Electrochemical Anodic Oxidation Treatment of Pool Water Containing Cyanuric Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Heterogeneous Photocatalysis, Photo-Fenton, and Photo-Persulfate Experiments
2.2. Anodic Oxidation Experiments
2.3. Voltammetric Study
2.4. Analytical Methods
3. Results and Discussion
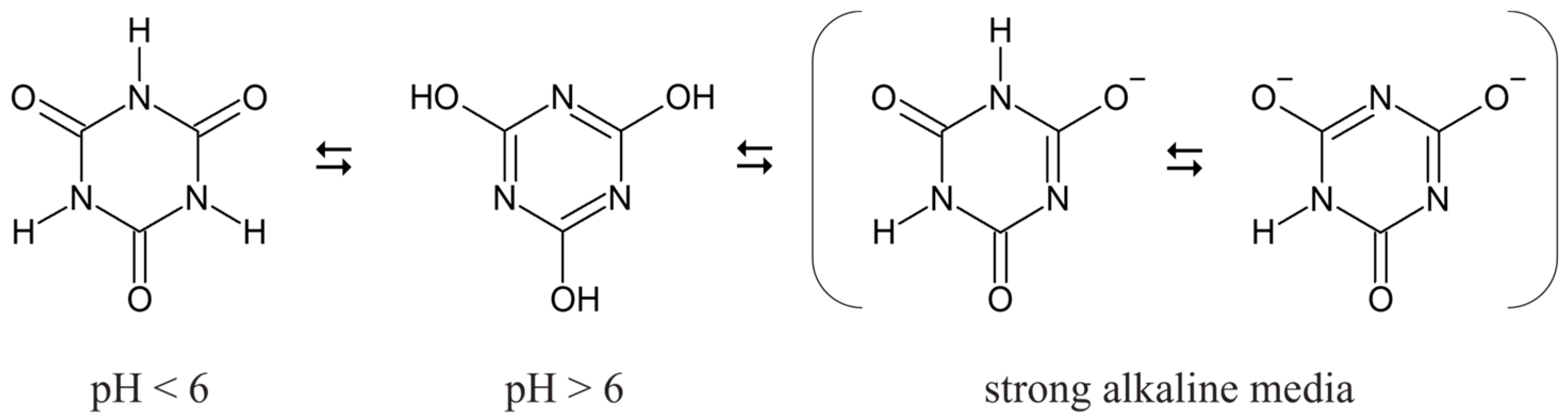
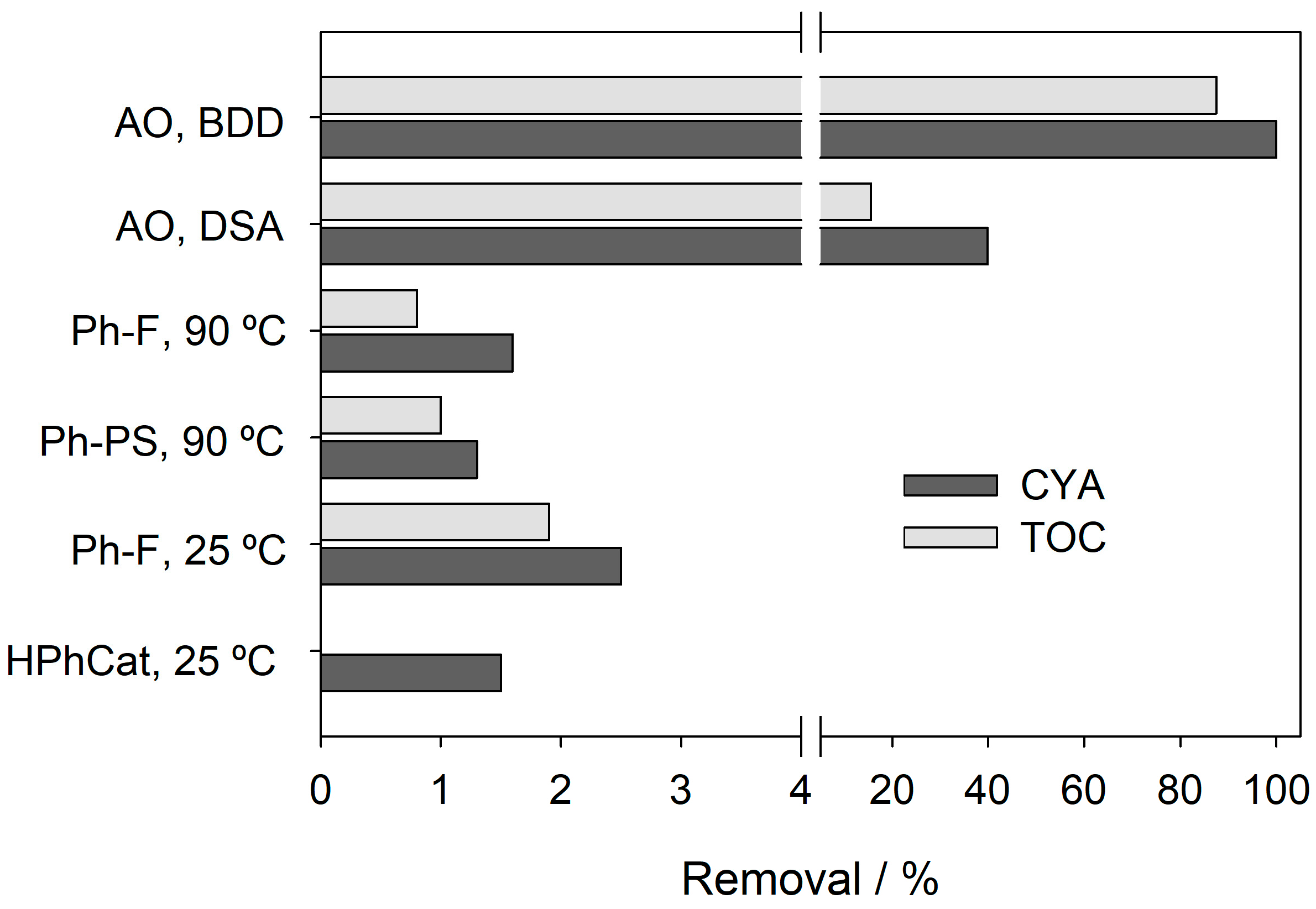
3.1. CYA Degradation by Advanced Oxidation Processes
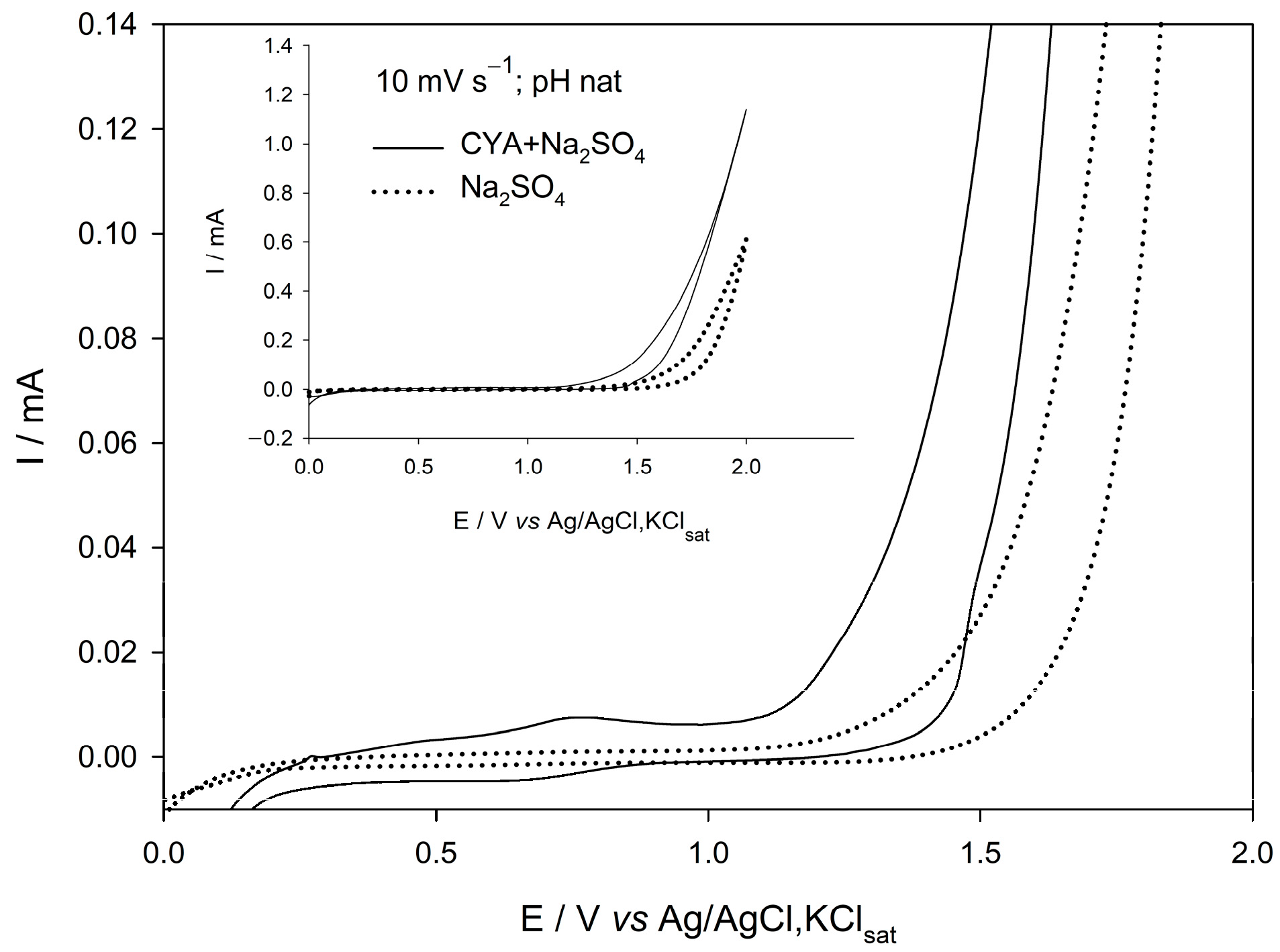
