reveals the early stage of reduction in the proximal parts of second and fifth metacarpals, while their distal pulley-like articulations remain fully functional [18]. This morphological condition preludes the telemetacarpal condition seen in modern Capreolinae (Figure 2A–C).

Table 1.
Number of recognised crown deer species of the world from the late Miocene to the late Pleistocene of the considered zoogeographic areas.
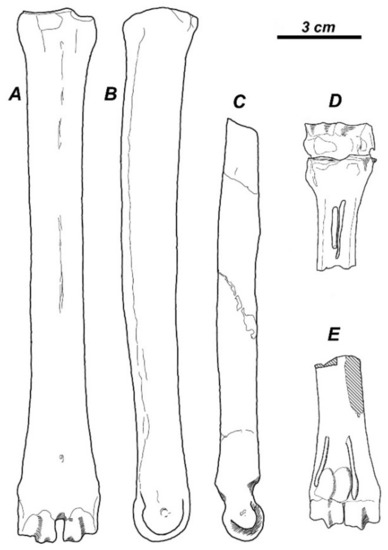
Figure 2.
Holometacarpal limbs in Cervidae: (A–C); Metadicrocerus variabilis from the late Miocene of Ukraine showing a strong development of distal articulation in lateral metacarpals: (A), dorsal vies of metacarpal III–IV; (B), side view of metacarpal III–IV; (C), side view of lateral metacarpal II [18]; (D,E), “Cervocerus novorossiae” from the Pliocene of Shansi with completely lost distal articulations of lateral metacarpals: (D), plantar view of proximal metacarpal III–IV with articulated carpal bones and proximal parts of lateral metacarpals II and V; E, plantar view of distal metacarpal III–IV with distal parts of metacarpals II and V [21].
The frontal bony ridges of Damacerus bessarabiae (Cervocerus novorossiae) regarded as evidence of transitional evolutionary position between “primitive” Muntiacus and modern Cervinae [17,22] are not homologous with the facial ridges of modern muntjacs [18]. Finally, the dentition of M. variabilis is characterised by a set of specific morphological features that distinguish it from Cervinae: rather high crowns of cheek teeth when compared to primitive Cervinae, the development of a protoconal fold in upper molars, advanced molarisation of lower fourth premolar combined with a relatively long lower premolar series, and presence of the Palaeomeryx fold in lower molars [18,31,32]. Most of these features are found in Capreolinae, with exception of the primitive morphology of P4 maintained in some late Miocene taxa and the Palaeomeryx fold that is lost in all modern Capreolinae.
The earliest representatives of the subfamily Capreolinae are reported from the late Miocene of Otovasca 1 (MN9, Moldova) and Răspopeni (MN10, Moldova) as Procapreolus sp. and Cervavitus sp., respectively [33]. A diversified group of primitive capreolines also comes from the late Miocene of the Iberian Peninsula [18]. This group of particularly archaic Capreolinae is represented by a small-sized muntjac-like deer (“Muntiacinae gen et sp. indet.”) from the late Miocene of Creventille-2 (Spain) [25]. The cervid from Creventille-2 was as large as modern barking deer (ca. 15 kg); however, it is characterised by relatively narrower and higher upper molars when compared to Asian muntjacs [34]. Deer of the genus Lucentia (L. iberica and L. pierensis) from the late Miocene of the Iberian Peninsula (MN11) are somewhat larger (27 and 53 kg correspondingly) but maintain the primitive two-pointed antlers with a very high position of bifurcation [25] (Figure 3A).
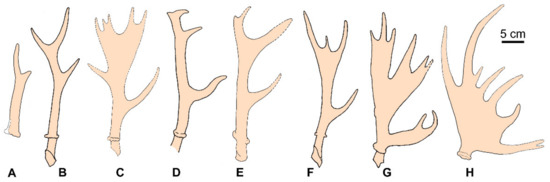
Figure 3.
Diversity of antlers of the late Miocene representatives of the subfamily Capreolinae: (A), Lucentia iberica [31]; (B), Procapreolus ucrainicus [22]; (C), Neomegaloceros gracilis [18]; (D), Pliocervus matheroni [18]; (E), Pavlodaria orlovi [32]; (F), Damacerus bessarabiae [18]; (G), Metadicrocerus variabilis [22]; (H), Cervodama pontoborealis [35].
The next stage of capreoline evolution characterised by three-pointed antlers is represented by the genus Procapreolus, which gave the most diversified evolutionary radiation among the early capreolines (nine species at least) and dispersed as far as the eastern regions of Eurasia [9,18,22,24,35]. The evolutionary radiation of Procapreolus is marked by the rather uniform shape of three-pointed lyre-shaped antlers and various combinations of advanced and primitive characteristics of dentition. Procapreolus maintained such an archaic feature as large upper canines [9,18]. The three-tined antlers with a high position of the first ramification and a distal more or less dichotomic fork represent (Figure 3B) the most common antler Bauplan of Capreolinae—maintained, for instance, by modern Capreolus. This is the initial stage for practically all known diversity of antler shape in telemetacarpal deer, including the Old World genus Alces and the American Capreolinae [18]. The transition from the three-pointed antler condition of Procapreolus to a more specialised antler shape is represented by Damacerus bessarabiae (the predicted body mass ca. 53 kg) from the late Miocene of Moldova that acquired an additional morphologically variable distal tine (Figure 3F). Despite the advanced specialisation of antlers that evolve a small distal palmation, D. bessarabiae maintains the primitive features of dentition, such as the unmolarised lower fourth premolar and the strong Palaeomeryx fold in lower molars. The next stage of evolution is represented by Metadicrocerus variabilis (= Cervavitus variabilis [17,22]), which maintained the basic three-pointed antler Bauplan but evolved multiple tine bifurcations of its palmated antlers (Figure 3G). It is necessary to mention, that a similar way of evolving multi-tined palmated antlers is assumed for Alces [2].
Neomegaloceros gracilis from the late Miocene of Ukraine [18,22] represents another direction of antler specialisation based on the three-pointed Procapreolus-like antler Bauplan: it evolved bifurcations and palmation-like flattening of the anterior branch of the distal fork (Figure 3C). Pliocervus matheroni from the Late Miocene of France evolved its four-tined antlers by further branching of the posterior tine of the distal fork (Figure 3D). Cervodama pontoborealis [35] from the Late Miocene—Early Pleistocene of Ukraine evolved the most specialised palmated antlers among the archaic capreolines that superficially remind antlers of modern Alces [17,22]. It is achieved by adding additional branches on the anterior side of the beam between the first and second initial ramifications (Figure 3H).
Despite the diversity of antler shapes and the progressive specialisation of the dentition, the ancient European capreolines show a certain evolutionary conservatism in body size, which varied in the rather limited range of 40 to 60 kg. Reports on the presence of Cervidae in the late Miocene of the Near East are more than scanty. The almost complete humerus of an “ancient deer” discovered in the Tortonian Bira Formation of the Jordan valley [36] belongs to a rather large ruminant similar in size to a modern red deer that does not fit the revealed pattern of the early evolutionary radiation of Capreolinae.
The well-documented late Miocene dispersals of archaic capreolines are recorded in the eastern Palearctic (Kazakhstan and Mongolia [23,32]) and north-east China [37]. Pavlodaria orlovi from the late Miocene of Gusinyi Pereliot (MN13, eastern Kazakhstan) is characterised by such a peculiar morphological feature as an ossified vomer that completely divides the nasal chamber, a specific characteristic that is seen in modern American Capreolinae [17]. The antler morphology of P. orlovi is very close to that of Pliocervus matheroni from western Europe, however, the dentition of the former species is characterised by the fully molarised lower fourth premolar [18].
The genus Procapreolus in Asia is represented by P. mongoliensis (Metacervulus mongoliensis [23]) from the Pliocene of Great Lakes Depression (Mongolia), by P. latifrons from the late Tertiary of north-eastern China [37], and rather small-sized P. jinensis (the predicted body mass is 22.5 kg) from the late Miocene of Yushe Formation [38].
The second archaic lineage of Capreolinae that reached the Sino-Malayan zone is represented by Cervavus ruetimeyeri Schlosser, 1903 (Procapreolus ruetimeyeri according to Schlosser [37]) from the late Miocene/early Pliocene of China. This small-sized deer (the predicted body mass is ca. 34 kg) is characterised by some morphological features relating it to Pliocervus and Pavlodaria, such as the subtriangular cross-section of the beam [18,32,37]. Schosser [37] ascribed to Cervavus ruetimeyeri large sabre-shaped upper canines. The cheek teeth of C. ruetimeyeri are similar to those of modern roe deer, with the exception of the relatively longer premolars [37].
One can assume that the origin of the puzzling cervid Platycemas infans Teilhard de Chardin & Trassaert, 1937 is related to Procapreolus [18]. P. infans is characterised by short antlers (the total length is about 4 cm) with a normally developed basal part of the antler corresponding to a deer of a body size similar to that of small modern roe deer [21]. The distal part of the antler is flattened and bears three small tines that show a Bauplan strongly resembling antlers of Procapreolus and Capreolus [18]. Perhaps, P. infans represents an evolutionary step toward the antler size reduction reminiscent of antler simplification and reduction in tropical capreolines from South America. The understanding of the evolution of P. infans may shed light on the origin of the antlerless deer of the genus Hydropotes.
