Abstract
Fluoride is a crucial inorganic anion found in drinking water, which may pose serious health hazards to human health if consumed in excess amounts. The quantification of fluoride in drinking water with high sensitivity, selectivity, and cross-sensitivity is critical. Given these factors, the present work proposes a spectroelectrochemical sensing platform for fluoride sensing using 5,10,15,20-tetraphenyl-21H,23H-porphine iron (III) chloride (FeTPP), and tetrabutylammonium perchlorate (TBAP) as the electrolyte. The proposed spectroelectrochemistry (SEC) is a hybrid platform that concurrently provides spectroscopic and electrochemical information about a system susceptible to oxidation and reduction. An ensemble–based multivariate prediction model was developed to simultaneously analyze electrochemical and spectroscopic data to predict fluoride concentration with enhanced reliability and precision. The prediction model provided promising results with a coefficient of determination of 0.9923 ± 0.0063 and a MSE of 0.369 ± 0.0596. These encouraging results demonstrate the promising performance of the proposed spectroelectrochemical platform in complex real-world applications.
1. Introduction
Spectroelectrochemistry (SEC) is a hybrid technique that simultaneously delivers spectroscopic and electrochemical data about a system prone to oxidation and reduction. Depending on the type of investigation and system under study, every spectroscopic method can theoretically be coupled with any electrochemical technique to provide unique information. This powerful hybrid approach allows simultaneous monitoring of spectral and electrochemical changes, providing comprehensive insights into electron-transfer mechanisms and molecular structures. SEC’s capacity to interpret intricate reaction dynamics has led to its enormous significance in a number of domains, including materials research, catalysis, bioelectrochemistry, etc. [1]. Predominantly in the field of sensing, the dual-domain integrated approach is highly promising for the selective identification of a species in the presence of various interferents [2,3,4,5]. Moreover, this concept, coupled with chemometrics/machine learning, can reduce the number of false-positive data in real-time through cross-verification and help in detecting multiple species with identifiable fingerprints in the optical and electrochemical domain [6,7,8].
Further, in designing an SEC sensor, the sensing material plays a pivotal role. Metalloporphyrins offer tunable and strong redox activity, allowing the generation of sensitive electrochemical signals along with strong optical absorption bands (Soret and Q-bands). The distinct optical absorption bands help in selective spectroelectrochemical readout [9]. Iron(III) tetraphenylporphyrin (FeTPP), with iron as its central metal ion, exhibits a distinct tendency to form complexes with perchlorate ions. Furthermore, it demonstrates the ability to coordinate with fluoride ions, expanding its utility in ligand-binding and sensing studies. Thus, SEC has proven to be a versatile tool in understanding both fundamental and applied aspects of electrochemistry; however, its potential remains underexplored in the field of sensing.
This work proposes a spectroelectrochemical-based platform for fluoride sensing using 5,10,15,20-tetraphenyl-21H,23H-porphine iron (III) chloride (FeTPP), and tetrabutylammonium perchlorate (TBAP) as electrolyte. The fluoride concentration varied from 0.5 ppm to 20 ppm for sensing studies. The sensing mechanism has been validated by performing interference studies in the presence of interfering ions: chloride (Cl−), bromide (Br−), iodide (I−), sulfate (SO42−), and nitrate (NO3−).
A machine learning algorithm was also developed to predict fluoride concentration in water. The absorbance data, combined with IV data, provided two complementary signals for a single sample. These datasets were integrated and fed into the developed algorithm, enabling accurate prediction of fluoride concentration with enhanced reliability and precision.
2. Materials and Methods
2.1. Materials
5,10,15,20-tetraphenyl−21H,23H−porphine iron(III) chloride (FeTPP), tetrabutylammonium perchlorate (TBAP), and dimethylformamide (DMF) were procured from Sigma Aldrich (Darmstadt, Germany) and were used as received. The IC TraceCERT® standard solutions of 1000 ppm of fluoride (F−), chloride (Cl−), bromide (Br−), iodide (I−), sulfate (SO42−), and nitrate (NO3−) were also procured from Sigma Aldrich. The dilutions of all the standards were performed using deionized water (18 MΩ cm−1).
