Abstract
Metal oxide nanocomposites have gained significant attention due to their unique physicochemical properties and applications in biomedicine, catalysis, sensing, and environmental remediation. Among them, zinc oxide (ZnO) nanoparticles (NPs) are widely studied, and functionalization with thiosemicarbazide (TSC) derivatives enhances their stability, reactivity, solubility, and biocompatibility. Ultrasonic-assisted synthesis offers an eco-friendly approach, enabling rapid nucleation, uniform dispersion, and controlled particle size while promoting efficient ligand coordination. Nanocomposites are typically characterized using various spectroscopic techniques, which confirm successful functionalization. This review highlights synthesis strategies, characterization techniques, and applications of ligand-functionalized metal oxide nanocomposites in environmental, catalytic, and biological fields.
1. Introduction
Metal-oxide-based nanocomposites have gained immense scientific and technological interest due to their exceptional physicochemical characteristics and their various applications [1]. These materials often exhibit enhanced surface reactivity, high mechanical stability, and tunable electronic structures that make them suitable for a broad spectrum of functional uses [2,3]. Among the wide range of metal oxides explored, ZnO NPs stand out as one of the most extensively studied materials. ZnO, an n-type semiconductor with a wide band gap of 3.37 eV and a high exciton binding energy of 60 meV, belongs to the family of metal oxide semiconductors [4]. At the nanoscale, ZnO exhibits outstanding physicochemical characteristics that enhance its suitability for various industrial applications. Owing to its high surface-to-volume ratio, ZnO nanostructures have been extensively investigated for their potential in photocatalytic [5,6,7,8], photovoltaic [9,10], biomedical [11,12,13,14], and sensing technologies [15,16,17]. Several methods have been explored for synthesizing ZnO nanostructures, including electrochemical deposition [18], solvothermal/hydrothermal routes, and conventional precipitation techniques [19,20]. Among these, chemical precipitation has become popular due to its simplicity, low cost, and ability to control particle size and morphology. It typically involves reacting aqueous zinc salts such as Zn(NO3)2 or ZnSO4 with alkaline solutions. Despite these advantages, the method often suffers from strong particle agglomeration, reliance on environmentally harmful surfactants, and the need for high-temperature calcination to obtain crystalline ZnO [21]. As an alternative, sonochemical precipitation offers a more efficient pathway by using ultrasonic irradiation to promote reactions between zinc salts and basic solutions. This approach reduces energy input, operates at lower temperatures, avoids toxic reagents, and eliminates post-synthesis heat treatment. Therefore, developing such one-step, low-energy, and environmentally friendly processes represents a promising route for producing ZnO nanostructures [22]. To further improve their physicochemical properties and expand their applicability, surface modification of ZnO NPs with organic ligands, such as TSC and its derivatives, has proven to be a highly effective strategy [23]. TSCs represent a versatile class of compounds exhibiting a wide range of biological activities [24]. Among these, their antimicrobial potential is particularly noteworthy, while numerous derivatives have also demonstrated significant antiviral, antimalarial [25] and anticancer effects [26]. The general structure of substituted thiosemicarbazone is given in Figure 1.
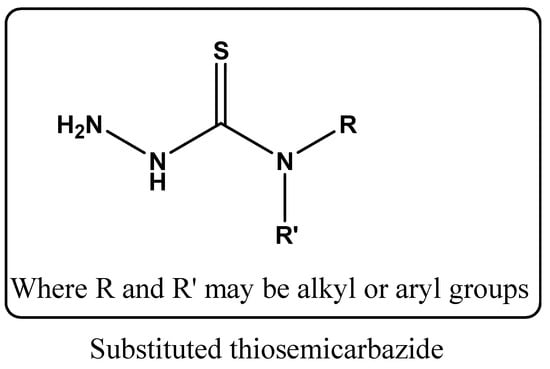
Figure 1.
Structure of substituted thiosemicarbazide.
These TSC moieties can coordinate with the ZnO surface through donor atoms, thereby enhancing the nanoparticles’ dispersion stability, solubility, and surface functionality [27]. Such conjugation not only provides better control over surface chemistry but also introduces additional reactive sites that are beneficial for catalytic and biological interactions.