Most of the archaic capreoline genera became extinct by the beginning of the Pliocene, with the exception of Procapreolus that survived in Europe until the late Pliocene [17,22,34] and, possibly, Croizetoceros that evolved the most complicated multi-tined antlers among Eurasian Capreolinae [39]. The only species of the genus, Croizetoceros ramosus, had a rather restricted Euro-Mediterranean distribution and persisted until the late early Pleistocene [8]. The assumption of Geist [1] that the modern Old World telemetacarpal deer represent a strongly depleted remnant of ancient evolutionary radiation is, therefore, supported by the late Miocene paleontological record of western Eurasia. The origin of a very limited number of highly specialised Old World Palearctic telemetacarpal deer that survived into the Pleistocene (the genera Alces, Capreolus, and Rangifer) is related to the eastern part of Eurasia [40,41]. The taxonomic diversity of Capreolinae in the Sino-Malayan zoogeographic area remains marginal and is represented by a single endemic capreoline genus, Hydropotes [1,24]. Most probably, the origin of Hydropotes is related to the episodical southward intrusion of a Capreolinae lineage triggered by the shifts of the biogeographic boundary between Palearctic and Oriental realms from north to south during the glacial and interglacial periods [42].
3.1.2. Early Evolutionary Radiation and Dispersals of Cervinae
Southeastern and Eastern Asia is often regarded as an area of highly diverse evolutionary radiation of plesiometacarpal deer (subfamily Cervinae) that remained almost intact in modern fauna [1,43]. This viewpoint is supported by the extremely rich fossil record. Nonetheless. The late Miocene evolutionary radiation of plesiometacarpal deer still requires a meticulous taxonomic revision and reappraisal of the enormous amount of data on Chinese late Miocene and Pliocene fossil deer [7,15,44,45]. The main question is related to the taxonomical status and systematic position of the Asian cervid remains traditionally ascribed to “Cervocerus” or “Cervavitus” [46]. Gustafson [47] examined the remains of “Cervocerus novorossiae” from the Zdansky collection in Uppsala including limb bones with complete lateral metacarpals and confirmed that the lateral metacarpals in Zdansky’s “Cervocerus” have lost contact with the carpal bones by reduction in their proximal ends; however, the metacarpal V appears to retain a contact (including an articular facet) with the posterior side of metacarpal IV, as it still does in the very reduced metacarpal V in modern Cervus [47]. According to Gustafson [47], this structure seems to foreshadow the metacarpal anatomy of Cervinae. The type of reduction in lateral metacarpals in Asian “Cervocerus novorossiae” [21] confirms this conclusion: the distal parts of the second and fifth metacarpals are much reduced (Figure 2D,E) and, unlike Metadicrocerus variabilis, have lost their distal articulations. The cluster analysis of craniodental and some postcranial characteristics provided by Zdansky [46] nested “Cervocerus novorossiae” from the late Tertiary of China within the modern plesiometacarpal deer [18].
Nonetheless, the situation is much more complicated since Zdansky [46] described under the species name “Cervocerus novorossiae” three different cervid forms: (i) a cervid with slender three-pointed antlers, convex frontal bones, and an angle of 70° between the pedicle and the parietal bone (Figure 4A); (ii) a cervid with robust three-pointed antlers, flattened frontal bones, and oblique position of the pedicles (the angle between the pedicle and the parietal bone is 55°) (Figure 4B); and (iii) a cervid with palmated four-tined antlers and an angle between the pedicle and the parietals of 70° [46] (pl. V). The taxonomy and systematic position of cervids described by Zdansky [46] as “Cervocerus novorossiae” is not resolved yet. This is the main reason why the so-called “pliocervines” from the Asian late Tertiary represent a rather eclectic and artificial group of several likely unrelated forms.
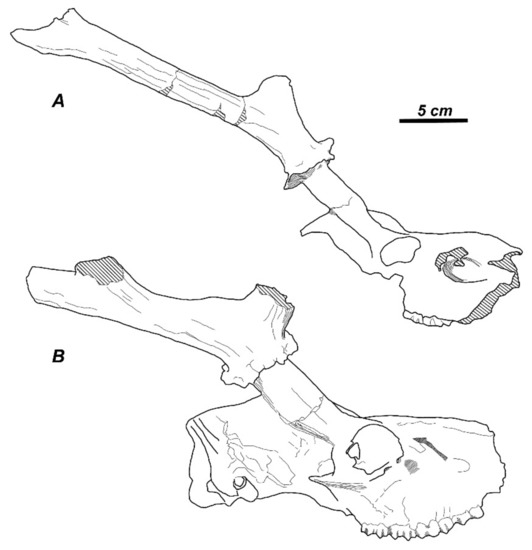
Figure 4.
Cranial remains of two cervid forms from the Hipparion fauna of Shansi ascribed by Zdansky [46] to “Cervoceros novorossiae”: (A), a small deer with slender antlers, convex frontals and parietals; (B), a large deer with flattened parietals and frontals, and robust antlers. Adapted from Zdansky [46].
Thus, the antler of Cervavitus demissus No. 12.705 [21] (Figure 18A) resembles Zdansky’s Eostyloceros [46]. The second antler ascribed to C. demissus (No. 22.957 [21]) is different and may belong to Capreolinae as it is characterised by a very high position of the first ramification.
The antlered frontlet from the late Miocene of the Lantian Formation, China [48] (Figure 1G) described as Cervavitus novorossiae belongs to Procapreolus. The fine antlered skull from Yushe Basin reported by Wang and Zhang [48] (Figure 3C1) as C. novorossiae shanxius Dong and Hu, 1994 is another cervid similar to Procapreolus, but its antlers are relatively short and robust as is in Capreolus. The second skull from Yushe Basin figured by Wang and Zhang [48] (Figure 3C2) belongs to a comparatively large cervid that is not identical to the type material of the rather small-sized C. novorossiae shanxius from Hounao (Yushe, Shanxi) [49]. “Cervavitus shanxius” is a genuine distinct species characterised by a more robust antler segment below the first ramification when compared to antler remains ascribed by Zdansky [46] to “Cervocerus novorossiae”, with a relatively lower position of the first ramification, and a rather thin antler beam above the first ramification [49] (Table S3, Figure 1, Figure 2, Figure 3 and Figure 4).
Another fine antlered frontlet from the Lantian Formation reported as C. novorossiae [48] (Figure 1J) is characterised by the comparatively low position of a very long basal tine and a distal fork formed by the stronger and longer posteromedial tine and the shorter anterolateral tine. The Lantian specimen is similar to “Cervavitus” flerowi Aubekerova, 1974 from the Pliocene of Esekartan (Kazakhstan) [50], but it differs in the somewhat lower position of the basal tine. Both cervid forms are regarded here as the representatives of the subfamily Cervinae. The dental remains from the Lantian Formation [48] belong to a rather large deer (ca. 115 kg), while the dental remains ascribed by Teilhard de Chardin and Trassaert [21] to “C. novorossiae” belong to a quite small cervid (ca. 36.4 kg). Therefore, an accurate revision of the above-mentioned material is needed.
“Cervavitus” fenqii from the early Pliocene of Dayakou [50] (Figure 3H) resembles the modern Axis axis but is distinguished by the small body size and the rather straight antler beam. “Cervocerus” ultimus is believed to be the last primitive holometacarpal deer similar to “Cervocerus novorossiae” that survived into the early Pleistocene of Southern China [51,52], However, Chen [53] considers that “C.” ultimus is related to modern cervids.
The late Miocene—early Pliocene paleontological record of Eastern Asia has also yielded archaic stem deer that represent a peculiar zoogeographic holdover of the Sino-Malayan zoogeographic zone of that period. The analysis of the inner bony ear of Eostyloceros—a genus containing several small-sized cervid forms with simple two-pointed antlers [17,23,24,46,53,54]—suggest it belongs to an archaic stem deer lineage [55].
The small-sized muntjac-like cervines with simple two-pointed antlers are exceptionally diverse in the fossil record of Eastern Asia and are represented by several genera [21,23,24], including the modern genus Muntiacus that appears for the first time in the late Miocene of Yuanmou (China) [56]. Muntiacus gave a rich diversity of species with body masses ranging from 10–11 kg (M. nanus and M. zhaotongensis [54]) to 40.5 kg (M. gigas [57]). However, as all small-sized tropical forest dwellers, all Muntiacus species were characterised by very low vagility and never dispersed from the Oriental zoogeographic realm. Unlike the archaic representatives of the subfamily Capreolinae, the late Miocene Cervinae has a rather low dispersal capacity (Figure 5).
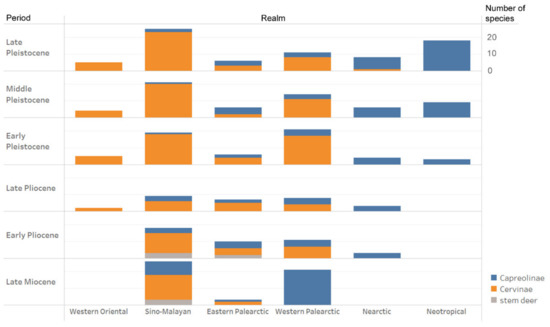
Figure 5.