2.2. Sensor Preparation
A 1.42 mM stock solution of FeTPP was prepared by continuously stirring 10 mg of FeTPP in 10 mL of DMF at 50 °C for 4 h to ensure dissolution and homogeneity. Further, 0.1 M TBAP was prepared in DMF, which was used as an electrolyte for the spectroelectrochemical studies. The sensor-probe/chemosensor for the detection of fluoride was prepared using FeTPP and TBAP in a ratio of 1:4. All sensing and interference studies were conducted using the as-prepared chemosensor.
2.3. Instruments Used
Cyclic voltammetry and in situ absorbance measurements were carried out using a customized portable set-up comprising (i) a three-electrode bulk electrochemical set-up and Precision Source/Measure Unit (Keysight B2902, Keysight Technologies, Santa Rosa, CA, USA) as the electrochemical platform, (ii) a spectroelectrochemical cell integrated with collimating lenses, (iii) a light source (RIDH2000, Hangzhou Brolight Technology Co., Ltd. (Brolight Tech), Hangzhou, China), and (iv) spectrophotometer (Brolight BIM-6002, RI Instruments & Innovation India (RI Group), Haldwani, India). The light source was positioned parallel to the electrode, introducing a beam that passed through the analyte solution and reached the detector, as shown in Figure 1. Customized disposable cuvettes with a path length of 1.0 cm and volume of 1 mL were used for all experiments.
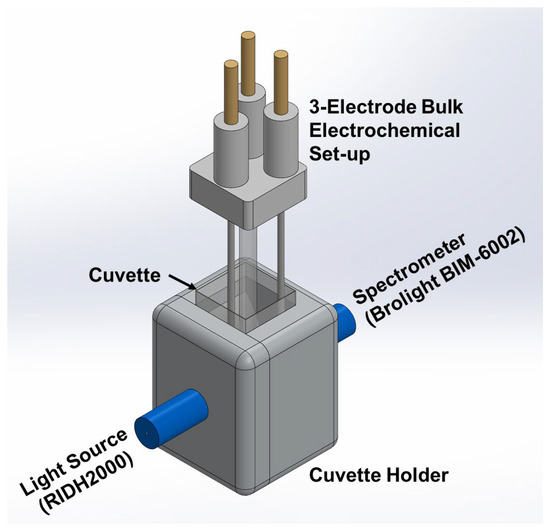
Figure 1.
Data acquisition set-up for spectroelectrochemical sensing of fluoride using iron porphyrin.
2.4. Data Analysis/Prediction
For data analysis, all the electrochemical and optical data were plotted using Origin 2024b software. An ensemble learning-based multivariate machine learning prediction model was developed for the prediction and quantification of fluoride in the presence of interfering ions. The developed model used linear regression (LR) for prediction. The model generated the ensemble results for the IV and recorded absorbance data. The multivariate prediction model was trained before validation using the subset of the concentration matrix from 0.5 to 20 ppm fluoride. The dataset was divided into 80% for training and 20% for testing based on random selection.
3. Results and Discussion
3.1. Spectroelectrochemical Characterization
Cyclic voltammetry was carried out for each sample in a four-wire configuration using a 4-wired configuration in KeySight Precision Source/Measure Unit 2902 (Merck KGaA, Darmstadt, Germany) and a three-electrode bulk electrochemical set-up. The potential applied across the electrode was linearly varied from −1.5 V to 1.5 V and back at a scan rate of 60 mV/s using Quick IV Measurement software. The absorbance spectra were simultaneously acquired in this potential window for a wavelength range of 400–750 nm using BSV 4.09 software. This configuration enabled the capture of dual signals for a single sample, i.e., optical and electrochemical signals. These spectroelectrochemical (SEC) measurements were recorded for different concentrations of fluoride and interfering ions using the as-prepared chemosensor.
3.2. Sensing Studies
FeTPP and TBAP were used for all the sensing and interference experiments in a ratio of 1:4 as a sensing probe. A total of 1 mL of solution was used for each experiment. A range of fluoride concentrations from 0.5 ppm to 20 ppm was prepared by serial dilution of IC TraceCERT® standard solutions of a 1000 ppm stock solution of fluoride. The interference studies were conducted using chloride (Cl−), bromide (Br−), iodide (I−), sulfate (SO42−), and nitrate (NO3−) as interferents, and their concentrations were considered within the permissible range, issued by WHO/BIS/EPA. The concentration matrix used for interference studies is given in Table 1.