Ultrasonic-assisted synthesis has found use as a sustainable, energy-efficient, and environmentally friendly approach for the fabrication of ZnO/TSC nanocomposites. The process is based on acoustic cavitation, where the formation and collapse of microscopic bubbles generate localized high temperatures and pressures, leading to accelerated reaction kinetics. This phenomenon promotes rapid nucleation, homogeneous particle distribution, and effective coordination between ZnO and organic ligands [28]. As a result, the ultrasound-assisted route offers controlled particle size, crystallinity, and morphology compared to conventional chemical or thermal synthesis techniques. Despite a growing number of studies on ZnO NPs and separate literature on TSC derivatives, TSC functionalization with ultrasonication-assisted ZnO NP synthesis is underexplored in the literature. This proceeding provides a focused mini-review of the recent progress in the synthesis, structural modification, and diverse applications of ZnO–ZnO-thiosemicarbazide nanocomposites. Special attention is given to the advantages of ultrasonic-assisted fabrication methods, which offer greener, faster, and more controllable pathways for producing highly functional hybrid nanomaterials compared to traditional synthetic approaches.
2. Methodology
In preparing this review, the relevant literature was collected from databases such as ScienceDirect, Scopus, Web of Science, SpringerLink, PubMed, and Google Scholar, covering publications from 2015 to 2025. Keywords including “metal oxide nanocomposites,” “ZnO NPs,” “thiosemicarbazide conjugates,” “sonochemical synthesis,” “ultrasonication-assisted synthesis”, and “biomedical applications” were used for the search. Articles were screened to include original research papers, review articles, and recent advances focusing on the synthesis, functionalization, and application of ZnO-based nanocomposites with thiosemicarbazide derivatives.
3. Synthesis of ZnO/TSC Conjugates by Ultrasonication
There are mainly two approaches used for the synthesis of ZnO/TSC conjugates, which are discussed below.
3.1. One-Pot Synthesis with In Situ Ligand Presence
Adding thiosemicarbazide/thiosemicarbazone during ZnO nucleation can lead to adsorption, chelation to surface Zn (II) ions, or partial capping. Ultrasonication offers a fast, energy-efficient, and environmentally friendly route for synthesizing ZnO nanoparticles. The intense cavitation generated during sonication promotes rapid nucleation, yielding smaller, uniformly dispersed particles with reduced agglomeration and improved crystallinity [29].
3.2. Two-Step Post-Synthesis Functionalization with TSC
In this method, ZnO NPs are functionalized by the ultrasonication method using suitable linkers, such as amino acids, silanes, thiols, etc. After that, ligands are attached through adsorption, electrostatic interaction, or covalent coupling. This gives greater control of ligand stoichiometry and orientation [30]. Generally, the mixture is sonicated for around 45–60 min and then stirred for around 14–16 h at a pH of 7–8. The ultrasonication-assisted synthesis of ZnO/TSC conjugates is represented in Figure 2.
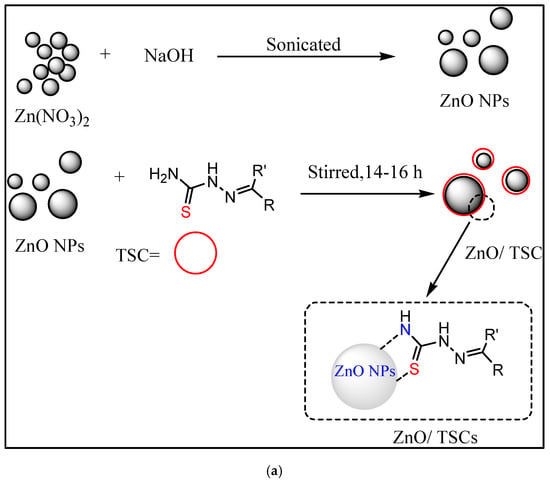
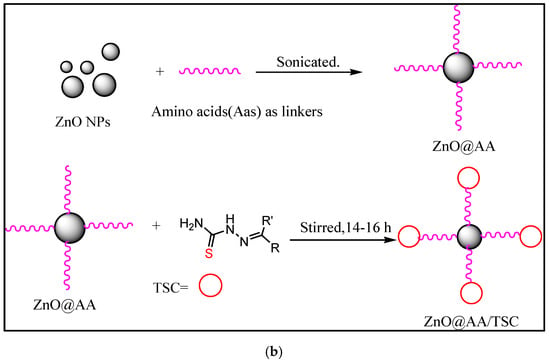
Figure 2.
