Assessing the Relationship Between Gesture Intuitiveness and Muscle Network Efficiency: A Comparison of NMF and Inter-Muscular Coherence Analysis Methods †
Abstract
1. Introduction
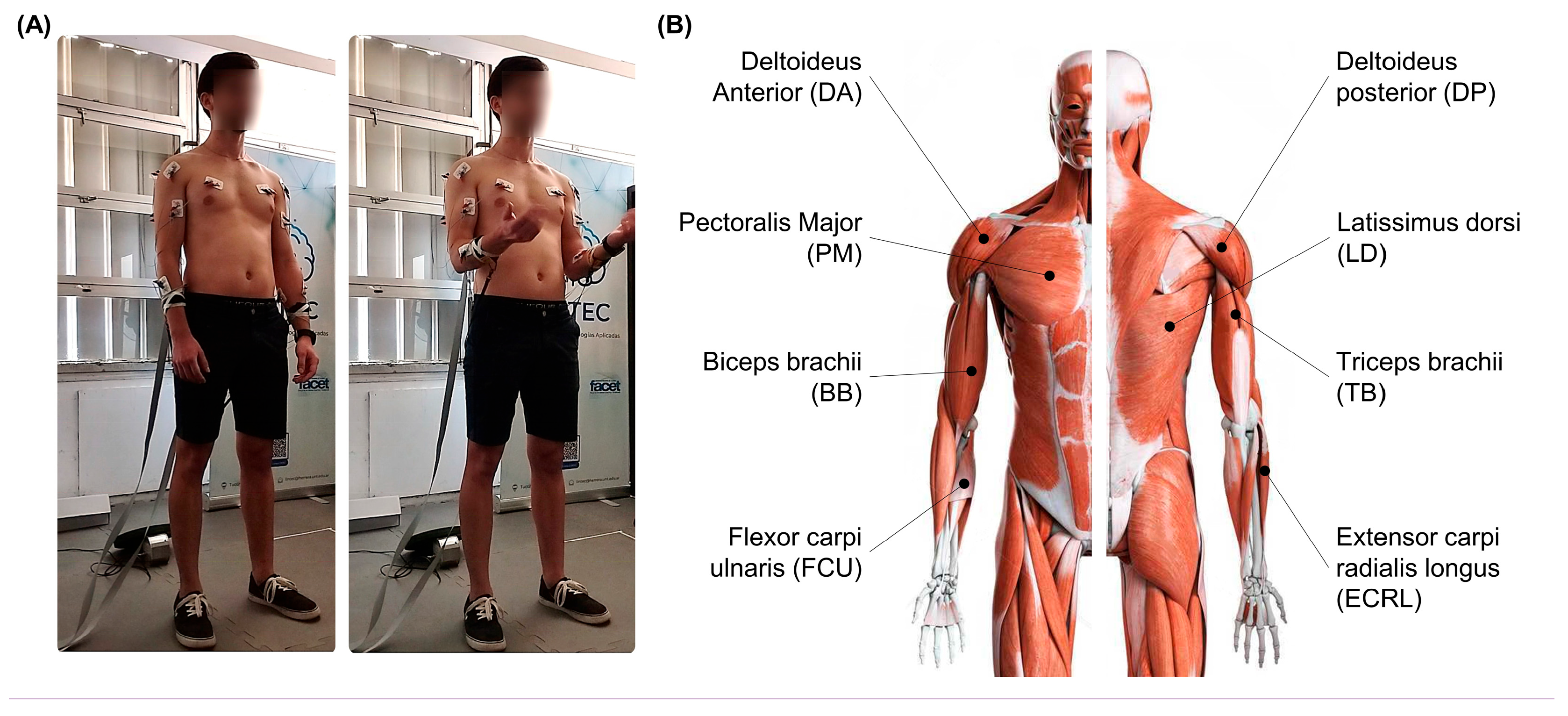
2. Methods
3. Results and Discussion
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villarreal-Narvaez, S.; Vanderdonckt, J.; Vatavu, R.D.; Wobbrock, J.O. A systematic review of gesture elicitation studies: What can we learn from 216 studies? In Proceedings of the 2020 ACM Designing Interactive Systems Conference, Eindhoven, The Netherlands, 6–10 July 2020; pp. 855–872. [Google Scholar] [CrossRef]
- Canuto, C.; Freire, E.O.; Molina, L.; Carvalho, E.A.; Givigi, S.N. Intuitiveness level: Frustration-based methodology for human robot interaction gesture elicitation. IEEE Access 2022, 10, 17145–17154. [Google Scholar] [CrossRef]
- Boonstra, T.W.; Danna-Dos-Santos, A.; Xie, H.B.; Roerdink, M.; Stins, J.F.; Breakspear, M. Muscle networks: Connectivity analysis of EMG activity during postural control. Sci. Rep. 2016, 5, 17830. [Google Scholar] [CrossRef] [PubMed]
- Kerkman, J.N.; Bekius, A.; Boonstra, T.W.; Daffertshofer, A.; Dominici, N. Muscle synergies and coherence networks reflect different modes of coordination during walking. Front. Physiol. 2020, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Kristeva, R.; Patino, L.; Omlor, W. Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. Neuroimage 2007, 36, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Desmyttere, G.; Mathieu, E.; Begon, M.; Simoneau-Buessinger, E.; Cremoux, S. Effect of the phase of force production on corticomuscular coherence with agonist and antagonist muscles. Eur. J. Neurosci. 2018, 48, 3288–3298. [Google Scholar] [CrossRef] [PubMed]