Biogeographic and geochronological distribution of fossil crown Cervidae in the world. The bar height corresponds to a number of species.
Late Miocene cervines with a relatively high dispersal capacity are rather exceptional. This is the case for Praesinomegaceros venustus (ca. 105 kg) from the late Miocene of Taralyk-Cher (Tuva, Russia) [27]. In addition, “Cervid indet. cf. Cervavitus novorossiae” reported by Sen et al. [58] from the late Miocene of Taghar (Afghanistan) should also be included, a taxon that shows a great similarity to “Axis speciosus" from the late Tertiary of China [18].
3.2. Plio–Pleistocene Dispersal and Evolutionary Diversifications of Old World Deer
3.2.1. Dispersals of Cervinae into the Indian Subcontinent
The Alpine–Himalayan mountain belt acts as an insurmountable zoogeographic obstacle for cervids during most of their evolutionary history [59]. The paleontological record shows that Cervidae failed to disperse to Southern Asia until Pliocene.
The Pliocene is a turning point in the cervid evolutionary history as their geographical distribution and taxonomical diversity become similar to modern cervid biogeography (Figure 3). Most modern genera of Cervinae are already present in the Pliocene of the Sino-Malayan area [43]. The representatives of the family Cervinae almost simultaneously dispersed into the Indian subcontinent and reached western Eurasia [60,61].
The Pliocene and early Pleistocene dispersals of Cervinae into the Indian subcontinent (Figure 5) took place from Central Asia via the Siva-Bactrian dispersal route (Figure 1). Praesinomegaceros bakri is the earliest cervid species recorded in the Pliocene of Siwalik Hills [61]. This is a direct descent of Praesinomegaceros venustus from the late Miocene of Central Asia that acquired simplified, relatively short antlers similar to those of modern hog deer and represents the relic phylogenetic lineage that survived in the Indian subcontinent [62]. Other cervid genera recorded in the Pliocene—early Pleistocene of the Siwalik Hills are Rucervus, Metacervocerus, and Panolia. The evolutionary diversification of Panolia from the rest of the Cervus-Rusa evolutionary stock is a consequence of the dispersal of the forerunner of Panolia into the Indian subcontinent and its further evolution in isolation [61]. The taxonomical distinctiveness of cervids from the Indian subcontinent from the eastern part of the Oriental realm lasted until the middle Pleistocene when the endemic Praesinomegaceros became extinct and Muntiacus and Hyelaphus dispersed from the eastern Oriental realm via the Siva-Malayan dispersal path that emerged during the global glaciation sea level drop [60,62].
3.2.2. Early Dispersals of Cervinae to Western Eurasia
After the late Miocene extinctions of capreoline deer, the early Pliocene European faunas were replenished by several species of the subfamily Cervinae, among which we find the representatives of Metacervocerus and Rucervus [29,34]. Unlike their counterparts from the Indian subcontinent, European Metacervocerus and Rucervus evolved more advanced evolutionary specialisations [63,64]. Most of the Pliocene plesiometacarpal deer of Europe may be classified as medium-sized deer (60–120 kg [29]). Possibly, the body mass of 60 kg is an important ecological threshold that increases the dispersal capacity in Cervinae [60]. However, there are two European Pliocene Cervinae deer that contradict this hypothesis. Praemuntiacus pidoplitschkoi (Eostyloceros pidoplitschkoi) from the early Pliocene of Central and Southeastern Europe is characterised by small two-tined antlers and its body size corresponds to the size of modern Muntiacus. P. pidoplitschkoi is found in the forest paleocommunities near mountains and river valleys. However, unlike primitive muntjac-like deer, P. pidoplitschkoi exhibits advanced features such as short pedicles and relatively broad frontal bones [34]. Thus, it may represent a quite specialized deer species that possibly evolved secondary adaptations to forest habitats
“Cervus” ruscinensis Deperet, 1890 from the early Pliocene of France is the second small-sized deer that attained the body size of modern hog deer [65]. The systematic position of this cervid remained unclear for more than a century. Recently, Mennecart et al. [55], demonstrated that the bony labyrinth morphology of “Cervus” ruscinensis defines it as an early offshoot of the subfamily Cervinae. Antlers of the Ruscinian deer are rather simple and consist of the main beam and a small vestige of anterior (basal) tine. Nonetheless, the cranial morphology of this cervid reveals extremely advanced specialisations. “C.” ruscinensis is remarkable by a combination of such a primitive characteristic as a strong caudal direction of pedicles and a short orbitofrontal portion of the splanchnocranium with a set of advanced characteristics such as short pedicles, the rather broad frontal bones (52.2% of the skull length measured from P2 to occipital condyles), and the particularly broad occiput [65]. The relatively broad frontal bones may be indicative of the secondary body size reduction. The dwarfed insular Praemegaceros cazioti from the ate Pleistocene of Corsica and Sardinia is a good example of a cervid with reduced body size that maintains particularly broad frontals in males (59.4–63.7%, M = 61.6%, n = 4) [66]. The relatively broad frontal bones in P. pidoplitschkoi may also suggest its body size reduction after dispersal into western Eurasia.
3.2.3. Pleistocene Diversification of Palearctic Cervinae
The early Pleistocene of Eurasia is marked by an explosive diversification and speciation of the subfamily Cervinae that became dominant in the faunas of the continent (Figure 5). The acceleration of the process of evolutionary and eco-morphological diversification of Cervinae is first recorded in the late Pliocene when plesiometacarpal deer dispersed almost over the entirety Eurasia, as is indirectly illustrated by the progressively increased species body mass range (Figure 6). The peak of speciation and diversity of eco-morphological forms of Cervinae is recorded during the early Pleistocene. This evolutionary diversification is characterised by an extreme diversity of antlers [6,7,8,21,27] and numerous dispersal of rather short-living species from the Sino-Malayan area to the western Palearctic [29]. Among there are several middle Pleistocene species of the genus Praemegaceros (P. verticornis, P. dawkinsi, P. solilhacus), Praedama giulii (= Eucladoceros giulii) which is only known from a very short period of the final stage of the early Pleistocene of Europe, Cervus nestii from the early Pleistocene of Upper Valdarno and Dmanisi, and Sinomegaceros insolitus (= Arvernoceros insolitus) known only from the early Pleistocene fauna of Dmanisi, Georgia [29]. This intensive speciation of deer in the Sino-Malayan area was triggered by the glacial–interglacial cycles that caused the repeated northward and southward shifts of the zoogeographic border between the Oriental realm and the Palearctic [42]. This is one of the reasons why it is difficult to apply the definitions of modern zoogeographic realms to southeastern and eastern Asia from a paleontological perspective. During the interglacial periods, tropical cervids of the eastern part of the Oriental realm dispersed northward. The glacial periods caused a retraction of areas of distribution of tropical species [42]; however, some remnant cervid populations survived in “zoogeographic pockets” where they were influenced by the climate deterioration to a lesser degree, and thus allowing the evolutionary transformation of isolated relic populations. This is the most plausible mechanism of the explosive cervid speciation in eastern Asia that is supported by the fossil evidence: most Pleistocene Cervinae from western Palearctic are presented in the paleontological record of eastern Asia [17,27,29]. Most of the new Pleistocene species were rather large or even giant forms (Sinomegaceros, Praemegaceros, Praedama, Megaloceros) showing various degrees of adaptations to open continental or periglacial landscapes [1,6,7,27,29]. As a consequence of the multiple successive westward dispersals of cervines in combination with glacial pulses, the highly specialised endemic species and genera—often rather small-sized—evolved in the climate refuge of the western Palearctic: Rucervus radulescui from the early Pleistocene of Romania [63]; Praemegaceros dawkinsi from the middle Pleistocene of England [7,29]; Praedama matritensis [67] and Haploidoceros mediterraneus [68] from the late Pleistocene of Spain. As a rule, the Pleistocene deer from the western Palearctic are evolutionary more advanced and specialised than their East Asian counterparts [29]. The genus Dama seems to be an exception since it is only known to be from the western Palearctic. The evolutionary specialisation of the cranial morphology of fallow deer is one of most advanced among plesiometacarpal deer [29]. The origin of Dama is possibly related to the Pliocene westward dispersal of Asian Metacervocerus [64] and therefore confirms the statement on higher evolutionary specialisations of Cervinae from the western Palearctic.
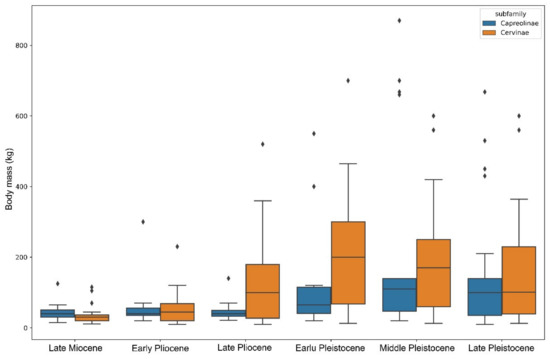
Figure 6.
