Abstract
Excesses and shortages of some metal ions such as Fe3+ and Cr2O72− pose a threat to human life. A Zn-based two-dimensional MOF has proven to be sensitive for Fe3+ and Cr2O72− in water with Ksv values of 1.8 × 104 and 6.5 × 104, respectively. The limit of detection (LOD) for these ions was found to be 2.5 μM and 0.15 μM for Fe3+ and Cr2O72− ions, respectively. A comparative study was conducted using two 3D MOFs in terms of their detection ability, synthesis and structure.
1. Introduction
In order to detect trace amounts of Fe3+ and Cr2O72− ions in water, relatively effective and fast methods of detection are required. Fluorescent sensors are suitable for detection as they are convenient and straightforward to use, with high sensitivity and accuracy [1]. Metal-organic frameworks (MOFs), or porous coordination polymers, are a class of highly ordered crystalline materials consisting of metal ions or metal clusters and organic linkers connected into three-dimensional lattices [2]. In recent years, metal-organic frameworks have become favorable sensing materials for cations/anions and small molecules [3] due to their several structural advantages over other materials [4]. The presence of metals such as Zn (II) and Cd(II) with π-conjugated organic linkers make MOFs good materials for fluorescence sensing [5]. Azolate-containing MOFs with uncoordinated nitrogen atoms can lead to strong interactions with heavy metals, which facilitates fluorescence quenching and consequently enhances the sensitivity of the MOF [6].
Given these considerations, a simple, small N,N-containing linker 5-aminotetrazole was selected as the secondary linker because it contains multiple binding sites, i.e., an amino group that can functionalize the MOF [7]. In this work, a 2D MOF (Zn (ATZ)(HCO2)) BUT-25 (BUT = Beijing University of Technology) with a layer-pillared structure was synthesized [8] with a slightly modified method and applied for the detection of Fe3+ and Cr2O72− ions in water. This MOF proved to be highly sensitive at detecting trace amounts of Fe3+ and Cr2O72− in aqueous solution, with a limit of detection (LOD) of 0.25 μM and 0.15 μM for Fe3+ and Cr2O72−, respectively. These values are less than the minimum values specified by US-EPA (15.7 μM and 2 μM (0.1 mg/L) [9,10]. In addition, a comparative study was performed with two different 3D MOFs, which also showed that BUT-25 is superior to the other two methods in terms of stability and recycling. Hence, we determined that BUT-25 can act as a selective and sensitive sensor for these ions.
2. Methods
The solid-state emission spectra of HATZ ligand and BUT-25 were investigated to check their fluorescence at room temperature. For the measurements, 20 mg of ground BUT-25 was added into 20 mL of water and sonicated until a uniform suspension formed. Subsequently, 0.1 mL of 5mM cation solutions and 5 mM anion solutions were introduced into cuvettes preloaded with 1mL of BUT-25 suspension, and the mixture was shaken for consistency. The Fe3+ and Cr2O72− detection limit was recorded with the decrease in fluorescence intensity of the BUT-25, which was observed after adding cation and anion aqueous solutions (Fe3+ and Cr2O72−) with varying concentrations into 1 mL MOF suspensions in DMF, respectively.
3. Result and Discussion
3.1. Structural Description
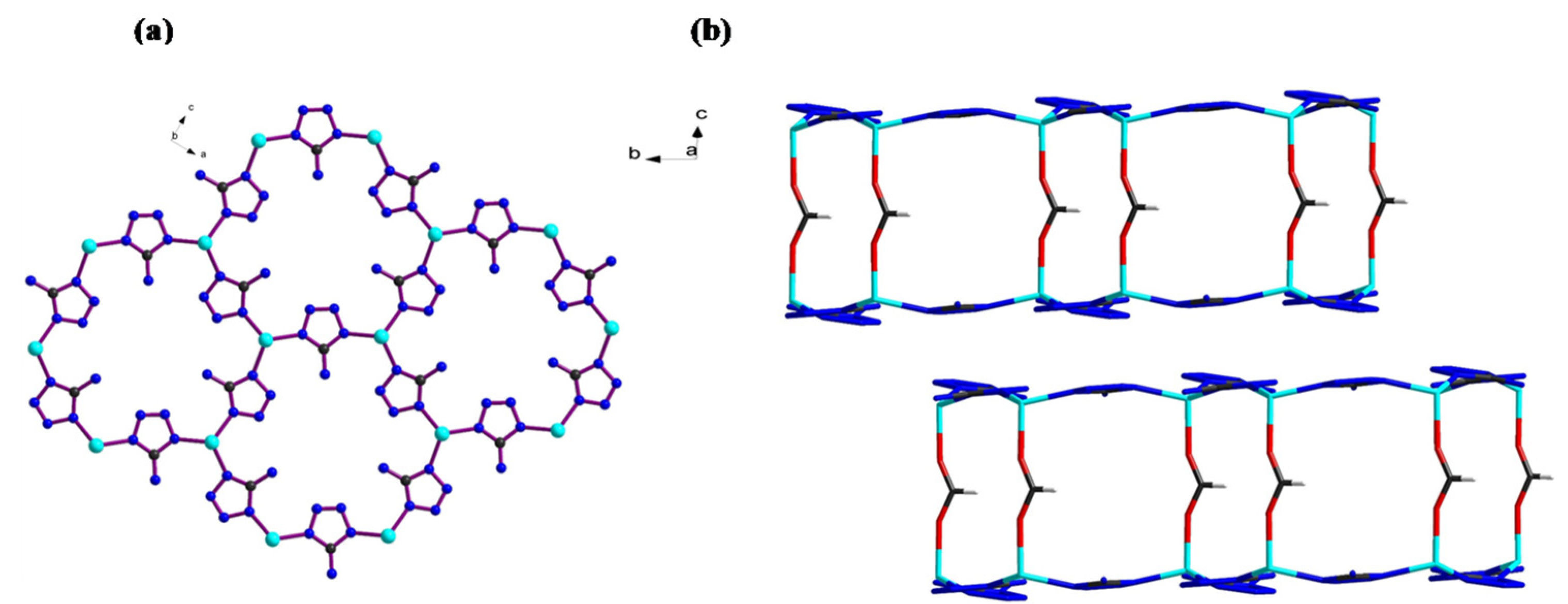