- Elie, D.; Barbier, F.; Ido, G.; Cremoux, S. Corticomuscular coherence and motor control adaptations after isometric maximal strength training. Brain Sci. 2021, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Omlor, W.; Patino, L.; Hepp-Reymond, M.C.; Kristeva, R. Gamma-range corticomuscular coherence during dynamic force output. Neuroimage 2007, 34, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Gwin, J.T.; Ferris, D.P. Beta-and Gamma-range human lower limb corticomuscular coherence. Front. Hum. Neurosci. 2012, 6, 258. [Google Scholar] [CrossRef] [PubMed]
- Mehrkanoon, S.; Breakspear, M.; Boonstra, T.W. The reorganization of corticomuscular coherence during a transition between sensorimotor states. Neuroimage 2014, 100, 692–702. [Google Scholar] [CrossRef] [PubMed]
- L’Abbate, T.; Armonaite, K.; Gianni, E.; Bertoli, M.; Conti, L.; Grifoni, J.; Cancelli, A.; Cottone, C.; Trombetta, E.; Tecchio, F.; et al. Corticomuscular coherence dependence on body side and visual feedback. Neuroscience 2022, 490, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Da Silva, F.L. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Rabbi, M.F.; Pizzolato, C.; Lloyd, D.G.; Carty, C.P.; Devaprakash, D.; Diamond, L.E. Non-negative matrix factorisation is the most appropriate method for extraction of muscle synergies in walking and running. Sci. Rep. 2020, 10, 8266. [Google Scholar] [CrossRef] [PubMed]
- Bigot, J.; Longcamp, M.; Dal Maso, F.; Amarantini, D. A new statistical test based on the wavelet cross-spectrum to detect time–frequency dependence between non-stationary signals: Application to the analysis of cortico-muscular interactions. Neuroimage 2011, 55, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Freire, E.; Cano, L.A.; Rivolta, L.; Albarracín, A.L.; Acosta, L.P.; Farfan, F.D. First Insights About the Relationship Between Gesture Intuitiveness and Muscle Synergy. In Advances in Bioengineering and Clinical Engineering (SABI 2023); Ballina, F.E., Armentano, R., Acevedo, R.C., Meschino, G.J., Eds.; IFMBE Proceedings; Springer: Cham, Switzerland, 2024; Volume 106. [Google Scholar] [CrossRef]