(a,b) Schematic representation of synthesis of ZnO/TSC conjugates by ultrasonication.
4. Biological and Photocatalytic Application of ZnO/TSC Nanocomposites
ZnO/TSC nanocomposites are attracting increasing attention because of the cooperative effects between the ZnO NPs and the attached organic ligands. The functionalization of ZnO with TSC derivatives not only enhances its surface activity and compatibility with biological systems. Recent research has revealed that these nanocomposites possess notable cytotoxic, antibacterial, antioxidant, and photocatalytic properties, making them valuable materials for biomedical applications and environmental purification [31]. Some of the following nanocomposites have been reviewed in the literature.
In a recent study, ZnO@Gln-TSC NPs were synthesized by using ZnO NPs and glutamic acid, and conjugated with thiosemicarbazide. ZnO@Gln-TSC nanoparticles with a size range of 20–70 nm (DLS: 374 nm; zeta potential: −31.7 mV) exhibited strong cytotoxicity against AGS cells, with an IC50 of 9.8 μg/mL, which was markedly lower than that of ZnO (130 μg/mL), TSC (80.5 μg/mL), and oxaliplatin (67.7 μg/mL), while in HEK293 cells the IC50 was 150.5 μg/mL, compared to 215 μg/mL for ZnO [32]. In another recent study, plant-derived ZnO NPs were conjugated with salicylaldehyde thiosemicarbazone (STSC), vanillin thiosemicarbazone (VTSC), and 3-acetylpyridine thiosemicarbazone (3-APTSC) using an ultrasonication-assisted method, and the resulting nanoconjugates were characterized by spectral and surface analyses, including UV–Vis, FT–IR, W–H plots, and BET studies. ZnO/3-APTSC showed an increased surface area (32.188 m2/g) compared to pure ZnO (26.687 m2/g) and exhibited the highest antioxidant activity (59.89% scavenging). Antibacterial assays revealed effective inhibition, while photocatalytic studies demonstrated significant malachite green dye degradation efficiencies of 61.6%, 90.6%, and 94% for ZnO/STSC, ZnO/VTSC, and ZnO/3-APTSC, respectively, within 90 min, highlighting their potential as effective photocatalysts [33]. In a similar study, CuO NPs were conjugated with TSC, and the CuO/TSC conjugates exhibited notably improved functional properties. CuO/3-APTSC achieved the highest DPPH radical-scavenging activity (59.02%), surpassing the bare CuO NPs. A similar trend was observed in photocatalysis: while pure CuO NPs degraded only about 57% of malachite green in 90 min, the conjugated systems showed markedly higher efficiencies—75% for CuO/STSC, 73% for CuO/VTSC, and 84% for CuO/3-APTSC—demonstrating the superior performance of the nanoconjugates over bare CuO NPs [34]. Nejabatdoust et al. reported the synthesis of ZnO NPs functionalized with glutamic acid and subsequently conjugated with thiosemicarbazide (ZnO@Glu–TSC). The resulting nanoconjugates were tested against multidrug-resistant Staphylococcus aureus and demonstrated antibacterial activity that was 2–8 times stronger than that of ciprofloxacin [35]. In another study, ZnO NPs were synthesized and subsequently functionalized with Cu (II) and Pd (II) complexes of 4-hydroxybenzaldehyde thiosemicarbazone to obtain ZnONPs@Cu-BTSC and ZnONPs@Pd-BTSC nanocomposites. XRD analysis confirmed the crystalline hexagonal wurtzite phase of ZnO, while UV–Vis and FT–IR spectra evidenced successful coordination of the metal–ligand complexes onto the ZnO surface. SEM–EDX observations showed uniform particle morphology and the presence of Cu and Pd elements, verifying effective surface modification. In catalytic reduction of 4-nitrophenol, the ZnONPs@Pd-BTSC nanocomposite displayed the highest activity, followed by ZnONPs@Cu-BTSC and bare ZnO NPs, indicating that metal functionalization significantly enhanced the catalytic efficiency [36]. Recently, ZnO NPs modified with a glutamine-linked TSC ligand (ZnO@Glu-TSC) have displayed distinct optical and structural characteristics, such as an absorption peak near 380 nm and a nanoscale wurtzite crystal framework. The functionalized material shows improved antibacterial activity against P. aeruginosa strains, which show MIC values typically ranging from 128 to 512 µg/mL and frequent synergistic responses among clinical isolates. In addition to inhibiting bacterial growth, the nanoconjugate markedly diminishes key virulence mechanisms, reducing biofilm mass by almost 80% and lowering pyocyanin synthesis by more than 75%. These findings show promising