Range of deer species body masses from the late Miocene to the late Pleistocene from all zoogeographic realms. Outliers (exceptionally large species) are represented by black diamonds.
Among important biogeographic barriers for Cervinae that likely restricted the dispersals of the subfamily are arid areas, ecological incompatibility with highly specialised tropical bovids (the main reason why cervids failed to disperse into the old mature ecosystems of Africa [1]), and high latitudes of Eurasia where few specialised capreolines (Alces and Rangifer) remained dominant. This may also explain why only a single plesiometacarpal deer, Cervus canadensis, dispersed to North America by the end of the last glaciation [69]. Although Cervinae (unlike Capreolinae) are known as good island colonisers [70], sea-straight with strong currents, such as the Strait of Gibraltar [71] and the Lombok Strait (part of the Wallace biogeographic line) [72,73] remained insurmountable biogeographic barriers for them. The only two known cervid species from the late Pleistocene of North Africa, Megaceroides algericus and Cervus elaphus, most probably, dispersed via the south-east coast of the Mediterranean Sea, the so-called “Libyan–Egyptian” way [71].
3.3. New World Dispersals and the Second Radiation of Capreolinae
3.3.1. Dispersals and Diversity of Capreolinae in Nearctic
The taxonomic diversity of Cervidae in the Plio–Pleistocene of North America is rather low (Table 1, Figure 5). Three already well-specialised cervid genera (Eocoileus, Bretzia, and Odocoileus) appeared in the Early Pliocene fossil record of North America almost simultaneously, a fact that suggests three independent dispersal events of Capreolinae from Eurasia during the early Pliocene [16]. The origin of Odocoileus possibly is related to Paracervulus capreolinus from the late Tertiary of China that already shows particular Odocoileus antler features: a small first tine shifted somewhat medially of the antler beam, and a posterior insertion of the distal branch (Figure 7).
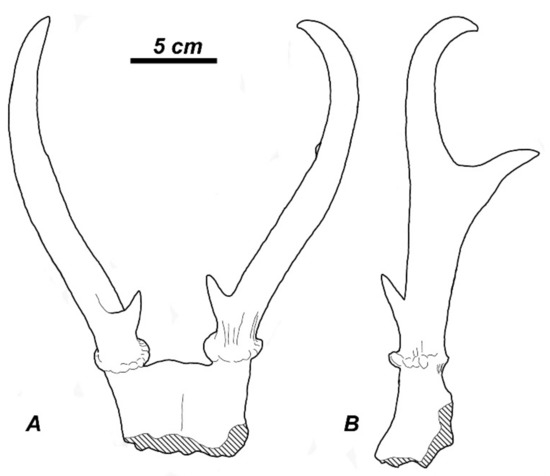
Figure 7.
Metacervulus capreolinus from the late Tertiary of China (holotype), a possible forerunner of the genus Odocoileus: (A), frontal view; (B), lateral view of left antler (adapted from Teilhard de Chardin and Trassaert [21]).
Eocoileus gentriorum (ca. 40 kg) from the early Pliocene of Florida is the least specialised deer of the New World that in many respects (three-pointed simple antlers, their position on skull, dental morphology) is reminiscent of the Old World genus Procapreolus [16]. The presence of Capreolus constantini in the Pliocene of Hidalgo (Central Mexico) reported by Jiménez-Hidalgo and Bravo-Cuevas [74] is questionable. The dental remains of a small deer from Hidalgo ascribed to C. constantini fits the size of E. gentriorum, while the arguments for the inclusion of the deer under discussion into the genus Capreolus remain unclear. Most probably, the remains of the small-sized Pliocene deer from Mexico belong to Eocoileus. Odocoileus and Bretzia are two long-lived and specialised taxa that were present on the North American mainland for more than three million years [16,47]. The medium-sized (ca. 50 kg) Bretzia with palmated antlers became extinct during the late Pleistocene, while Odocoileus is represented by two modern species, O. virginianus and O. hemionus [16,47,75]. The longevity of the North American cervid genera is comparable to that of Rucervus and Panolia from the Indian subcontinent, while the low evolutionary diversification of the North American deer reminds the evolutionary stasis of cervids from the Plio–Pleistocene of the western Oriental realm. One can assume that the New World cervids soon after their dispersal to North America achieved in a relatively short time the evolutionary stasis similar to the phenomenon described by Sondaar and Van der Geer [76] for insular cervids. Possibly, this curious biogeographic phenomenon on a large continental scale was caused by the relative biogeographic isolation of the Plio–Pleistocene cervids of the North American mainland which prevented new dispersals of cervids from Eurasia. The ecological opportunism and flexibility permitted North American cervids to overcome climate fluctuations without any significant evolutionary modifications. This is a very different biogeographic condition from what we see in the Palearctic during the Plio–Pleistocene when each new glacial–interglacial cycle triggered diversifications of cervid forms at the edge of the eastern part of the Oriental realm, their dispersals over the Palearctic, and subsequent competition with populations of older indigenous species that were already negatively affected by the climate perturbations.
The Pleistocene paleontological record of North America yielded remains of the highly specialised capreoline genera Rangifer, Cervalces, and Alces [16]. These dispersals of new genera from the Eurasian continent did not perturb the existence of the old North American capreolines since they did not compete for the same ecological resources. Torontoceros hypogaeus Churcher and Peterson, 1982 from the late Pleistocene of Canada shows some features (robust and short pedicles, smooth antler surface, and high position of second tine [77]) that relate it to forest caribou Rangifer tarandus. The strong divergence of the antler beams of Torontoceros hypogaeus may be interpreted as an adaptation to the open landscape. Torontoceros is regarded here as a possible junior synonym of Rangifer.
3.3.2. Neotropical Radiation of Capreolinae
During most of their evolutionary history, Eurasian Capreolinae maintained, with very few exceptions, a rather conservative range of species body mass compared to Cervinae (Figure 6). This discrepancy in eco-evolutionary diversification became very important from the late Pliocene onwards. The increase in the species’ body size range in Capreolinae recorded during the Pleistocene (Figure 6) marks their dispersals, evolutionary radiation, and ecological diversification in South America.
The evolutionary diversification of Capreolinae in South America represents an exceptional case of colonisation of an entire continent by cervids in the absence of large competing herbivores [16]. The cervid evolutionary radiation in South America had rather an exponential characteristic (Table 1, Figure 5) and produced more genera than in the Nearctic realm in less time: the dispersal of cervids into South America took place during the early Pleistocene (ca. 2.4 Ma) when the Panama Land Bridge emerged [16]. In some respects, Pleistocene South America could be seen as a miniature model of cervid evolution in Eurasia. As in Eurasia, there is a centre of evolutionary radiation of cervids in the tropical latitudes, as well as an evolutionary diversification zone at the border of the Neotropical realm and the Paleo-Patagonian realm [78], which was influenced by the Patagonian ice sheet that covered the southern part of the Andes [79,80]. The ecological diversification of the Neotropical radiation of Capreolinae shows great similarity with the late Miocene radiation of Cervinae: in both cases, the species with a body mass around 20 kg are the most numerous (Figure 8). Most probably, the paleontological data of very small deer remain incomplete, since only one finding of Pudu sp. was recently discovered in the late Pleistocene of Chile [81]. The radiation of the late Miocene Capreolinae in western Eurasia evolved in drier conditions, forcing a more advanced dentition morphology and a larger body size (Figure 8B) [18].
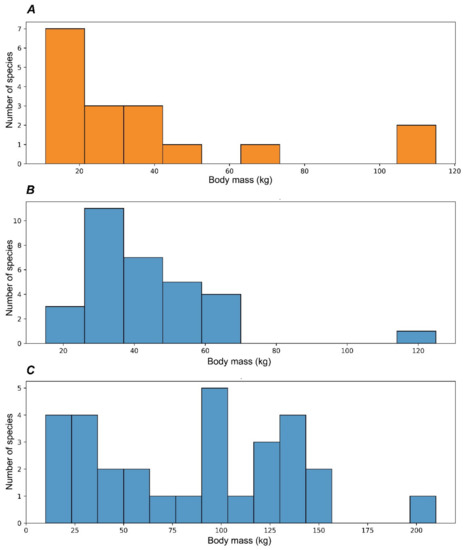
Figure 8.
Distribution of deer species body mass within evolutionary radiations: (A), the late Miocene radiation of the subfamily Cervinae in Eurasia; (B), the late Miocene radiation of the subfamily Capreolinae in Eurasia; (C), the Pleistocene radiation of the subfamily Capreolinae in South America.
The fossilised South American deer with a body mass above 75 kg represent a different evolutionary phenomenon: adaptations to diverse ecological niches in the Paleo-Patagonian realm, more or less corresponding to the evolutionary diversification of cervids in the Palearctic. Unlike Eurasian Capreolinae, South American Pleistocene deer developed a great diversity of antlers that in some cases represent astonishing instances of convergence with the Pleistocene Old World deer.