Table 1.
Concentration matrix of analyte and interfering ions for the interference studies.
The electrochemical measurements were performed using cyclic voltammetry, where the voltage was varied linearly from −1.5 V to 1.5 V at different scan rates to study the dominant reduction and oxidation. This revealed multiple oxidation and reduction, attributed to the metal-centered reductions with three consecutive one-electron transfers for the final formation of Fe(I) or neutral Fe species [10]. During the cyclic voltammetry, the reduction in FeTPP occurred in three steps, and the resulting Fe(I) species in Fe(I)TPP was generated, which is highly reactive. After these reductions, fluoride ions (F−) can bind to the iron center of FeTPP, forming a stable Fe-F bond, regardless of whether the iron is in the Fe(II), Fe(III), or Fe(I) oxidation state. Among different ions, fluoride has a high tendency to form complexes with trivalent metal ions such as iron [11,12]. The emergence of a new peak in the absorption in the Q band supports the proposed reaction mechanism.
The reaction with fluoride is represented as
where X represents any counter-ion from the electrolyte (e.g., TBAP), which balances the charge in the solution.
Fe(X)TPP + F− → Fe-F + X−
The absorbance spectra and IV spectra for iron porphyrin are shown in Figure 2a,b for fluoride concentration varying from 0.5 ppm to 20 ppm. The addition of TBAP resulted in the appearance of absorption bands around 505 nm, 531 nm, and 694 nm and the disappearance of absorption bands associated with Q-bands. These spectral changes are related to redox-active interactions with the Fe center in FeTPP, which can influence the metal’s oxidation state and the associated electronic transitions. Furthermore, with the addition of fluoride, the emergence of new peaks was observed at 614 nm and 649 nm, with the disappearance of the peak at 698 nm.
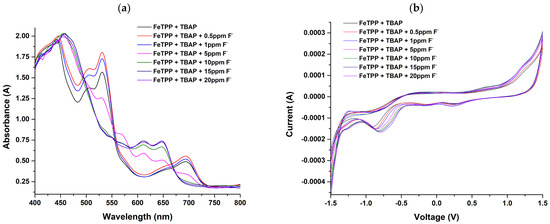
Figure 2.
(a) Absorbance spectra; (b) IV spectra of 5,10,15,20−tetraphenyl−21H,23H−porphine iron (III) chloride (FeTPP) for varying concentrations of fluoride from 0.5 ppm to 20 ppm.
3.3. Ensemble Learning-Based Multivariate Prediction Model
The developed ensemble learning-based prediction model took both IV and absorbance data as input and predicted the concentration of fluoride. Linear regression was used with an average of 10 runs for fluoride sensing, along with the interfering ions. The R2 and MSE for the developed prediction model are given in Table 2.

Table 2.
R2 and MSE for the linear regression of fluoride sensing in water.
The selectivity studies were performed in the presence of different interfering ions, and the response was studied in terms of absorbance recorded at 614.147 nm, as presented in Figure 3a, which shows high selectivity of the proposed sensor for fluoride as compared to other potential interferences. The effect of the electrochemical technique on the cross-sensitivity of the proposed scheme was also studied. A voltage peak shift vs. current graph is shown in Figure 3b. It is evident that in the case of fluoride ions, there is a voltage shift along with a high magnitude of current as compared to other interfering ions. Thus, this helps to understand and distinguish different interfering ions in real-world scenarios. The use of the dual technique, i.e., electrochemical data embedded with spectroscopic data, helps avoid false alarms in complex interfering mixtures in real-world applications.
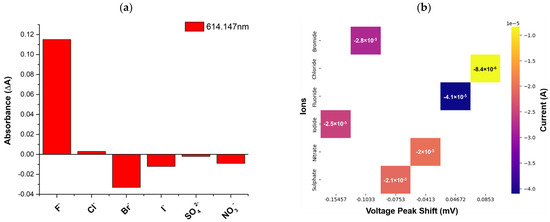
Figure 3.