multifunctional nano-antimicrobials capable of weakening both the survival and pathogenic behaviour of persistent bacterial strains [37]. Similarly, Danisman-Kalindemirtaş and co-workers reported the successful incorporation of two Zn(II)–thiosemicarbazone derivatives (ZnTcA and ZnTcB) into bovine serum albumin nanocarriers through UV-assisted crosslinking, a strategy used to stabilize these otherwise unstable therapeutic agents. Their characterization studies showed that Alb-ZnTcA formed nanoparticles with an average size of about 32 nm, whereas Alb-ZnTcB produced slightly larger particles around 43 nm. When evaluated against MCF-7 breast cancer cells, both albumin-bound formulations demonstrated markedly greater cytotoxicity than the free compounds, with enhanced activity observed even at lower concentrations, highlighting the advantages of albumin-mediated delivery. Among the two, Alb-ZnTcA exhibited the most pronounced anticancer effect, likely due to its smaller size enabling more efficient cellular internalization. Mechanistic analyses further revealed that both nanoformulations predominantly induced apoptosis rather than necrosis, suggesting activation of intrinsic apoptotic pathways and thereby effectively suppressing the uncontrolled proliferation of cancer cells [38].
5. Mechanistic Insight of ZnO/TSC Nanoconjugates
Ultrasonication produces acoustic cavitation, where bubbles collapse violently, generating localized hot spots that enhance ZnO nucleation and promote smaller particle sizes and high defect density. These defects significantly affect photocatalytic and antibacterial activity by modulating ROS yield [39]. Thiosemicarbazide ligands coordinate ZnO through the C=S and –NH2 groups, forming Zn–S and Zn–N interactions that stabilize surface states and modulate electron density. Electron-donating substituents (–CH3, –OCH3) on TSC improve charge transfer, while electron-withdrawing groups (–Cl, –NO2) may reduce electron density and alter ROS pathways [40,41,42]. Enhanced cytotoxicity or antibacterial performance in many studies arises from synergistic effects: (i) smaller sonochemically produced ZnO NPs with high defect density; (ii) TSC ligands promoting stronger ROS production and membrane interactions. Comparative trends from recent studies have shown that TSC-functionalized ZnO NPs often exhibit higher photocatalytic degradation efficiency (e.g., of malachite green, methylene blue), likely due to improved charge separation at the ZnO–TSC ligand interface.
6. Conclusions
ZnO/TSC nanocomposites have emerged as a versatile class of hybrid materials exhibiting superior physicochemical and biological characteristics compared to ZnO NPs. The incorporation of thiosemicarbazide derivatives enhances their structural stability, surface reactivity, and overall biocompatibility. Moreover, the use of ultrasonic-assisted synthesis provides a sustainable and efficient approach for producing uniformly dispersed nanostructures with well-controlled morphology. Recent investigations have demonstrated that these nanocomposites possess remarkable cytotoxic, antibacterial, antioxidant, and photocatalytic activities, making them highly promising for biomedical as well as environmental remediation applications. Ongoing research into tailored ligand design and optimized sonochemical fabrication methods is expected to further enhance their performance and broaden their scope of practical applications. Although interest in this area is increasing, only a limited number of studies directly link TSC chemistry with sonochemical ZnO NPs synthesis. This mini-review consolidates the available literature, highlights these inconsistencies, and proposes essential reporting guidelines and experimental controls. By connecting synthesis parameters with material properties and functional outcomes, it aims to support more reproducible experiments, clarify mechanistic insights, and accelerate progress in this field. Future studies should focus on establishing standardized protocols, deeper mechanistic investigations, and long-term toxicity assessments to ensure safe and scalable application of ZnO/TSC nanocomposites.
Author Contributions
Conceptualization, T.K.; methodology, E.V. and T.K.; data curation, E.V. and N.F.; writing—original draft preparation, writing—review and editing, E.V.; supervision, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were generated during the study.
