Abstract
The study is devoted to the remediation of contaminated soil with the presence of potentially toxic elements by flotation. The research summarizes the results concerning the verification of the effectiveness of selected flotation agents at different doses. Kerosene and potassium ethyl xanthogenate were used as reagents. Application of these two reagents simultaneously and flotation without the use of a collector were also tested. The flotation tests showed that similar results were obtained with kerosene, which is a non-polar collector, as with the polar potassium ethyl xanthogenate, which as an anionic collector is designed specifically for metal flotation. Interesting results were also achieved in flotation without the use of a collector.
1. Introduction
The soil is a component of a bounded space in which living communities are influenced by the following basic abiotic factors: rocks, water, atmosphere, and climate. To understand the importance of soil, it is necessary to highlight both the spatial heterogeneity of soil in the landscape and the temporal variability of its properties throughout the seasons [1]. The importance of soil becomes most evident when it is endangered. In the European Union, the main processes that degrade soil are erosion, loss of organic matter, salinization, loss of biodiversity, development, landslides, flooding, and contamination [1,2]. Consequences of soil degradation include reduced fertility, carbon release, loss of biodiversity, decreased water retention, disrupted gas and nutrient cycling, and poorer contaminant degradation. Thus, land degradation directly impacts water and air quality, biodiversity, and climate change. It can also compromise public health and threaten food and feed safety [1,2].
2. Materials and Methods
The samples of soil were taken in the area of former important metallurgical works, located in the city centre of Ostrava. The samples were manipulated in accordance with Czech technical standard based on the international ISO standard (ČSN ISO) 11,464 (836160) Soil quality—Sample preparation for physicochemical analyses [3].
After the solid parts (stones, twigs, etc.) were removed from the soil, the samples were air-dried, homogenized, and then ground in a laboratory vibratory mill (VM 4-386). The material was then sieved using a Retsch AS 200 vibratory screener for particles smaller than 0.25 mm. These experiments were conducted in the laboratories of the Department of Environmental Engineering at VSB-Technical University of Ostrava (VŠB–TUO).
The study investigated the flotation efficiency of individual elements using different flotation agents. At the beginning of the flotation experiment, a representative soil sample was collected and analyzed by Wavelength Dispersive X-ray Fluorescence (WD XRF) spectrometry. This analysis focused on the presence of risk elements (Zn, Cr, Ni, Cu, As, and Pb). Their concentrations are shown in Table 1.

Table 1.
Concentration of risk elements in the original sample.
Flotation tests were carried out using kerosene and potassium ethyl xanthogenate and were carried out on a pneumo-mechanical flotator VRF-1, which was manufactured by a company RD Příbram, Příbram, Czech Republic. The doses of flotation collectors were chosen on the basis of professional publications [4]. The initial 4 flotations were executed using kerosene. Subsequent to this, 3 additional flotations were executed, each employing potassium ethyl xanthogenate. Subsequently, a flotation process was executed in the absence of any collector. The final flotation was a test using both kerosene and potassium ethyl xanthogenate. The flotation tests were conducted under the following conditions: the slurry was initially agitated in a flotation cell without forced aeration for a duration of 10 min. The frother, composed of pine oil, was administered 1 min prior to the initiation of the flotation process. The flotation process was then executed for a duration of 5 min. The aeration of the slurry was measured at 300 dm3/m2min. A Büchner funnel was used to filter out the resulting products. The flotation concentrate drained from liquids and waste was dried in a drying oven at 26 °C until it reached a constant weight. All flotation experiments were carried out under the same conditions, while only the doses of the reagents varied (Table 2):

Table 2.
Doses of the individual reagents.
- weight: 100 g soil,
- agitation: 10 min,
- frother: pine oil,
- frother dose: 1300 g/t,
- flotation time: 5 min,
- rotation speed: 1350 rpm.