The genus Antifer from the late Pliocene to late Pleistocene of the Pampean region and southern Brazil is represented by several rather large species with multi-tined flattened antlers [26,28] that strikingly resemble the antler shape of Cervodama pontoborealis. According to Alcaraz and Zurita [82] and Villavicencio Figueroa [28], the genus Antifer was adapted to cold open and rather dry habitats and therefore represents an ecological analogue of the Eurasian large-sized deer. Antifer crassus described by Rusconi [83] from the Pleistocene of Argentina was the largest deer that ever existed in South America, an ecological analogue of the Eurasian giant deer. The isolated tooth of a large-sized deer (the estimated body mass attained ca. 210 kg) from the late Pleistocene of Pesqueira (Brazil) described by Rotti et al. [84] as “Cervidae indet.”, most probably, belongs to A. crassus.
Morenelaphus brachyceros from the middle and late Pleistocene of the southern part of South America represents an even more striking example of evolutionary convergence with Eurasian Cervinae. M. brachyceros was one of the largest cervid species of South America that attained a body size comparable to the West European red deer (ca. 120 kg) and evolved complicated multi-tined antlers of 70 cm in length [83,85]. The juxtaposition of antlers of Morenelaphus and those of European Cervus elaphus reveals the development of similar functional structures: the bifurcated large basal tine in Morenelaphus is an eye-protecting functional analogy of the brow and bez tines in red deer; the middle tine in Morenelaphus is a functional analogue of trez-tine (Figure 9). As red deer, M. brachyceros evolved distal crown tines.
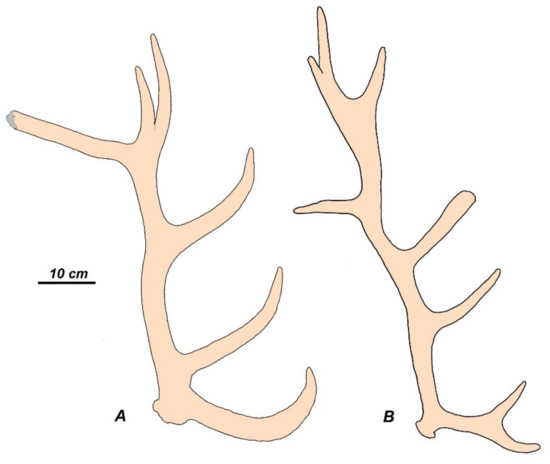
Figure 9.
Example of convergent evolution of antlers of Pleistocene deer from western Europe (subfamily Cervinae) and South America (subfamily Capreolinae): (A), Cervus elaphus angulatus from Steinheim, Germany (Nr. 16201, State Museum of Natural History, Stuttgart); (B), Morenelaphus brachyceros from the Pleistocene of Tapalquen, Argentina (adapted from Carette [22]).
Epieuryceros proximus is another specialised South American cervid showing a certain convergence with European Praedama savini. This middle-sized deer evolved rather large antlers with a flattened basal tine and a middle tine [26]. Alcaraz and Zurita [81] argued that E. proximus was adapted to open shrub environments and a rather moist climate.
Epieuryceros cf. proximus described by Alcaraz and Zurita [81] from the middle and late Pleistocene of Argentina is represented by antlers that show a superficial similarity with Praemegaceros dawkinsi from the British middle Pleistocene: the distal portion of the antler is flattened and seemingly is extended into a palmation, while the proximal tine is present as a small vestige. Paraceros fragilis from the late Pleistocene of northeastern Argentina and Uruguay represents a particular eco-morphological type of a small-sized cervid with lyre-shaped four-pointed antlers [28,85] reminiscent of “Dama-like” deer from the early Pleistocene of western Eurasia.
Some Pleistocene deer of South America show unusual mountain eco-morphological adaptations that have no analogues in the Old World. These mountain forms of cervids are Agalmaceros blicki (ca. 77 kg) from the late Pleistocene of Ecuador [86] and Charitoceros tarijensis from the Pleistocene of Bolivia and Peru that attained body mass of up to 90–100 kg [28].
4. Discussion
The representatives of Capreolinae and Cervinae often follow different evolutionary ways to achieve similar morpho-functional adaptations. The type of reduction in lateral metacarpals is the best-known example [10]. The evolution of dentition is another example of the evolutionary divergence between Cervinae and Capreolinae. The increase in the cheek teeth grinding surface as an adaptation to relatively coarse forage in the subfamily Cervinae and is achieved through the molarisation of the fourth lower premolar in the most specialised taxa (Dama, Megaloceros, etc.), while the second and third premolars always remain primitive and maintain their initial shearing function [9,29]. However, the increase in grinding surface in cervines most often is achieved through the relative increase in molar series length and the relative shortening of premolars [9]. This morpho-functional feature evolved in parallel practically in all genera of the subfamily Cervinae regardless of the type of molarisation of the lower fourth premolar [9,29,60,66,68]. Capreolines are characterized by relatively invariable long and large premolars and in most cases achieve a very high degree of molarisation of the fourth lower premolar [29]. In the most advanced forms (Alces, Rangifer), the third lower premolar is also molarised [9]. The main specialisation features of dentition evolved by the telemetacarpal deer in the late Miocene [18]. Cervinae and Capreolinae are distinguished by the different initial antler Bauplan and represent two different ways of antler branching development: the dichotomous branching in Capreolinae and the tree-like branching of antlers in Cervinae [11,18]. Unlike Cervinae, the antlers of capreolines (especially Old World Capreolinae) generally are more conservative from an evolutionary point of view and often maintain the basic three-tined Bauplan that may be recognized even in advanced species with large, complicated antlers such as moose and reindeer [2,18]. Such conservatism in Capreolinae antler evolution is often compensated by the position of pedicles on the skull roof that represent a peculiar evolutionary feature of the Old World capreolines [18,47]. The “conservatism” (with a single exception: the genus Alces) in body size evolution of Eurasian telemetacarpal deer is another circumstance that requires explanation. The distribution of species body mass within the subfamilies Capreolinae and Cervinae reveals an important difference between these two systematic groups: the species with a mean body size below 150 kg are more frequent in telemetacarpal deer, while the body size class of medium–large deer (150–450 kg) is very poorly represented (Figure 10).
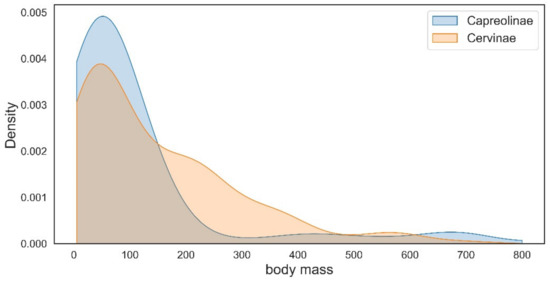
Figure 10.
The distribution of body size within the cervid subfamilies Cervinae and Capreolinae.
The paleobiogeographic history of telemetacarpal and plesiometacarpal deer may explain such differences between Capreolinae and Cervinae. The evolutionary radiation of telemetacarpal deer in western Eurasia took place in the conditions of the Mediterranean biome emerging during the late Miocene. The biome of southwest Europe, where the highest diversity of primitive capreolines is found, gradually moved from mixed warm forests to the Mediterranean biome [87]. According to Allen [88] and Rundel et al. [89], the emergence of Mediterranean-type climates was triggered by the growth of the east Antarctic ice sheet and progressive global cooling. Unlike cervines, capreolines did not have extensive refuges within the tropical climate latitudes and all their evolutionary radiation evolved in rather dry biomes of Mediterranean type.
Possibly, the telemetacarpal type of lateral metacarpals reduction is also linked to the cursorial adaptation in relatively dry environments with hard ground: the maintained articulations of lateral digits with the preserved distal portions of the second and fifth metacarpals could be an adaptation preventing their displacement and wounding.
The low number of small muntjac-like Old World capreolines and the predominance of forms characterised by a body size of modern roe deer may be regarded as a consequence of the physiological constraints imposed by the Mediterranean plants rich in fibre. The body size in ruminants is an important eco-physiological parameter that defines the range of fibre content in the forage that may be tolerated by a ruminant [5,90]. The excessively high content of fibre in forage may lead a ruminant to starvation even if its rumen is full of ingested food of low-quality. This is the reason why all deer adapted to the biomes of Mediterranean type tend to have a body size similar to that of modern fallow deer. This is the body size of most archaic capreolines from western Eurasia (Figure 8B). One can assume that such a biogeographic restriction shaped the main features of early capreolines, such as the more advanced features of dental morphology, the quite conservative antler Bauplan that is related to relative body size uniformity, and the pause period in the antler growth cycle [1,2] that mirrors the seasonal conditions of the early evolutionary radiation. The extinction of the archaic telemetacarpal deer from western Eurasia was most probably indirectly triggered by the Messinian salinity crisis, which generally deeply affected the late Miocene faunas of southern and southwestern Europe [91,92]. The drop in the level of the Mediterranean Sea and land connections between Europe and Africa created favourable conditions for multiple dispersals of bovid species [92] that eventually outcompeted most archaic telemetacarpal deer from western Eurasia. Starting from Pliocene, the subfamily Cervinae becomes the dominant ruminant group of western, northern, and southeastern Eurasia. The biogeographic importance of survived lineages of Capreolinae became marginal and is marked by adaptations to the ecological niches in high latitudes of Eurasia that were rather extreme for Cervidae and permitted them to avoid the competition with flourishing plesiometacarpal deer. This is the case of Eocoileus, Odocoileus, and Bretzia, but also some other lineages that eventually colonised North America via Beringia during the Pliocene and Pleistocene.