3.2. Voltammetric Study
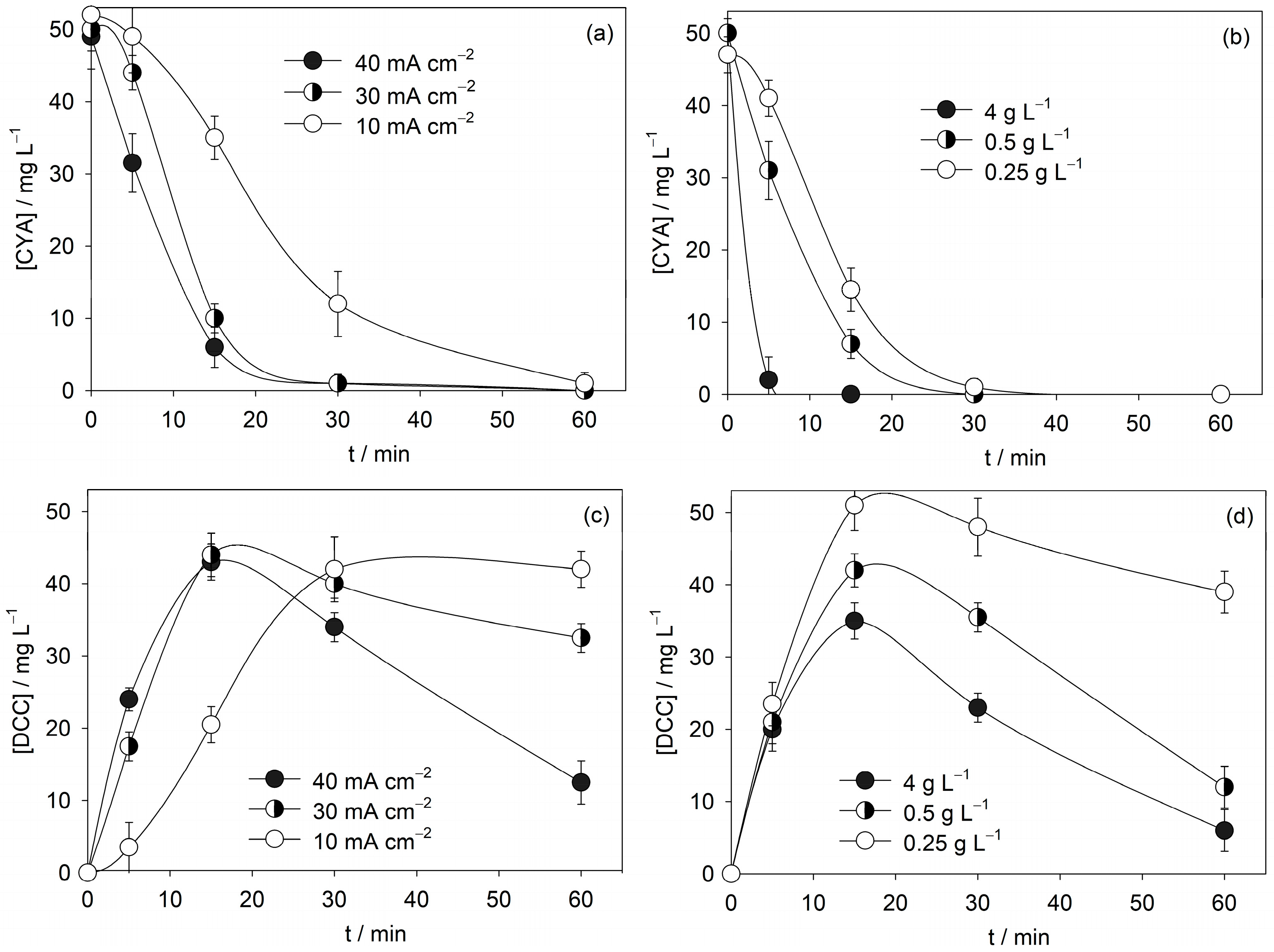
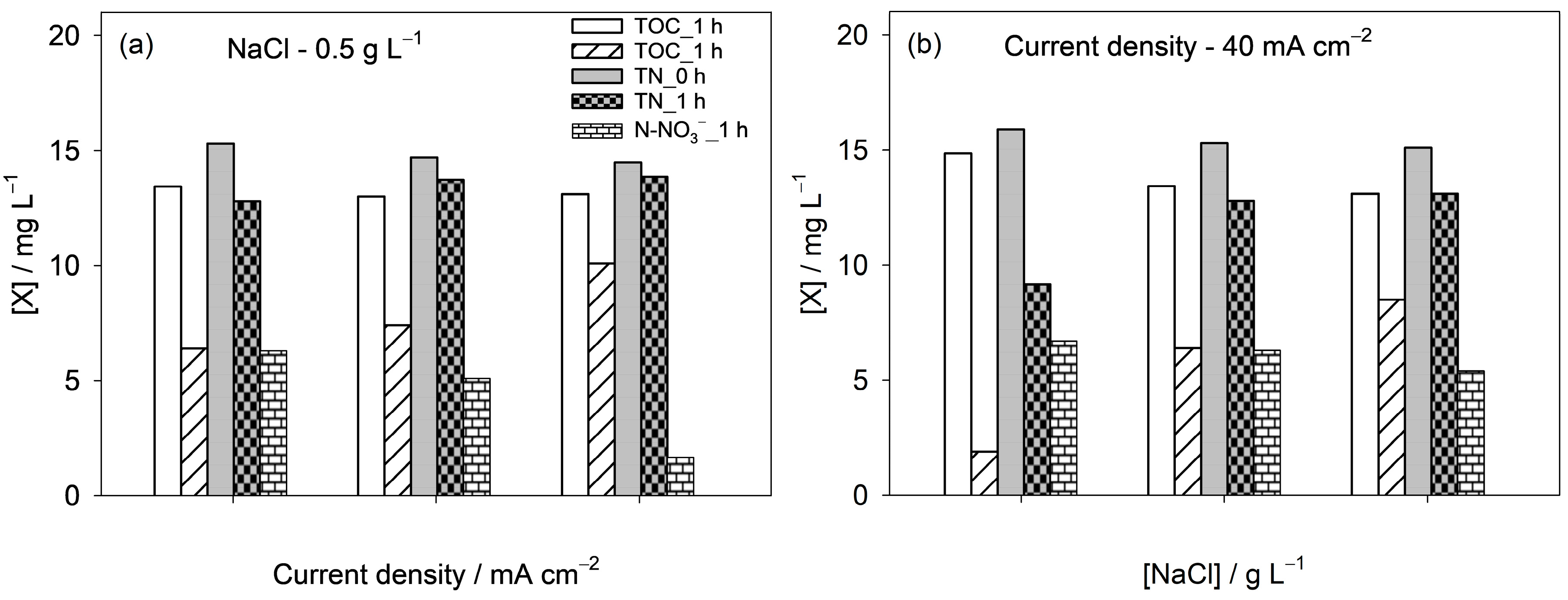
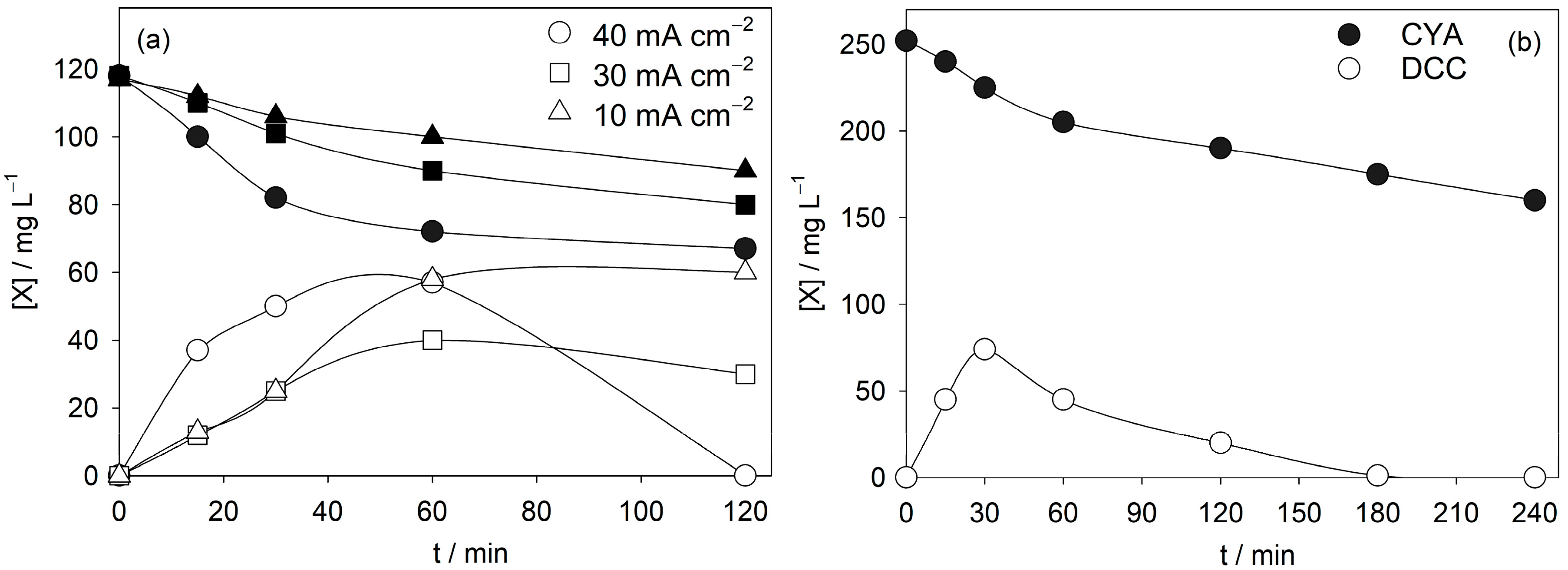
3.3. Influence of Operational Parameters on CYA Anodic Oxidation
3.4. CYA Anodic Oxidation in Swimming Pool Waters
4. Conclusions
- (1)
- CYA degradation was evaluated by different AOPs, and AO with BDD anodes showed the highest efficiency, while heterogeneous photocatalysis, photo-Fenton, and photo-persulfate were largely ineffective.