3. Results
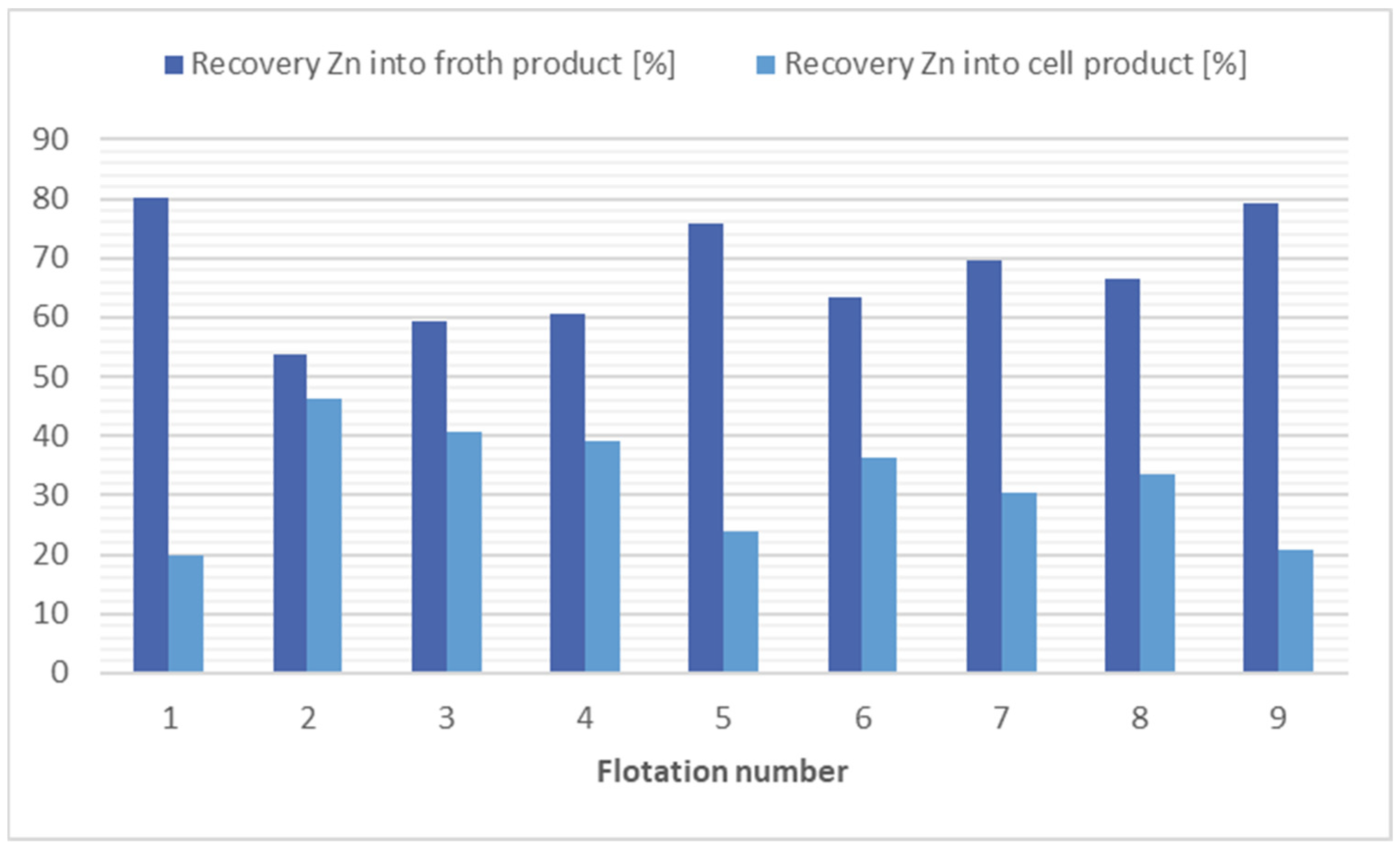
In the flotation process, the most effective separation method for zinc is using kerosene (4000 g/t), flotation 1, or kerosene (12,000 g/t) with potassium ethyl xanthogenate (200 g/t), flotation 9. However, considering economic and ecological factors, the best option is flotation 8, in which no collector was applied and still a significant reduction in zinc concentration (1.52×) was observed. The results of flotation tests with the purpose of zinc separation are presented in Figure 1. The results of flotation tests with the purpose of all risk metals separation are presented in Table 3.
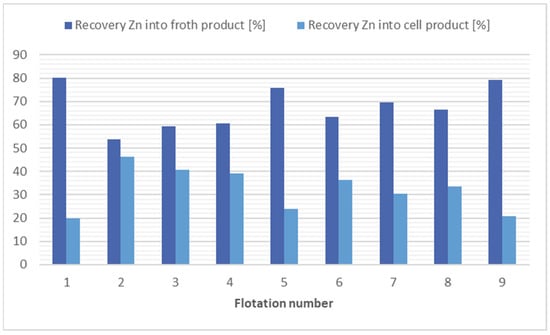
Figure 1.
Comparison of flotation results for zinc separation.

Table 3.
Comparison of flotation results.