- Gallese, V.; Lakoff, G. The brain’s concepts: The role of the sensory-motor system in conceptual knowledge. Cogn. Neuropsychol. 2005, 22, 455–479. [Google Scholar] [CrossRef] [PubMed]
- Kita, S.; Alibali, M.W.; Chu, M. How do gestures influence thinking and speaking? The gesture-for-conceptualization hypothesis. Psychol. Rev. 2017, 124, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Savaki, H.E.; Raos, V. Action perception and motor imagery: Mental practice of action. Prog. Neurobiol. 2019, 175, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Hostetter, A.B.; Bahl, S. Comparing the cognitive load of gesture and action production: A dual-task study. Lang. Cogn. 2023, 15, 601–621. [Google Scholar] [CrossRef]
- Todorov, E.; Jordan, M.I. Optimal feedback control as a theory of motor coordination. Nat. Neurosci. 2002, 5, 1226–1235. [Google Scholar] [CrossRef] [PubMed]


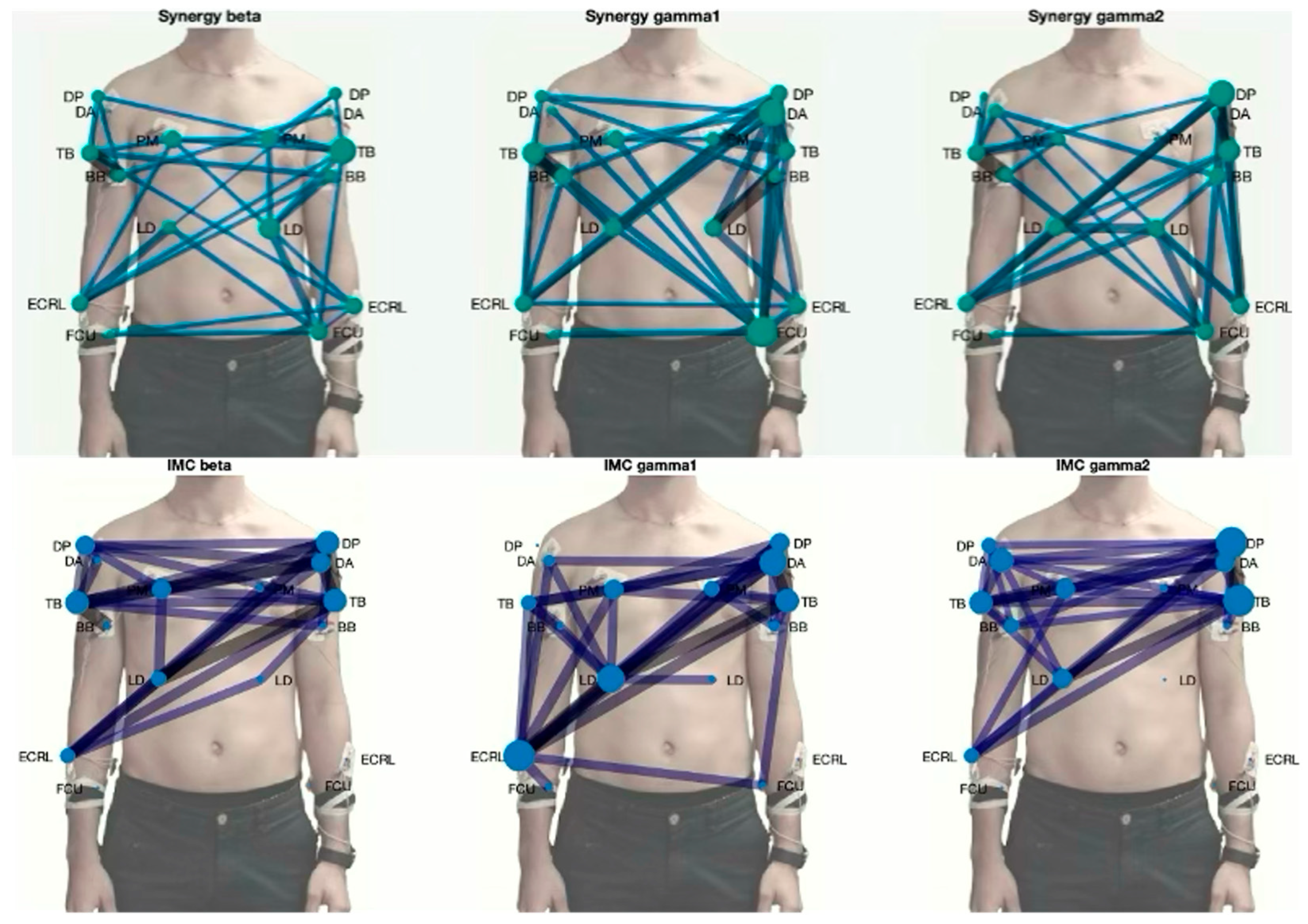
| Gesture | #1 | #2 | #3 | #4 | #5 | #6 | #7 |
|---|---|---|---|---|---|---|---|
| IL | 1.0000 | 0.0965 | 0.3533 | 0.4657 | 0.3916 | 0.0491 | 0.1962 |
| Gesture | Metric | Beta | Gamma 1 | Gamma 2 | |||
|---|---|---|---|---|---|---|---|
| SMN | CMN | SMN | CMN | SMN | CMN | ||
| #1 | WGE | 0.1133 | 0.0472 | 0.0855 | 0.0357 | 0.0846 | 0.0529 |
| #2 | 0.1287 | 0.0531 | 0.0665 | 0.0290 | 0.1156 | 0.0489 | |
| #3 | 0.1037 | 0.0691 | 0.0893 | 0.0510 | 0.0699 | 0.0674 | |
| #4 | 0.0506 | 0.0652 | 0.0916 | 0.0550 | 0.0770 | 0.0523 | |
| #5 | 0.0704 | 0.0779 | 0.0972 | 0.0610 | 0.0739 | 0.0391 | |
| #6 | 0.0719 | 0.0612 | 0.0347 | 0.0525 | 0.0974 | 0.0501 | |
| #7 | 0.0609 | 0.0611 | 0.0461 | 0.0549 | 0.0518 | 0.0434 | |
| #1 | EAS | 0.4628 | 0.2250 | 0.4179 | 0.1736 | 0.4174 | 0.2542 |
| #2 | 0.5423 | 0.2829 | 0.3351 | 0.1804 | 0.5584 | 0.2153 | |
| #3 | 0.4237 | 0.3809 | 0.4193 | 0.2525 | 0.3719 | 0.3707 | |