Acknowledgments
The authors acknowledge the support extended by the Department of Chemistry, Integral University, Lucknow, and the R&D cell of the university for the Manuscript Communication Number (IU/R&D/2025-MCN0004076).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kumar, R.R.; Kumar, K.U.; Haranath, D. Synthesis, characterization, and applications of ZnO–TiO2 nanocoposites. In Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2022; pp. 271–314. [Google Scholar] [CrossRef]
- Kannan, P.; Maduraiveeran, G. Metal oxides nanomaterials and nanocomposite-based electrochemical sensors for healthcare applications. Biosensors 2023, 13, 542. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Osibote, O.A. Green synthesis of metal oxide nanoparticles, and their various applications. J. Hazard. Mater. Adv. 2024, 13, 100401. [Google Scholar] [CrossRef]
- Baig, A.; Siddique, M.; Panchal, S. A Review of Visible-Light-Active Zinc Oxide Photocatalysts for Environmental Application. Catalysts 2025, 15, 100. [Google Scholar] [CrossRef]
- Lee, S.J.; Jung, H.J.; Koutavarapu, R.; Lee, S.H.; Arumugam, M.; Kim, J.H.; Choi, M.Y. ZnO supported Au/Pd bimetallic nanocomposites for plasmon improved photocatalytic activity for methylene blue degradation under visible light irradiation. Appl. Surf. Sci. 2019, 496, 143665. [Google Scholar] [CrossRef]
- Prabhu, S.; Megala, S.; Harish, S.; Navaneethan, M.; Maadeswaran, P.; Sohila, S.; Ramesh, R. Enhanced photocatalytic activities of ZnO dumbbell/reduced graphene oxide nanocomposites for degradation of organic pollutants via efficient charge separation pathway. Appl. Surf. Sci. 2019, 487, 1279–1288. [Google Scholar] [CrossRef]
- Segovia, M.; Alegría, M.; Aliaga, J.; Celedon, S.; Ballesteros, L.; Sotomayor-Torres, C.; González, G.; Benavente, E. Heterostructured 2D ZnO hybrid nanocomposites sensitized with cubic Cu2O nanoparticles for sunlight photocatalysis. J. Mater. Sci. 2019, 54, 13523–13536. [Google Scholar] [CrossRef]
- Selvaraj, S.; Mohan, M.K.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and photocatalytic activity of Gd doped ZnO nanoparticles for enhanced degradation of methylene blue under visible light. Mater. Sci. Semicond. Process. 2019, 103, 104622. [Google Scholar] [CrossRef]
- Taherkhani, M.; Naderi, N.; Fallahazad, P.; Eshraghi, M.J.; Kolahi, A. Development and Optical Properties of ZnO Nanoflowers on Porous Silicon for Photovoltaic Applications. J. Electron. Mater. 2019, 48, 6647–6653. [Google Scholar] [CrossRef]
- Zhang, W.; Chang, S.; Yao, S.; Wang, H. Preparation and Characterization of Submicron Star-Like ZnO as Light Scattering Centers for Combination with ZnO Nanoparticles for Dye-Sensitized Solar Cells. J. Electron. Mater. 2019, 48, 4895–4901. [Google Scholar] [CrossRef]
- Beyene, Z.; Ghosh, R. Effect of zinc oxide addition on antimicrobial and antibiofilm activity of hydroxyapatite: A potential nanocomposite for biomedical applications. Mater. Today Commun. 2019, 21, 100612. [Google Scholar] [CrossRef]
- Feng, J.N.; Guo, X.P.; Chen, Y.R.; Lu, D.P.; Niu, Z.S.; Tou, F.Y.; Hou, L.J.; Xu, J.; Liu, M.; Yang, Y. Time-dependent effects of ZnO nanoparticles on bacteria in an estuarine aquatic environment. Sci. Total Environ. 2019, 698, 134298. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Mandal, S.; Roy, A.; Chakrabarty, S.; Chakrabarti, G.; Pradhan, S.K. Enhanced antifungal activity of fluconazole conjugated with Cu-Ag-ZnO nanocomposite. Mater. Sci. Eng. C 2019, 110, 110160. [Google Scholar] [CrossRef] [PubMed]