The structures of all the synthesized MOFs have been reported previously [8,11]. BUT-25 consists of honeycomb-shaped bilayers, in which a Zn atom is tetrahedrally connected to three N atoms from three deprotonated ATZ- (5-aminotetrazole) and one oxygen atom from formate ions (HCOO–). Adjacent Zn atoms are connected by deprotonated ATZ−, while bilayers are connected via formate ions, as shown in Figure 1a,b. For comparison, BUT-26 was synthesized through the sequential addition of thiophene-2,5-dicarboxylic acid (H2TDC) to the crystals of BUT-25. The formate ions were replaced by H2TDC without disturbing the original structure of the honeycomb-shaped layers. It appeared that these layers adjusted themselves to accommodate the sequentially inserted linker, as shown in Figure 1c. BUT-27 was synthesized through the sequential addition of isopthathalic acid (H2IPA) to the crystals of BUT-25. The linker did not replace any of the BUT-25 linkers; rather, it converted into a totally new structure similar to zeolite LTA topology, as shown in Figure 1d.
Figure 1.
(a) honeycomb-shaped layer of BUT-25 (b) bilayers connected by formate ions in BUT-25.
3.2. Fluorescence Properties
Interestingly, Fe3+ and Cr2O72− quenched the fluorescence of BUT-25 by nearly 80% and 90%, respectively; the rest of the ions featured lower quenching efficiencies, as shown in Figure S2. It is clear from Figure S3b,d that the S-V plots for Fe3+ and Cr2O72− ions exhibited a good linear correlation (R2 = 0.994, 0.993) in the concentration range of 0.1–0.1 µM for Cr2O72− and 1–10 µM for Fe3+ ions. As the concentration increased, the S-V plots deviated from linearity and bent upwards, as shown in Figure S4a,b, indicating its high potential for practical application.
3.3. Sensing Mechanism
The PXRD patterns of BUT-25 before and after the fluorescence experiments were in accordance with each other, excluding the possibility of structural damage (Figure S1) [12]. The intense color change visible to the naked eye after the fluorescence tests indicates a strong interaction between the MOF and the target ions, as shown in Figures S5b,c, which might be responsible for detecting Cr2O72− and Fe3+ ions [13]. The UV-Vis absorption spectra of selected anions clearly shows that the absorption spectrum of Cr2O72− consists of two bands in the 200–600 nm range, which covered the excitation wavelength (290 nm) and emission spectrum of BUT-25, while absorption spectrum of Fe3+ ion covered the excitation wavelength of BUT-25, as shown in Figures S6 and S7, pointing towards fluorescence quenching of BUT-25 due to the inner filter effect and resonance energy transfer mechanism [14]. This overlap would reduce the excitation of BUT-25, resulting in weaker fluorescence in the presence of Cr2O72− and Fe3+ ions. However, no such overlap was observed for other anions and cations. Different metal cations may show different quenching behavior of luminescence because all cations feature different unsaturated electronic configurations. In comparison to BUT-26 and BUT-27, the performance of BUT-25 was quite satisfying in stability and recycling. The stability of both 3D structures was unsatisfactory as they could not recycle, even after washing with different organic solvents, such as DMF.
4. Conclusions
We successfully employed a 2D-MOF BUT-25 for the detection of Fe3+ and Cr2O72− ions in water with Ksv values of 1.8 × 104 and 6.5 × 104, respectively, which is quite a high value compared with many reported MOFs. The 2D-MOF BUT-25 also shows good selectively, accompanied by color change visible to naked eye and recycling. The post-synthetic modification of BUT-25 through the sequential insertion of dicarboxylic linkers provides an example of how to post-synthetically modify a 2D MOF into a 3D MOF, which is helpful in widening the applications of MOF.