(a) Selectivity studies for different interfering ions at 614.147 nm; (b) Cross-sensitivity studies conducted using IV spectra recorded during cyclic voltammetry.
4. Conclusions
A spectroelectrochemical platform using a three-electrode bulk electrochemical set-up for detecting fluoride in water is proposed with better selectivity and cross-sensitivity. The selective and sensitive detection of fluoride was carried out using iron porphyrin (FeTPP). The hybrid spectroelectrochemical technique not only aids in enhancing the sensitivity but also enables the proposed scheme to avoid interfering ions, offering better cross-sensitivity for real-world applications.
Author Contributions
S.R.: Formal analysis, Investigation, Methodology, Writing—Original draft; S.B.: Conceptualization, Formal analysis, Methodology, Supervision, Writing—Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available on request.
Acknowledgments
The authors express gratitude to CSIO Analytical Facilities for sample characterization.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Garoz-Ruiz, J.; Perales-Rondon, J.V.; Heras, A.; Colina, A. Spectroelectrochemical Sensing: Current Trends and Challenges. Electroanalysis 2019, 31, 1254–1278. [Google Scholar] [CrossRef]
- Ghoorchian, A.; Afkhami, A.; Madrakian, T.; Rameshan, R.; Rameshan, C.; Hajian, A. Absorbance-based spectroelectrochemical sensor for determination of ampyra based on electrochemical preconcentration. Sens. Actuators B Chem. 2020, 324, 128723. [Google Scholar] [CrossRef]
- Maizels, M.; Seliskar, C.J.; Heineman, W.R. Spectroelectrochemical Sensing Based on Multimode Selectivity Simultaneously Achievable in a Single Device. 7. Sensing of Fe(CN)64−. Electroanalysis 2000, 12, 1356–1362. [Google Scholar] [CrossRef]
- Shi, Y.; Slaterbeck, A.F.; Seliskar, C.J.; Heineman, W.R. Spectroelectrochemical Sensing Based on Multimode Selectivity Simultaneously Achievable in a Single Device. 1. Demonstration of Concept with Ferricyanide. Anal. Chem. 1997, 69, 3679–3686. [Google Scholar] [CrossRef]
- Heineman, W.R.; Seliskar, C.J.; Bryan, S.A. Spectroelectrochemical Sensor for Pertechnetate Applicable to Hanford and Other DOE Sites; University of Cincinnati: Cincinnati, OH, USA; Pacific Northwest National: Richland, WA, USA, 2012. Available online: https://www.osti.gov/biblio/1050972 (accessed on 12 August 2025).
- Chapman, J.; Truong, V.K.; Elbourne, A.; Gangadoo, S.; Cheeseman, S.; Rajapaksha, P.; Latham, K.; Crawford, R.J.; Cozzolino, D. Combining Chemometrics and Sensors: Toward New Applications in Monitoring and Environmental Analysis. Chem. Rev. 2020, 120, 6048–6069. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Bagchi, S. Multiview Ensemble Learning Framework for Real-Time UV Spectroscopic Detection of Nitrate in Water with Chemometric Modelling. J. Chemom. 2025, 39, e70033. [Google Scholar] [CrossRef]
- Martynko, E.; Kirsanov, D. Application of chemometrics in biosensing: A brief review. Biosensors 2020, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Harriman, A.; Richoux, M.C.; Neta, P. Redox chemistry of metalloporphyrins in aqueous solution. J. Phys. Chem. 1983, 87, 4957–4965. [Google Scholar] [CrossRef]
- Ryan, M.D.; DeSilva, C. The voltammetric study of the reduction of tetraalkylammonium perchlorate by Fe (TPP)2−. J. Porphyr. Phthalocyanines 2007, 11, 519–523. [Google Scholar] [CrossRef]
- Shamsipur, M.; Chaichi, M.J. Effects of some trivalent metal ions on the fluoride—Induced chemiluminescence from a phenylphosphate—Substituted dioxetane Lumigen PPD. Luminescence 2002, 17, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Wamser, C.C. Fundamentals of Organic Reaction Mechanisms. 1976. Available online: https://cir.nii.ac.jp/crid/1130000795072601856 (accessed on 19 April 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).