The evolutionary radiation of Capreolinae in South America reveals the high evolutionary potential of the subfamily in the absence of strong ecological competitors. The low number of large cervid forms (more than 150 kg) in the paleontological record of South America may be explained by the relatively short time of evolution (ca. 2 million years) and lack of the collective large predators in the Pleistocene faunas of South America. So far, there are only two known fossil findings from South America that were ascribed to Canis dirus [28,93] that may suggest an episodic short-termed presence of this species in South America.
5. Conclusions
The origin of crown deer is related to two equally diversified late Miocene evolutionary radiations, which gave rise to modern subfamilies Cervinae and Capreolinae. The evolution of those crown subfamilies of Cervidae generally followed the same evolutionary directions and evolved some convergent features, such as the reduction in lateral metacarpals. The late Miocene evolutionary radiation of Capreolinae (telemetacarpal deer) took place in the emerging Mediterranean biome that defined the main morphological characteristics of the subfamily, including the adaptation to savanna-like biomes in the most specialised late Miocene forms that successfully dispersed eastward. The dramatic extinction of most of late Miocene telemetacarpal lineages in western Eurasia was likely caused by the faunal transformations triggered by the Messinian salinity crisis. The evolutionary radiation of plesiometacarpal deer (the subfamily Cervinae) took place in the much more stable late Miocene tropical conditions of the eastern part of the Oriental realm. The further evolutionary diversifications and dispersals of plesiometacarpal deer were triggered by the climate oscillations (glacial–interglacial cycles) that caused the repeated northward dispersal of tropical cervines during warm phases and their subsequent adaptations to new conditions when the biogeographic border between the Palearctic and the eastern part of Oriental realm shifted southward. This evolutionary diversification of Cervinae attained its maximum during the early Pleistocene and produced a large number of short-living species. Unlike the Plio–Pleistocene deer from Palearctic Eurasia, the cervids from the western part of the Oriental realm (plesiometacarpal deer) and North America (telemetacarpal deer) show remarkable taxonomic longevity and evolutionary stasis. The dispersal of Capreolinae into South America was followed by an exponential evolutionary diversification during a relatively very short time period. The characteristics of the evolutionary diversification of cervids in South America mirrors the features of the Cervinae radiation in the eastern part of the Oriental realm and the Pleistocene speciation of Palearctic cervines.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/earth3040066/s1, Table S1: the complete list and biogeographic distribution of crown Cervidae of the world from late Miocene to late Pleistocene; Table S2: Bibliographic sources that provided the data on fossil deer biostratigraphic and zoogeographic distribution and predicted body mass (see Table S1); Table S3: Biostratigraphic and zoogeographic distribution of deer from the Late Miocene to the Late Pleistocene and their body mass diversity: data processing and visualisation (Python 3).
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data analyzed in this study were a re-analysis of existing data, which are openly available at locations cited in the reference section and Supplementary Mterials.
Acknowledgments
The author thanks the three anonymous reviewers for their valuable suggestions and comments that improved the quality of the article.
Conflicts of Interest
The author declares no conflict of interest.
References
- Geist, V. Deer of the World: Their Evolution, Behaviour, and Ecology; Stackpole Books: Mechanicsburg, OH, USA, 1998; 421p. [Google Scholar]
- Bubenik, A.B. Epigenetical, morphological, physiological, and behavioral aspects of evolution of horns, pronghorns, and antlers. In Horns, Pronghorns, and Antlers: Evolution, Morphology, Physiology, and Social Significance; Bubenik, G.A., Bubenik, A.B., Eds.; Springer: New York, NY, USA, 1990; pp. 3–113. [Google Scholar]
- Clutton-Brock, T.H. The functions of antlers. Behaviour 1982, 79, 108–124. [Google Scholar] [CrossRef]
- Kaiser, T.M.; Croitor, R. Ecological interpretations of early Pleistocene deer (Mammalia, Cervidae) from Ceyssaguet (Haute-Loire, France). Geodiversitas 2004, 26, 661–674. [Google Scholar]
- Janis, C. The evolutionary strategy of the Equidae and the origins of rumen and cecal digestion. Evolution 1976, 30, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Lister, A.M. Diversity and evolution of antler form in Quaternary deer. In Biology and Management of the Cervidae; Wemmer, C.M., Ed.; Smithsonian Institution Press: London, UK, 1987; pp. 81–98. [Google Scholar]
- Azzaroli, A. The Deer of the Weybourn Crag and Forest Bed of Norfolk; British Museum: London, UK, 1953; Volume 1. [Google Scholar]
- Heintz, E. Les cervidés villafranchiens de France et d’Espagne. Mem. Du Mus. Natl. D’Hist. Nat. Nouv. Ser. Ser. C Sci. De La Terre 1970, 22, 1–303. [Google Scholar]
- Vislobokova, I.A. Fossil deer of Eurasia. Trans. Paleontol. Inst. 1990, 240, 1–206. [Google Scholar]
- Brooke, V. On the Classification of the Cervidae with the synopsis of the existing Species. Proc. Zool. Soc. Lond. 1878, 46, 883–928. [Google Scholar] [CrossRef]
- Lydekker, R. Deer of All Lands: A History of the Family Cervidae Living and Extinct; Rowland Ward, Ltd.: London, UK, 1898; 329p. [Google Scholar]
- Bouvrain, G.; Geraads, D.; Jehenne, Y. Nouvelles données relatives à la classification de Cervidae (Artiodactyla, Mammalia). Zool. Anz. 1989, 223, 82–90. [Google Scholar]
- Randi, E.; Mucci, N.; Pierpaoli, M.; Douzery, E. New phylogenetic perspectives on the Cervidae (Artiodactyla) are provided by the mitochondrial cytochrome b gene. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1998, 265, 793–801. [Google Scholar] [CrossRef]
- Gilbert, C.; Ropiquet, A.; Hassanin, A. Mitochondrial and nuclear phylogenies of Cervidae (Mammalia, Ruminantia): Systematics, morphology, and biogeography. Mol. Phylogenet. Evol. 2006, 40, 101–117. [Google Scholar] [CrossRef]
- Czyżewska, T. Deer from Węże and their relationship with the Pliocene and Recent Eurasiatic Cervidae. Acta Palaeontol. Pol. 1968, 8, 537–593. [Google Scholar]
- Webb, S.D. Evolutionary history of New World Cervidae. In Antelopes, Deer, and Relatives: Fossil Record, Behavioral Ecology, Systematics, and Conservation; Vrba, E.S., Schaller, G.B., Eds.; Yale University Press: New York, NY, USA, 2000; pp. 38–64. [Google Scholar]
- Vislobokova, I.A.; Kalmykov, N.P. On the history of roe deer. In Paleotheriology; Sokolov, V.E., Tatarinov, L.P., Eds.; Nauka: Moscow, Russia, 1994; pp. 214–235. (In Russian) [Google Scholar]
- Croitor, R. Early evolutionary radiation and diversity of the Old World telemetacarpal deer (Capreolinae, Cervidae, Mammalia). Neues Jahrb. Geol. Palaontol.-Abh. 2021, 300, 33–67. [Google Scholar] [CrossRef]
- Holt, B.G.; Lessard, J.P.; Borregaard, M.K.; Fritz, S.A.; Araújo, M.B.; Dimitrov, D.; Fabre, P.H.; Graham, C.H.; Graves, G.R.; Jønsson, K.A.; et al. An update of Wallace’s zoogeographic regions of the world. Science 2013, 339, 74–78. [Google Scholar] [CrossRef]
- Janis, C.M. Correlation of cranial and dental variables with body size in ungulates and macropodoids. In Body Size in Mammalian Paleobiology: Estimation and Biological Implications; Damuth, J., MacFadden, B.J., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 255–299. [Google Scholar]