- (2)
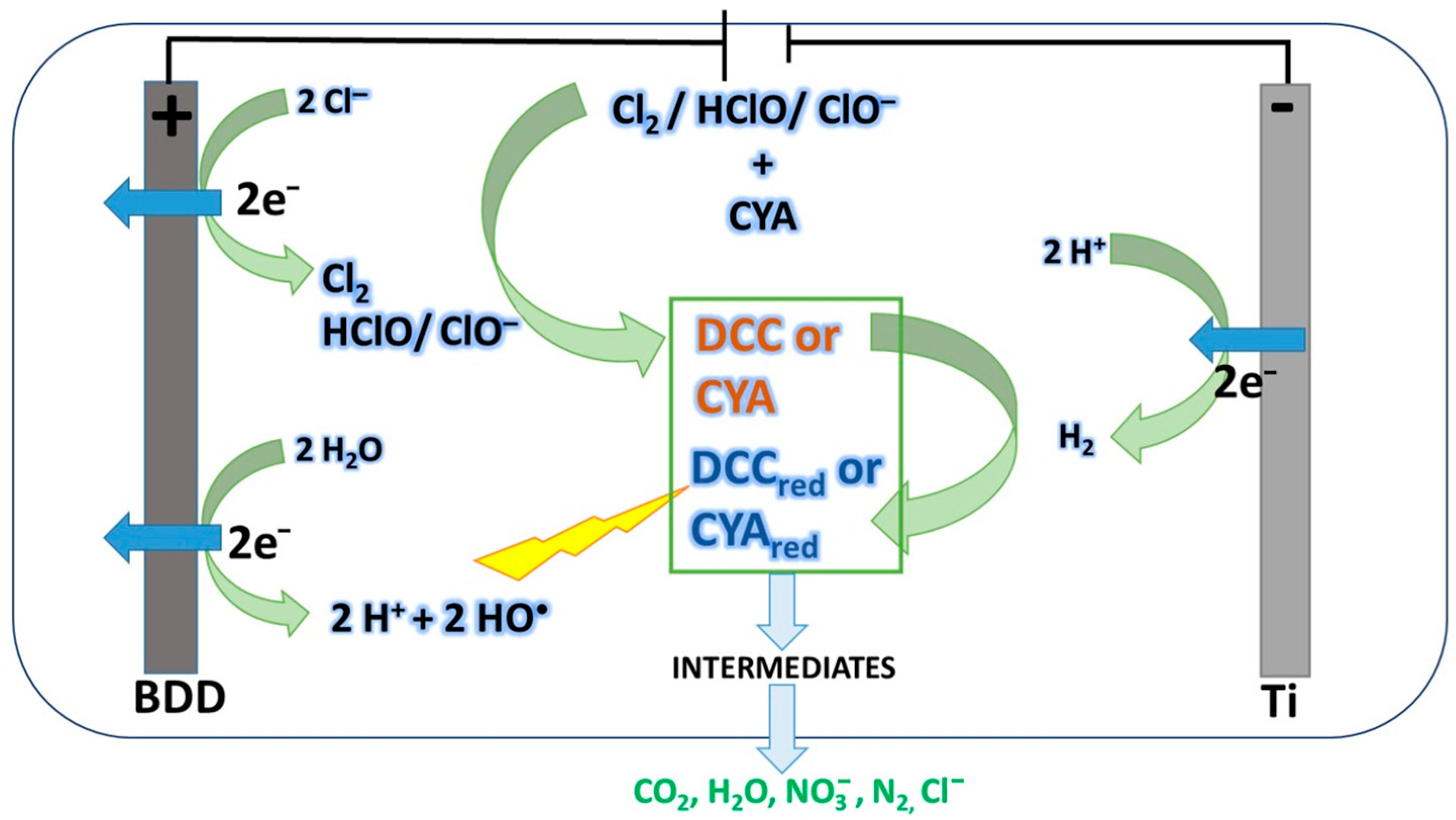
- Cyclic voltammetry confirmed that, in the presence of CYA, the oxidation of chloride is delayed, consistent with the formation of organochlorine intermediates.