Author Contributions
K.T.: methodology, investigations, data curation, and writing—original draft. A.: investigation, writing—review and editing. J.-R.L.: writing—review and editing, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We acknowledge the financial support from the Natural Science Foundation of China (Grant Nos. 21771012, 21601008), the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (Grant No. 51621003), and the Science and Technology Project of Beijing Municipal Education Committee (KZ201810005004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, B.; Ma, H.; Zhang, B.; Qian, J.; Cao, T.; Feng, H.; Li, W.; Dong, Y.; Qin, W. Dually emitting carbon dots as fluorescent probes for ratiometric fluorescent sensing of pH values, mercury (II), chloride and Cr (VI) via different mechanisms. Microchim. Acta 2019, 186, 341. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2011, 112, 1126–1162. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F. Luminescent metal-organic frameworks as potential sensory materials for various environmental toxic agents. Coord. Chem. Rev. 2019, 401, 213065. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Goswami, R.; Smith, V.J.; Bajaj, H.C.; Neogi, S. Pore Wall-Functionalized Luminescent Cd (II) Framework for Selective CO2 Adsorption, Highly Specific 2, 4, 6-Trinitrophenol Detection, and Colorimetric Sensing of Cu2+ Ions. ACS Sustain. Chem. Eng. 2018, 6, 10295–10306. [Google Scholar] [CrossRef]
- Zhou, E.-L.; Qin, C.; Tian, D.; Wang, X.-L.; Yang, B.-X.; Huang, L.; Shao, K.-Z.; Su, Z.-M. A difunctional metal–organic framework with Lewis basic sites demonstrating turn-off sensing of Cu2+ and sensitization of Ln3+. J. Mater. Chem. C 2018, 6, 7874–7879. [Google Scholar] [CrossRef]
- Zhang, H.; Sheng, T.; Hu, S.; Zhuo, C.; Li, H.; Fu, R.; Wen, Y.; Wu, X. Stitching 2D polymeric layers into flexible 3D metal–organic frameworks via a sequential self-assembly approach. Cryst. Growth Des. 2016, 16, 3154–3162. [Google Scholar] [CrossRef]
- Talha, K.; He, T.; Xie, L.-H.; Wang, B.; Zhao, M.-J.; Zhang, Y.-Z.; Chen, Q.; Li, J.-R. A three-dimensional metal–organic framework with high performance of dual cation sensing synthesized via single-crystal transformation. New J. Chem. 2020, 44, 11829–11834. [Google Scholar] [CrossRef]
- Swaidan, A.; Borthakur, P.; Boruah, P.K.; Das, M.R.; Barras, A.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Szunerits, S.; Boukherroub, R. A facile preparation of CuS-BSA nanocomposite as enzyme mimics: Application for selective and sensitive sensing of Cr (VI) ions. Sens. Actuators B Chem. 2019, 294, 253–262. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, H.; Sun, L.; Yan, Y.; Wang, B.; Liang, Z.; Wang, L.; Li, J. Water Stable Metal–Organic Framework Based on Phosphono-containing Ligand as Highly Sensitive Luminescent Sensor toward Metal Ions. Cryst. Growth Des. 2018, 18, 7683–7689. [Google Scholar] [CrossRef]
- Alamgir, A.; Ahamd, N.; Xie, L.-H.; Zhang, X.; Li, J.-R. Construction of Zeolite A Type Multivariate Metal-Organic Framework for Selective Sensing of Fe3+ and Cr2O72. Cryst. Eng. Comm. 2021, 23, 4923–4929. [Google Scholar]
- Sun, S.; Wang, F.; Sun, Y.; Guo, X.; Ma, R.; Zhang, M.; Guo, H.; Xie, Y.; Hu, T. Construction of a Dual-Function Metal–Organic Framework: Detection of Fe3+, Cu2+, Nitroaromatic Explosives, and a High Second-Harmonic Generation Response. Ind. Eng. Chem. Res. 2019, 58, 17784–17791. [Google Scholar] [CrossRef]
- Liu, L.-L.; Yu, Y.-Z.; Zhao, X.-J.; Wang, Y.-R.; Cheng, F.-Y.; Zhang, M.-K.; Shu, J.-J.; Liu, L. A robust Zn (ii)/Na (i)-MOF decorated with [(OAc)2 (H2 O)2]n2n− anions for the luminescence sensing of copper ions based on the inner filter effect. Dalton Trans. 2018, 47, 7787–7794. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Bai, Z.; Li, Y.; Wang, Y.; Chen, L.; Xu, L.; Diwu, J.; Chai, Z.; Wang, S. Hydrolytically stable luminescent cationic metal organic framework for highly sensitive and selective sensing of chromate anions in natural water systems. ACS Appl. Mater. Interfaces 2017, 9, 16448–16457. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).