- Teilhard de Chardin, P.; Trassaert, M. The Pliocerie Camelidae, Giraffidae, and Cervidae of South Eastern Shansi. Palaeontol. Sin. New Ser. C 1937, 1, 1–69. [Google Scholar]
- Korotkevich, E.L. Late Neogene Deer of the North Black Sea Area; Naukova Dumka: Kyiv, Ukraine, 1970; 196p. [Google Scholar]
- Vislobokova, I.A. The fossil deer of Mongolia. Jt. Sov. Mong. Palaeontol. Exped. 1983, 23, 5–77. [Google Scholar]
- Dong, W. The fossil record of deer in China. In Deer of China: Biology and Management; Ōtaishi, N., Sheng, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 95–102. [Google Scholar]
- Azanza, B. Los Cervidae (Artiodactyla, Mammalia) del Mioceno de las Cuencas del Duero, Tajo, Calatayud-Teruel y Levance. Mem. Mus. Paleont. Univ. Zaragoza 2000, 8, 1–376. [Google Scholar]
- Alcaraz, M.A. Sistemática y Evolución de los Cérvidos (Mammalia, Artiodactyla) del Pleistoceno de las Áreas Extraandinas de Argentina. Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentina, 2010. [Google Scholar]
- Vislobokova, I.A. Giant Deer: Origin, Evolution, Role in the Biosphere. Paleontol. J. 2012, 46, 643–775. [Google Scholar] [CrossRef]
- Villavicencio Figueroa, N.A. Late Quaternary Megafaunal Extinctions in South America: Chronology, Environmental Changes and Human Impacts at Regional Scales. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2016. [Google Scholar]
- Croitor, R. Plio-Pleistocene Deer of Western Palearctic: Taxonomy, Systematics, Phylogeny; Institute of Zoology of the Academy of Sciences of Moldova: Chișinău, Moldova, 2018; 140p. [Google Scholar]
- Cirilli, O.; Machado, H.; Arroyo-Cabrales, J.; Barrón-Ortiz, C.I.; Davis, E.; Jass, C.N.; Jukar, A.M.; Landry, Z.; Marín-Leyva, A.H.; Pandolfi, L.; et al. Evolution of the Family Equidae, Subfamily Equinae, in North, Central and South America, Eurasia and Africa during the Plio-Pleistocene. Biology 2022, 11, 1258. [Google Scholar] [CrossRef]
- Azanza, B.; Montoya, P. A new deer from the lower Turolian of Spain. J. Paleontol. 1995, 69, 1163–1175. [Google Scholar] [CrossRef]
- Vislobokova, I.A. The systematic position of a deer from Pavlodar and the origin of Neocervinae. Paleontol. J. 1980, 1980, 91–106. (In Russian) [Google Scholar]
- Lungu, A.; Rzebik-Kowalska, B. Faunal Assemblages, Stratigraphy and Taphonomy of the Late Miocene Localities in the Republic of Moldova; Institute of Systematics and Evolution of Animals, Polish Academy of Sciences: Kraków, Poland, 2011; 62p. [Google Scholar]
- Croitor, R.; Zakharov, D.; Mararescul, V. Deer from the Early Pliocene Prioziornoe, Kuchurgan River Valley (Moldova, Eastern Europe). Neues Jahrb. Geol. Paläontol. 2020, 297, 1–43. [Google Scholar] [CrossRef]
- Pidoplichko, N.G.; Flerov, C.C. The new form of deer from the Pliocene of Southern Ukraine. Rep. Acad. Sci. USSR 1952, 84, 1239–1242. [Google Scholar]
- Rozenbaum, A.G.; Shaked Gelband, D.; Stein, M.; Mienis, H.K.; Rabinovich, R. First evidence of “ancient deer” (cervid) in the late Miocene Bira Formation, Northern Israel. PLoS ONE 2017, 12, e0185268. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, M. Tertiary vertebrates from Mongolia. Geol. Surv. China Ser. C 1924, 1, 1–119. [Google Scholar]
- Dong, W.; Ye, J. Two cervid species from the Late Neogene of Yushe Basin, Shanxi province, China. Vertebr. PalAsiat. 1996, 4, 135–144. [Google Scholar]
- Pfeiffer-Deml, T. Deer from the Pliocene site of Bad Deutsch-Altenburg 26 (Lower Austria, Leithagebirge): Conclusions based on skeletal morphology. Ann. Des Nat. Mus. Wien. Ser. A 2016, 118, 133–173. [Google Scholar]
- Vislobokova, I.; Dmitrieva, E.; Kalmykov, N. Artiodactyls from the Late Pliocene of Udunga, Western Trans-Baikal, Russia. J. Vertebr. Paleontol. 1995, 15, 146–159. [Google Scholar] [CrossRef]
- Bondarev, A.A.; Tesakov, A.S.; Simakova, A.N.; Dorogov, A.L. Reindeer (Rangifer) from Early Pleistocene of the South of Western Siberia. In Proceedings of the Materials of the LXIII Session of the Palaeontological Society “Integrative Palaeontology: Development Prospects for Geological Objectives”, Sankt-Petersburg, Russia, 3–7 April 2017. (In Russian). [Google Scholar]
- Tong, H. Composition des faunes de mammifères quaternaires en Chine selon un gradient Nord-Sud. L’anthropologie 2006, 110, 870–887. [Google Scholar] [CrossRef]
- Di Stefano, G.; Petronio, C. Systematics and evolution of the Eurasian Plio-Pleistocene tribe Cervini (Artiodactyla, Mammalia). Geol. Romana 2002, 36, 311–334. [Google Scholar]
- Petronio, C.; Krakhmalnaya, T.; Bellucci, L.; Di Stefano, G. Remarks on some Eurasian pliocervines: Characteristics, evolution, and relationships with the tribe Cervini. Geobios 2007, 40, 113–130. [Google Scholar] [CrossRef]
- Vislobokova, I.A. Family Cervidae Gray, 1821. In Biostratigraphy of the Late Pliocene–Early Pleistocene of Tajikistan; Nikiforova, K.V., Vangengeim, E.A., Eds.; Nauka: Moscow, Russia, 1998; pp. 72–98. (In Russian) [Google Scholar]
- Zdansky, O. Fossile Hirsche Chinas. Palaeont. Sin. Ser. C 1925, 2, 1–94. [Google Scholar]
- Gustafson, E.P. An Early Pliocene North American Deer: Bretzia Pseudalces, Its Osteology, Biology, and Place in Cervid History; Museum of Natural History, University of Oregon: Eugene, OR, USA, 2015; 75p. [Google Scholar]
- Wang, L.-H.; Zhang, Z.-Q. Late Miocene Cervavitus novorossiae (Cervidae, Artiodactyla) from Lantian, Shaanxi Province. Vertebr. PalAsiat. 2014, 52, 303–315. [Google Scholar]
- Dong, W.; Hu, C. The Late Miocene Cervidae from Hounao, Yushe Basin, Shanxi. Vertebr. PalAsiat. 1994, 32, 209–227. [Google Scholar]
- Aubekerova, P.A. Pliocene artiodactyls of southeastern Kazakhstan. Paleontol. J. 1974, 1974, 92–100. [Google Scholar]
- Chen, S.; Pang, L.; He, C.; Wei, G.; Huang, W.; Yue, Z.; Zhang, X.; Zhang, H.; Qin, L. New discoveries from the classic Quaternary mammalian fossil area of Yanjinggou, Chongqing, and their chronological explanations. Chin. Sci. Bull. 2013, 58, 3780–3787. [Google Scholar] [CrossRef]
- Dong, W. Reconsideration of the systematics of the Early Pleistocene Cervavitus (Cervidae, Artiodactyla, Mammalia). Estudios Geol. 2011, 67, 603–611. [Google Scholar] [CrossRef]
- Chen, G.F. Artiodactyla. In Jianshi Hominid Site; Zheng, S.H., Ed.; Science Press: Beijing, China, 2004; pp. 254–307. [Google Scholar]
- Deng, T.; Wang, S.Q.; Shi, Q.Q.; Li, Y.K.; Li, Y. A new species of Eostyloceros (Cervidae, Artiodactyla) from the late Miocene of the Linxia Basin in Gansu, China. Zootaxa 2014, 3893, 363–381. [Google Scholar] [CrossRef]
- Mennecart, B.; DeMiguel, D.; Bibi, F.; Rössner, G.E.; Métais, G.; Neenan, J.M.; Wang, S.; Schulz, G.; Müller, B.; Costeur, L. Bony labyrinth morphology clarifies the origin and evolution of deer. Sci. Rep. 2017, 7, 13176. [Google Scholar] [CrossRef]
- Dong, W.; Pan, Y.; Liu, J. The earliest Muntiacus (Artiodactyla, Mammalia) from the Late Miocene of Yuanmou, southwestern China. C. R. Palevol 2004, 3, 379–386. [Google Scholar] [CrossRef]
- Stimpson, C.M.; Utting, B.; O’Donnell, S.; Huong, N.M.; Kahlert, T.; Manh, B.V.; Khanh, P.S.; Rabett, R.J. An 11 000-year-old giant muntjac subfossil from Northern Vietnam: Implications for past and present populations. R. Soc. Open Sci. 2019, 6, 181461. [Google Scholar] [CrossRef]
- Sen, S.; Blieck, A.; Bouvrain, G.; Brunet, M.; Geraads, D.; Heintz, E.; Koufos, G.D. Late Miocene mammals from Taghar, Khurdkabul Basin, Afghanistan. Ann. Paleontol. 1997, 83, 233–266. [Google Scholar]
- Heintz, E.; Brunet, M.; Battail, B.; Jehenne, Y. The Main Features of the Cervid Palaeobiogeography. Quartärpaläontologie 1990, 8, 79–82. [Google Scholar]