- (3)
- The effect of operational parameters was systematically studied. Increasing current density enhanced TOC removal and nitrate formation, while TN remained nearly constant, showing that nitrogen is redistributed between organic species and nitrate rather than removed from the system. Higher NaCl concentrations promoted both TOC and TN removal, evidencing the role of active chlorine species.
- (4)
- In real swimming pool water, anodic oxidation with BDD achieved 38% CYA removal in 4 h at 40 mA cm−2, starting from a severely overstabilized matrix (251 mg L−1 CYA), without requiring additional electrolyte. This confirms the relevance of the process under realistic conditions.
- (5)
- Overall, the results demonstrate that AO with BDD can effectively control CYA overstabilization while simultaneously regenerating disinfectant capacity, offering a practical alternative to water replacement and avoiding the use of external chemicals.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AO | Anodic oxidation |
| BDD | Boron-doped diamond |
| CYA | Cyanuric acid |
| DCC | Dichloroisocyanuric acid |
| DSA | Dimensionally stable anode |
| HPhCat | Heterogeneous photocatalysis |
| HPLC | High-performance liquid chromatography |
| Ph-F | Photo-Fenton |
| Ph-PS | Photo-persulfate |
| PS | Persulfate |
| TN | Total nitrogen |
| TOC | Total organic carbon |
References
- Adhikary, R.K.; Mahfuj, M.S.E.; Starrs, D.; Croke, B.; Glass, K.; Lal, A. Risk of human illness from recreational exposure to microbial pathogens in freshwater bodies: A systematic review. Expo. Health 2022, 14, 325–343. Available online: https://link.springer.com/article/10.1007/s12403-021-00447-z (accessed on 7 April 2025). [CrossRef]
- Chowdhury, S.; Alhooshani, K.; Karanfil, T. Disinfection byproducts in a swimming pool: Occurrences, implications, and future needs. Water Res. 2014, 53, 68–109. [Google Scholar] [CrossRef] [PubMed]
- Teo, T.L.L.; Coleman, H.M.; Khan, S.J. Chemical contaminants in swimming pools: Occurrence, implications and control. Environ. Int. 2015, 76, 16–31. [Google Scholar] [CrossRef]
- Yang, L.; Chen, X.; She, Q.; Cao, G.; Liu, Y.; Chang, V.W.C.; Tang, C.Y. Regulation, formation, exposure, and treatment of disinfection by-products (DBPs) in swimming pool waters: A critical review. Environ. Int. 2018, 121, 1039–1057. [Google Scholar] [CrossRef]
- García-López, E.; Marcí, G.; Serpone, N.; Hidaka, H. Photoassisted oxidation of the recalcitrant cyanuric acid substrate in aqueous ZnO suspensions. J. Phys. Chem. C 2007, 111, 18025–18032. [Google Scholar] [CrossRef]
- Yanagisawa, I.; Oyama, T.; Serpone, N.; Hidaka, H. Successful scission of a recalcitrant triazinic ring. The photoassisted total breakup of cyanuric acid in ozonized TiO2 aqueous dispersions in the presence of an electron acceptor (H2O2). J. Phys. Chem. C 2008, 112, 18125–18133. [Google Scholar] [CrossRef]
- Long, L.; Bu, Y.; Chen, B.; Sadiq, R. Removal of urea from swimming pool water by UV/VUV: The roles of additives, mechanisms, influencing factors, and reaction products. Water Res. 2019, 161, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Royal Decree 742/2013, 27 September, Establishing the Technical-Sanitary Criteria of Swimming Pools; Official State Bulletin of the Government of Spain: La Rioja, Spain, 2013; Volume 244, pp. 83123–83135. Available online: https://www.boe.es/eli/es/rd/2013/09/27/742 (accessed on 24 April 2025).