- Lozhkomoev, A.; Kazantsev, S.; Kondranova, A.; Fomenko, A.; Pervikov, A.; Rodkevich, N.; Bakina, O. Design of antimicrobial composite nanoparticles ZnxMe (100-x)/O by electrical explosion of two wires in the oxygen-containing atmosphere. Mater. Des. 2019, 183, 108099. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Bhasin, K.; Baskoutas, S. Chemical sensing applications of ZnO nanomaterials. Materials 2018, 11, 287. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Kakarla, R.R.; Reddy, C.; Aminabhavi, T.M. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef]
- Young, S.-J.; Yuan, K.-W. ZnO Nanorod Humidity Sensor and Dye-Sensitized Solar Cells as a Self-Powered Device. IEEE Trans. Electron Devices 2019, 66, 3978–3981. [Google Scholar] [CrossRef]
- Hachem, K.; Ansari, M.J.; Saleh, R.O.; Kzar, H.H.; Al-Gazally, M.E.; Altimari, U.S.; Hussein, S.A.; Mohammed, H.T.; Hammid, A.T.; Kianfar, E. Methods of chemical synthesis in the synthesis of nanomaterial and nanoparticles by the chemical deposition method: A review. BioNanoScience 2022, 12, 1032–1057. [Google Scholar] [CrossRef]
- Droepenu, E.K.; Wee, B.S.; Chin, S.F.; Kok, K.Y.; Maligan, M.F. Zinc oxide nanoparticles synthesis methods and its effect on morphology: A review. Biointerface Res. Appl. Chem. 2022, 12, 4261–4292. [Google Scholar]
- Brangule, A. Nano ZnO: Structure, Synthesis Routes, and Properties. In Materials Research Foundations; Materials Research Forum LLC: Millersville, PA, USA, 2023; Volume 146. [Google Scholar]
- Ali, A.; Phull, A.R.; Zia, M. Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns. Nanotechnol. Rev. 2018, 7, 413–441. [Google Scholar] [CrossRef]
- Jajko-Liberka, G.; Anagha, M.G.; Chytrosz-Wróbel, P.; Kubisiak, P.; Kulig, W.; Cwiklik, L.; Kotarba, A. Sonochemical synthesis of nanoparticles from bioactive compounds: Advances, challenges, and future perspectives. Ultrason. Sonochem. 2025, 121, 107559. [Google Scholar] [CrossRef]
- Jarestan, M.; Khalatbari, K.; Pouraei, A.; Sadat Shandiz, S.A.; Beigi, S.; Hedayati, M.; Majlesi, A.; Akbari, F.; Salehzadeh, A. Preparation, characterization, and anticancer efficacy of novel cobalt oxide nanoparticles conjugated with thiosemicarbazide. 3 Biotech 2020, 10, 230. [Google Scholar] [CrossRef]
- Lasek, P.; Kosikowska, U.; Kołodziej, P.; Kubiak-Tomaszewska, G.; Krzyżanowska, N.; Szostek, T.; Struga, M.; Feldo, M.; Bogucka-Kocka, A.; Wujec, M. New Thiosemicarbazide Derivatives with Multidirectional Biological Action. Molecules 2024, 29, 1529. [Google Scholar] [CrossRef]
- Han, M.İ.; İnce, U.; Gündüz, M.G.; Küçükgüzel, Ş.G. Synthesis, Antimicrobial Evaluation, and Molecular Modeling Studies of New Thiosemicarbazide-Triazole Hybrid Derivatives of (S)-Naproxen. Chem. Biodivers. 2022, 19, e202100900. [Google Scholar] [CrossRef]
- Chen, R.; Huo, L.; Jaiswal, Y.; Huang, J.; Zhong, Z.; Zhong, J.; Williams, L.; Xia, X.; Liang, Y.; Yan, Z. Design, synthesis, antimicrobial, and anticancer activities of acridine thiosemicarbazides derivatives. Molecules 2019, 24, 2065. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc oxide nanoparticles: Antibacterial activity and toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Behal, J.; Maru, M.S.; Katwal, R.; Pathak, D.; Kumar, V. Ultrasonic assisted green synthesis approach for nanotechnological materials. J. Alloys Compd. Commun. 2024, 3, 100013. [Google Scholar] [CrossRef]