| #4 | 0.2617 | 0.3635 | 0.4062 | 0.2428 | 0.4278 | 0.2587 | |
| #5 | 0.3489 | 0.2788 | 0.3633 | 0.2288 | 0.4098 | 0.1976 | |
| #6 | 0.3200 | 0.2978 | 0.2218 | 0.2077 | 0.4524 | 0.2001 | |
| #7 | 0.3607 | 0.3136 | 0.2226 | 0.2040 | 0.3089 | 0.1868 | |
| Metric | Beta | Gamma 1 | Gamma 2 | |||
|---|---|---|---|---|---|---|
| SMN | CMN | SMN | CMN | SMN | CMN | |
| WGE | −0.143 | 0.048 | 0.524 * | 0.143 | 0.048 | 0.143 |
| EAS | −0.048 | −0.143 | 0.619 ** | 0.048 | −0.048 | 0.238 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire, E.O.; Cano, L.A.; Gerez, G.D.; Víscido, M.P.; Albarracín, A.L.; Farfán, F.D. Assessing the Relationship Between Gesture Intuitiveness and Muscle Network Efficiency: A Comparison of NMF and Inter-Muscular Coherence Analysis Methods. Eng. Proc. 2024, 81, 22. https://doi.org/10.3390/engproc2024081022
Freire EO, Cano LA, Gerez GD, Víscido MP, Albarracín AL, Farfán FD. Assessing the Relationship Between Gesture Intuitiveness and Muscle Network Efficiency: A Comparison of NMF and Inter-Muscular Coherence Analysis Methods. Engineering Proceedings. 2024; 81(1):22. https://doi.org/10.3390/engproc2024081022
Chicago/Turabian StyleFreire, Eduardo Oliveira, Leonardo Ariel Cano, Gonzalo Daniel Gerez, Manuel Parajón Víscido, Ana Lía Albarracín, and Fernando Daniel Farfán. 2024. "Assessing the Relationship Between Gesture Intuitiveness and Muscle Network Efficiency: A Comparison of NMF and Inter-Muscular Coherence Analysis Methods" Engineering Proceedings 81, no. 1: 22. https://doi.org/10.3390/engproc2024081022
APA StyleFreire, E. O., Cano, L. A., Gerez, G. D., Víscido, M. P., Albarracín, A. L., & Farfán, F. D. (2024). Assessing the Relationship Between Gesture Intuitiveness and Muscle Network Efficiency: A Comparison of NMF and Inter-Muscular Coherence Analysis Methods. Engineering Proceedings, 81(1), 22. https://doi.org/10.3390/engproc2024081022