- Falk, R.A.; Blatchley III, E.R.; Kuechler, T.C.; Meyer, E.M.; Pickens, S.R.; Suppes, L.M. Assessing the impact of cyanuric acid on Bather’s risk of gastrointestinal illness at swimming pools. Water 2019, 11, 1314. [Google Scholar] [CrossRef]
- Bobadilla, M.C.; González, E.P.V.; Lorza, R.L.; Gómez, F.S. Effecting partial elimination of isocyanuric acid from swimming pool water systems. Water 2019, 11, 712. [Google Scholar] [CrossRef]
- Oh, Y.-C.; Jenks, W.S. Photocatalytic degradation of a cyanuric acid, a recalcitrant species. J. Photochem. Photobiol. A Chem. 2004, 162, 323–328. [Google Scholar] [CrossRef]
- Abdelli, G.; Leitner, N.K.V. Oxidation of cyanuric acid in aqueous solution by catalytic ozonation. Ozone Sci. Eng. 2016, 38, 233–241. [Google Scholar] [CrossRef]
- Pei, L.Z.; Xie, Y.K.; Pei, Y.Q.; Cai, Z.Y.; Fan, C.G. Determination of cyanuric acid by electrochemical cyclic voltammetry method using CuGeO3 nanowires as modified electrode materials. J. Nanotechnol. Eng. Med. 2013, 4, 031003. [Google Scholar] [CrossRef]
- Chan, C.Y.; Chan, H.S.; Wong, P.K. Integrated photocatalytic-biological treatment of triazine-containing pollutants. Chemosphere 2019, 222, 371–380. [Google Scholar] [CrossRef]
- Mahlalela, L.C.; Casado, C.; Marugan, J.; Septien, S.; Ndlovu, T.; Dlamini, L.N. Photocatalytic degradation of atrazine in aqueous solution using hyperbranched polyethyleneimine templated morphologies of BiVO4 fused with Bi2O3. J. Environ. Chem. Eng. 2020, 8, 104215. [Google Scholar] [CrossRef]
- Carbajo, J.; García-Muñoz, P.; Tolosana-Moranchel, A.; Faraldos, M.; Bahamonde, A. Effect of water composition on the photocatalytic removal of pesticides with different TiO2 catalysts. Environ. Sci. Pollut. Res. 2014, 21, 12233–12240. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Lucas-Granados, B.; Muñoz-Portero, M.J.; Garcia-Antón, J. Elimination of pesticide atrazine by photoelectrocatalysis using a photoanode based on WO3 nanosheets. Chem. Eng. J. 2018, 350, 1114–1124. [Google Scholar] [CrossRef]
- Niu, B.; Cai, J.; Song, W.; Zhao, G. Intermediate accumulation and toxicity reduction during the selective photoelectrochemical process of atrazine in complex water bodies. Water Res. 2021, 205, 117663. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, Y.; Zhu, J.; Wang, Z.; Li, J. Study on the efficacy and mechanism of Fe-TiO2 visible heterogeneous Fenton catalytic degradation of atrazine. Chemosphere 2020, 252, 126333. [Google Scholar] [CrossRef]
- Oturan, N.; Brillas, E.; Oturan, M.A. Unprecedented total mineralization of atrazine and cyanuric acid by anodic oxidation and electro-Fenton with a boron-doped diamond anode. Environ. Chem. Lett. 2012, 10, 165–170. [Google Scholar] [CrossRef]
- Liu, G. Recalcitrance of cyanuric acid to oxidative degradation by OH Radical: Theoretical investigation. RSC Adv. 2014, 70, 37359–37364. [Google Scholar] [CrossRef]
- Malpass, G.R.P.; Salazar-Banda, G.R.; Miwa, D.W.; Machado, S.A.S.; Motheo, A.J. Comparing atrazine and cyanuric acid electro-oxidation on mixed oxide and boron-doped diamond electrodes. Environ. Technol. 2013, 34, 1043–1051. [Google Scholar] [CrossRef]
- Polcaro, A.M.; Vacca, A.; Mascia, M.; Palmas, S. Oxidation at boron-doped diamond electrodes: An effective method to mineralize triazines. Electrochim. Acta 2005, 50, 1841–1847. [Google Scholar] [CrossRef]
- Bensalah, N.; Dbira, S.; Bedoui, A. The contribution of mediated oxidation mechanisms in the electrolytic degradation of cyanuric acid using diamond anodes. J. Environ. Sci. 2016, 45, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Salazar, R.; Brillas, E.; Sirés, I. Finding the best Fe2+/Cu2+ combination for the solar photoelectro-Fenton treatment of simulated wastewater containing the industrial textile dye Disperse Blue 3. Appl. Catal. B Environ. 2012, 115–116, 107–116. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Gao, Z.; You, S.; Wei, L. Electro-oxidation and UV irradiation coupled method for in-site removing pollutants from human body fluids in swimming pool. J. Hazard. Mater. 2024, 464, 132963. [Google Scholar] [CrossRef]