- Croitor, R. Description of a new deer species (Cervidae, Mammalia) from the Early Pliocene of Eastern Europe, with a review of early dispersals and palaeobiogeography of the subfamily Cervinae. Neues Jahrb. Geol. Paläontol.-Abh. 2017, 283, 85–108. [Google Scholar] [CrossRef]
- Croitor, R.; Khan, M.A.; Abbas, S.G.; Babar, M.A.; Asim, M.; Akhtar, M. Description of new Pliocene to Early Pleistocene deer (Cervidae, Mammalia) remains from the Siwalik Hills in Pakistan with a discussion on paleobiogeography of cervids from the Indian subcontinent. Geobios 2022, 74, 21–41. [Google Scholar] [CrossRef]
- Croitor, R.; Abbas, S.G.; Babar, M.A.; Khan, M.A. A new deer species (Cervidae, Mammalia) from the upper Siwaliks (Pakistan). Quat. Int. 2021, 595, 1–11. [Google Scholar] [CrossRef]
- Croitor, R. A description of two new species of the genus Rucervus (Cervidae, Mammalia) from the Early Pleistocene of Southeast Europe, with comments on Hominin and South Asian ruminants dispersals. Quaternary 2018, 1, 17. [Google Scholar] [CrossRef]
- Croitor, R.; Robinson, C. A revision of “Cervus” punjabiensis Brown, 1926 (Cervidae, mammalia) from the Upper Siwaliks of Chandigarh, India. Quat. Int. 2020, 550, 147–158. [Google Scholar] [CrossRef]
- Depéret, C. Les Animaux Pliocènes du Roussillon; Baudry: Paris, France, 1890; Volume 3, pp. 5–194. [Google Scholar]
- Croitor, R.; Bonifay, M.-F.; Bonifay, E. Origin and evolution of the late Pleistocene island deer Praemegaceros (Nesoleipoceros) cazioti (Depéret) from Corsica and Sardinia. Bull. Mus. D’anthropol. Préhist. Monaco 2006, 46, 35–68. [Google Scholar]
- Van der Made, J. The dwarfed “giant deer” Megaloceros matritensis n. sp. from the Middle Pleistocene of Madrid—A descendant of M. savini and contemporary to M. giganteus. Quat. Int. 2019, 520, 110–139. [Google Scholar] [CrossRef]
- Croitor, R.; Sanz, M.; Daura, J. The endemic deer Haploidoceros mediterraneus (Bonifay) (Cervidae, Mammalia) from the Late Pleistocene of Cova del Rinoceront (Iberian Peninsula): Origin, ecomorphology, and paleobiology. Hist. Biol. 2020, 32, 409–427. [Google Scholar] [CrossRef]
- Meiri, M.; Lister, A.M.; Collins, M.J.; Tuross, N.; Goebel, T.; Blockley, S.; Zazula, G.D.; van Doorn, N.; Dale Guthrie, R.; Boeskorov, G.G.; et al. Faunal record identifies Bering isthmus conditions as constraint to end-Pleistocene migration to the New World. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132167. [Google Scholar] [CrossRef]
- Sondaar, P.Y. Insularity and its effect on mammal evolution. In Major Patterns in Vertebrate Evolution; Hecht, M.K., Goody, P.C., Hecht, B.M., Eds.; Springer: Boston, MA, USA, 1977; pp. 671–707. [Google Scholar]
- Croitor, R. Systematical position and paleoecology of the endemic deer Megaceroides algericus Lydekker, 1890 (Cervidae, Mammalia) from the late Pleistocene-early Holocene of North Africa. Geobios 2016, 49, 265–283. [Google Scholar] [CrossRef]
- Raven, H.C.; Gregory, W.K. Wallace’s line and the distribution of Indo-Australian mammals. Bull. Am. Mus. Nat. Hist. 1935, 68, 179–293. [Google Scholar]
- Martins, R.F.; Schmidt, A.; Lenz, D.; Wilting, A.; Fickel, J. Human-mediated introduction of introgressed deer across Wallace’s line: Historical biogeography of Rusa unicolor and R. timorensis. Ecol. Evol. 2018, 8, 1465–1479. [Google Scholar] [CrossRef]
- Jiménez-Hidalgo, E.; Bravo-Cuevas, V.M. A roe deer from the Pliocene of Hidalgo, central Mexico. Acta Palaeontol. Pol. 2014, 60, 807–813. [Google Scholar]
- Gunnell, G.F.; Foral, A. New species of Bretzia (Cervidae; Artiodactyla) from the latest Pleistocene or earliest Holocene of Nebraska and South Dakota. J. Mammal. 1994, 75, 378–381. [Google Scholar] [CrossRef]
- Sondaar, P.Y.; Van der Geer, A.A.E. Evolution and Extinction of Plio-Pleistocene Island Ungulates. Quaternaire hors-sér. 2005, 2, 241–256. [Google Scholar]
- Churcher, C.S.; Peterson, R.L. Chronologic and environmental implications of a new genus of fossil deer from Late Wisconsin deposits at Toronto, Canada. Quat. Res. 1982, 18, 184–195. [Google Scholar] [CrossRef]
- Gallo, V.; Avilla, L.S.; Pereira, R.C.; Absolon, B.A. Distributional patterns of herbivore megamammals during the Late Pleistocene of South America. An. Acad. Bras. Ciênc. 2013, 85, 533–546. [Google Scholar] [CrossRef]
- McCulloch, R.D.; Bentley, M.J.; Purves, R.S.; Hulton, N.R.; Sugden, D.E.; Clapperton, C.M. Climatic inferences from glacial and palaeoecological evidence at the last glacial termination, southern South America. J. Quat. Sci. 2000, 15, 409–417. [Google Scholar] [CrossRef]
- Rabassa, J.; Coronato, A. Glaciations in Patagonia and Tierra del Fuego during the Ensenadan stage/age (early Pleistocene–earliest middle Pleistocene). Quat. Int. 2009, 210, 18–36. [Google Scholar] [CrossRef]
- Gonzalez, E.; Labarca, R.; Chavez-Hoffmeister, M.; Pino, M. First fossil record of the smallest deer cf. Pudu Molina, 1782 (Artiodactyla, Cervidae), in the Late Pleistocene of South America. J. Vertebr. Paleontol. 2014, 34, 483–488. [Google Scholar] [CrossRef]
- Alcaraz, M.; Zurita, A. Nuevos registros de cérvidos poco conocidos: Epieuryceros cf. proximus Castellanos y Antifer sp. (Mammalia, Artiodactyla, Cervidae). Rev. Mus. Argent. Cienc. Nat. 2004, 6, 41–48. [Google Scholar] [CrossRef]
- Rusconi, C. Tercera noticia sobre los vertebrados fosiles de Las Arenas Puelchenses de Villa Ballester. An. Soc. Cíent. Argent. 1934, 117–118, 28–30. [Google Scholar]
- Rotti, A.; Vezzosi, R.I.; Mothé, D.; dos Santos Avilla, L. Rising from the ashes: The biggest South American deers (Cetartiodactyla: Cervidae) once roamed Northeast Brazil. J. S. Am. Earth Sci. 2021, 108, 103–154. [Google Scholar] [CrossRef]
- Carette, E. Cérvidos actuales y fóssiles de Sid America: Revisión de las formas extinguidas pampeanas. Rev. Mus. La Plata 1922, 26, 393–472. [Google Scholar]
- Tomiati, C.; Abbazzi, L. Deer fauna from Pleistocene and Holocene localities of Ecuador (South America). Geobios 2002, 35, 631–645. [Google Scholar] [CrossRef]
- Mosbrugger, V. Climate change and water cycle-some lessons from the geological past. In Climatic Changes and Water Resources in the Middle East and North Africa; Zereini, F., Hötzl, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 3–13. [Google Scholar]
- Allen, H.D. Mediterranian environments. In The Physical Geography of Africa; Adams, W.M., Goudie, A.S., Orme, A.R., Eds.; Oxford University Press: Oxford, UK, 1996; pp. 307–325. [Google Scholar]
- Rundel, P.W.; Arroyo, M.T.; Cowling, R.M.; Keeley, J.E.; Lamont, B.B.; Vargas, P. Mediterranean biomes: Evolution of their vegetation, floras, and climate. Ann. Rev. Ecol. Evol. Syst. 2016, 47, 383–407. [Google Scholar] [CrossRef]
- Clauss, M.; Frey, R.; Kiefer, B.; Lechner-Doll, M.; Loehlein, W.; Polster, C.; Rössner, G.E.; Streich, W.J. The maximum attainable body size of herbivorous mammals: Morphophysiological constraints of foregut, and adaptations of hindgut fermenters. Oecologia 2003, 136, 14–27. [Google Scholar] [CrossRef]
- Hsü, K.J.; Montadert, L.; Bernoulli, D.; Cita, M.B.; Erickson, A.; Garrison, R.E.; Kidd, R.B.; Mèlierés, F.; Müller, C.; Wright, R. History of the Mediterranean salinity crisis. Nature 1977, 267, 399–403. [Google Scholar] [CrossRef]
- Van der Made, J.; Morales, J.; Montoya, P. Late Miocene turnover in the Spanish mammal record in relation to palaeoclimate and the Messinian Salinity Crisis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 238, 228–246. [Google Scholar] [CrossRef]
- Dundas, R.G. Quaternary records of the dire wolf, Canis dirus, in North and South America. Boreas 1999, 28, 375–385. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