- Noman, M.T.; Petru, M.; Militký, J.; Azeem, M.; Ashraf, M.A. One-Pot Sonochemical Synthesis of ZnO Nanoparticles for Photocatalytic Applications, Modelling and Optimization. Materials 2020, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Wang, X.; Zhang, A.; Gao, X.; Yagoub, A.E.-G.A.; Ma, H.; Zhou, C. Ultrasound-Assisted Synthesis of Potentially Food-Grade Nano-Zinc Oxide in Ionic Liquids: A Safe, Green, Efficient Approach and Its Acoustics Mechanism. Foods 2022, 11, 1656. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Natarajan, P.M.; Umapathy, V.R.; Swamikannu, B.; Sivaraman, N.M.; Krishnasamy, L.; Palanisamy, C.P. Advancements in the Synthesis and Functionalization of Zinc Oxide-Based Nanomaterials for Enhanced Oral Cancer Therapy. Molecules 2024, 29, 2706. [Google Scholar] [CrossRef]
- Beigi, S.; Salehzadeh, A.; Habibollahi, H.; Shandiz, S.A.S.; Safa, F. The Effect of ZnO Nanoparticles Functionalized with Glutamine and Conjugated with Thiosemicarbazide on Triggering of Apoptosis in the Adenocarcinoma Gastric Cell Line. Adv. Biomed. Res. 2024, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Veg, E.; Raza, A.; Bansal, P.; Rai, S.; Sharma, S.; Gupta, R.; Khan, T. Synthesis, and characterization of ZnO–thiosemicarbazone nanoconjugates for enhanced biological and photocatalytic activity through surface synergy. Inorg. Chem. Commun. 2025, 181, 115265. [Google Scholar] [CrossRef]
- Veg, E.; Raza, A.; Bansal, P.; Rai, S.; Sharma, S.; Dwivedi, S.; Fatima, N.; Gupta, R.; Joshi, S.; Khan, A.R.; et al. Ultrasonically Synthesized CuO–Thiosemicarbazone Nanoconjugates: A Study on Interfacial Interactions and Synergistic Biological and Photocatalytic Effects. Surf. Interfaces 2025, 72, 107271. [Google Scholar] [CrossRef]
- Nejabatdoust, A.; Zamani, H.; Salehzadeh, A. Functionalization of ZnO nanoparticles by glutamic acid and conjugation with thiosemicarbazide alters expression of efflux pump genes in multiple drug-resistant Staphylococcus aureus strains. Microb. Drug Resist. 2019, 25, 966–974. [Google Scholar] [CrossRef]
- Bale, V.K.; Katreddi, H.R. Synthesis, characterization and catalytic activity of zinc oxide nanoparticles functionalized with metallo-thiosemicarbazones. J. Nanostruct. Chem. 2022, 12, 159–173. [Google Scholar]
- Rabani, H.M.; Isazadeh, K.; Ghasemi, M.F.; Habibi, A. Novel dual-targeting of biofilm formation and pyocyanin production in clinical Pseudomonas aeruginosa isolates using glutamine-modified thiosemicarbazone-conjugated ZnO nanoparticles. Discover Nano 2025, 20, 202. [Google Scholar] [CrossRef]
- Danişman-Kalindemirtaş, F.; Özerkan, D.; Kariper, İ.A.; Cilasun, G.E.; Ülküseven, B.; Erdem-Kuruca, S. Albumin-based nanocarriers loaded with novel Zn (II)-thiosemicarbazone compounds chart a new path for precision breast cancer therapy. Anti-Cancer Drugs 2025, 36, 208–219. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mohamed, A.A. Advances in ultrasound-assisted synthesis of photocatalysts and sonophotocatalytic processes: A review. Iscience 2024, 27, 108583. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Khan, A.; Shehzad, M.T.; Hameed, A.; Ahmed, N.; Halim, S.A.; Khiat, M.; Anwar, M.U.; Hussain, J.; Csuk, R.; et al. Synthesis and characterization of new thiosemicarbazones, as potent urease inhibitors: In vitro and in silico studies. Bioorganic Chem. 2019, 87, 155–162. [Google Scholar] [CrossRef]
- Veg, E.; Hashmi, K.; Ahmad, M.I.; Joshi, S.; Khan, A.R.; Khan, T. Some Biological Applications and Mechanistic Insights of Benzaldehyde-Substituted Thiosemicarbazones and Their Metal Complexes: A Review. Nat. Sci. 2025, 5, e70005. [Google Scholar] [CrossRef]
- Khan, T.; Raza, S.; Hashmi, K.; Ahmad, M.I.; Khan, A.R. Structural modifications for biological activity enhancements in thiosemicarbazone scaffolds and their metal complexes. Synlett 2025, 36, 2732–2762. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).