- Pasciucco, E.; Pasciucco, F.; Castagnoli, A.; Iannelli, R.; Pecorini, I. Removal of heavy metals from dredging marine sediments via electrokinetic hexagonal system: A pilot study in Italy. Heliyon 2024, 10, e27616. [Google Scholar] [CrossRef]


| Solution | [CYA] (mg L−1) | Process | Experimental Conditions |
|---|---|---|---|
| CYA aqueous solution | 50 | Heterogeneous photocatalysis | [TiO2] = 0.5 g L−1; pH0 = 6.7; T = 25 °C; V = 750 mL; t = 240 min |
| Photo-Fenton | [H2O2]0 = Stoichiometric; pH0 = 3; [Fe2+]0 = 10 mg L−1; T = 25 and 90 °C; V = 750 mL; t = 240 min | ||
| Photo-persulfate | [PS]0 = Stoichiometric; T = 90 °C; pH0 = 6.7; V = 750 mL; t = 240 min | ||
| Anodic oxidation | AO experiments set 1: Anode: DSA or BDD—25 cm2; Cathode: Stainless steel—25 cm2; Applied current density = 40 mA cm−2; [NaCl] = 4 g L−1; pH0 = 6.7; V = 250 mL; t = 240 min for DSA and 60 min for BDD | ||
| AO experiments set 2: Anode: BDD—25 cm2; Cathode: Stainless steel—25 cm2; Applied current density = 10, 30 and 40 mA cm−2; [NaCl] = 0.25, 0.5, and 4 g L−1; pH0 = 6.7; V = 250 mL; t = 60 min | |||
| Real swimming pool water | 118 | Anodic oxidation | Anode: BDD—25 cm2; Cathode: Stainless steel—25 cm2; Applied current density = 10, 30 and 40 mA cm−2; pH0 = 7.2; V = 250 mL; t = 120 min |
| 251 | Anode: BDD—25 cm2; Cathode: Stainless steel—25 cm2; Applied current density = 40 mA cm−2; pH0 = 7.0; V = 250 mL; t = 240 min |
| [NaCl]/g L−1 | TN0/mg L−1 | TNfinal/mg L−1 | N-NO3−final/mg L−1 | N-NO2−final/mg L−1 | N-NH4+final/mg L−1 |
|---|---|---|---|---|---|
| 0.5 | 15.3 | 12.8 | 6.3 | 0.1 | 0.8 |
| 4 | 15.9 | 9.2 | 6.7 | 0.1 | 0.4 |
| Pool Water Parameters | Sample 1 | Sample 2 | ||
|---|---|---|---|---|
| Before Treatment | After 2-h Treatment | Before Treatment | After 4-h Treatment | |
| CYA/mg L−1 | 118 | 65 | 251 | 155 |
| TOC/mg L−1 | 66.5 | 28.1 | 85.5 | 52.1 |
| Inorganic carbon/mg L−1 | 2.2 | 1.1 | 3.2 | 1.2 |
| pH | 7.2 | 6.9 | 7.0 | 6.6 |
| Conductivity/µS cm−1 | 632 | 743 | 1234 | 1432 |
| NO3−/mg L−1 | 13 | 75 | 16 | 97 |
| NH4+/mg L−1 | 3.5 | 5.1 | 3.0 | 5.0 |
| NO2−/mg L−1 | ≤1 | ≤1 | ≤1 | ≤1 |
| Free chlorine/mg L−1 | ≤1 | 3.4 | ≤1 | 5.1 |
| Total chlorine/mg L−1 | ≤1 | 4.1 | ≤1 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbajo, J.; Silveira, J.E.; Gomes, I.; Fernandes, A.; Ciríaco, L.; García-Costa, A.L.; Zazo, J.A.; Casas, J.A. Electrochemical Anodic Oxidation Treatment of Pool Water Containing Cyanuric Acid. Pollutants 2025, 5, 39. https://doi.org/10.3390/pollutants5040039
Carbajo J, Silveira JE, Gomes I, Fernandes A, Ciríaco L, García-Costa AL, Zazo JA, Casas JA. Electrochemical Anodic Oxidation Treatment of Pool Water Containing Cyanuric Acid. Pollutants. 2025; 5(4):39. https://doi.org/10.3390/pollutants5040039
Chicago/Turabian StyleCarbajo, Jaime, Jefferson E. Silveira, Inês Gomes, Annabel Fernandes, Lurdes Ciríaco, Alicia L. García-Costa, Juan A. Zazo, and Jose A. Casas. 2025. "Electrochemical Anodic Oxidation Treatment of Pool Water Containing Cyanuric Acid" Pollutants 5, no. 4: 39. https://doi.org/10.3390/pollutants5040039
APA StyleCarbajo, J., Silveira, J. E., Gomes, I., Fernandes, A., Ciríaco, L., García-Costa, A. L., Zazo, J. A., & Casas, J. A. (2025). Electrochemical Anodic Oxidation Treatment of Pool Water Containing Cyanuric Acid. Pollutants, 5(4), 39. https://doi.org/10.3390/pollutants5040039