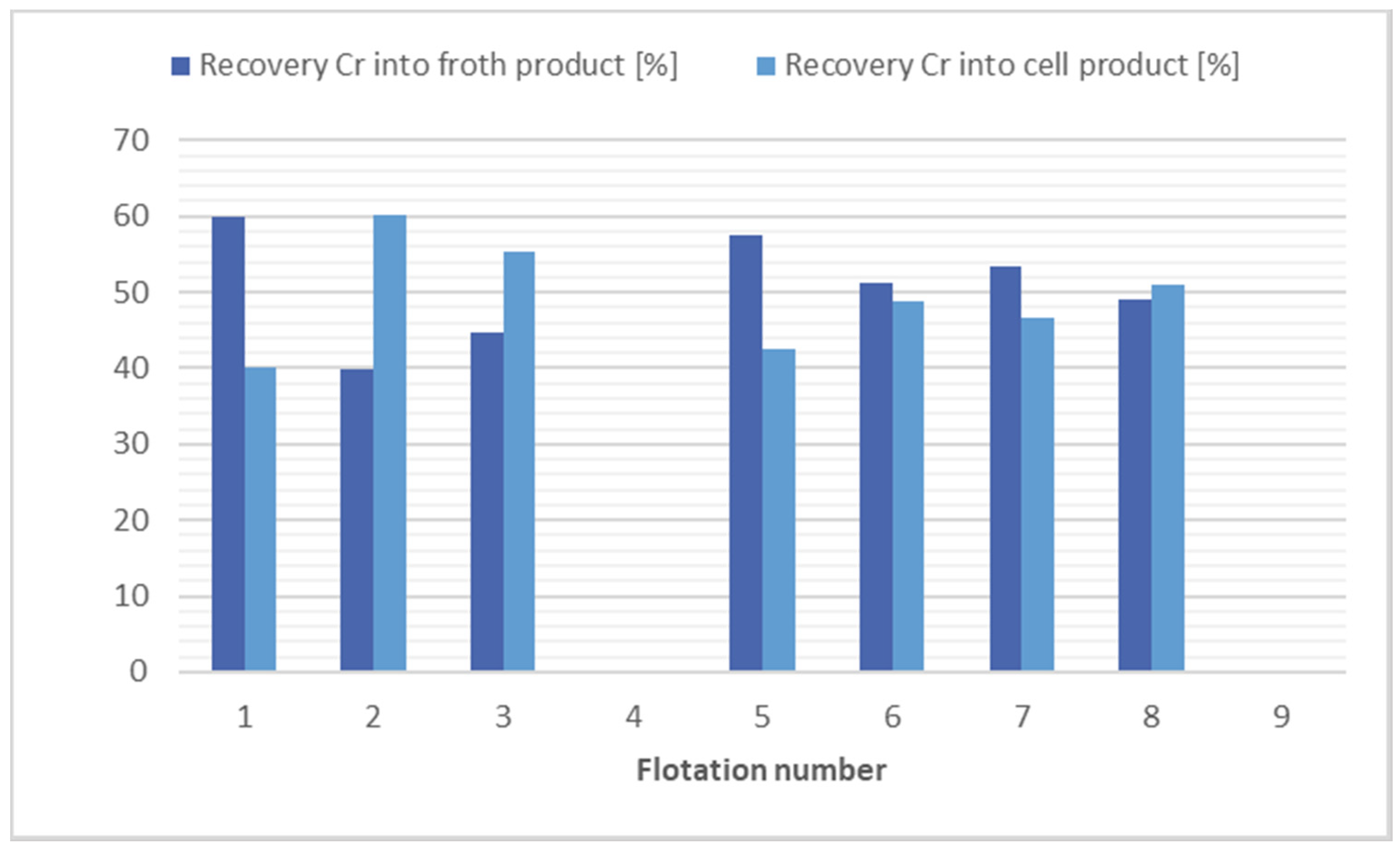
The results of the flotation test on soil samples for chromium separation suggest that the flotation process is ineffective under the prevailing conditions. Virtually none of the flotations carried out resulted in a significant reduction in the concentration of this metal. Only a separation of the mass into froth product and cell products was observed. The results of the flotation tests for chromium separation are shown in Figure 2.
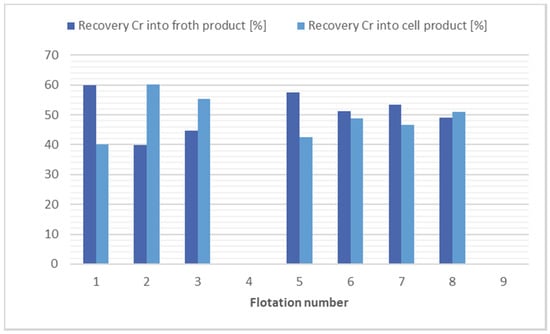
Figure 2.
Comparison of flotation results for chromium separation.
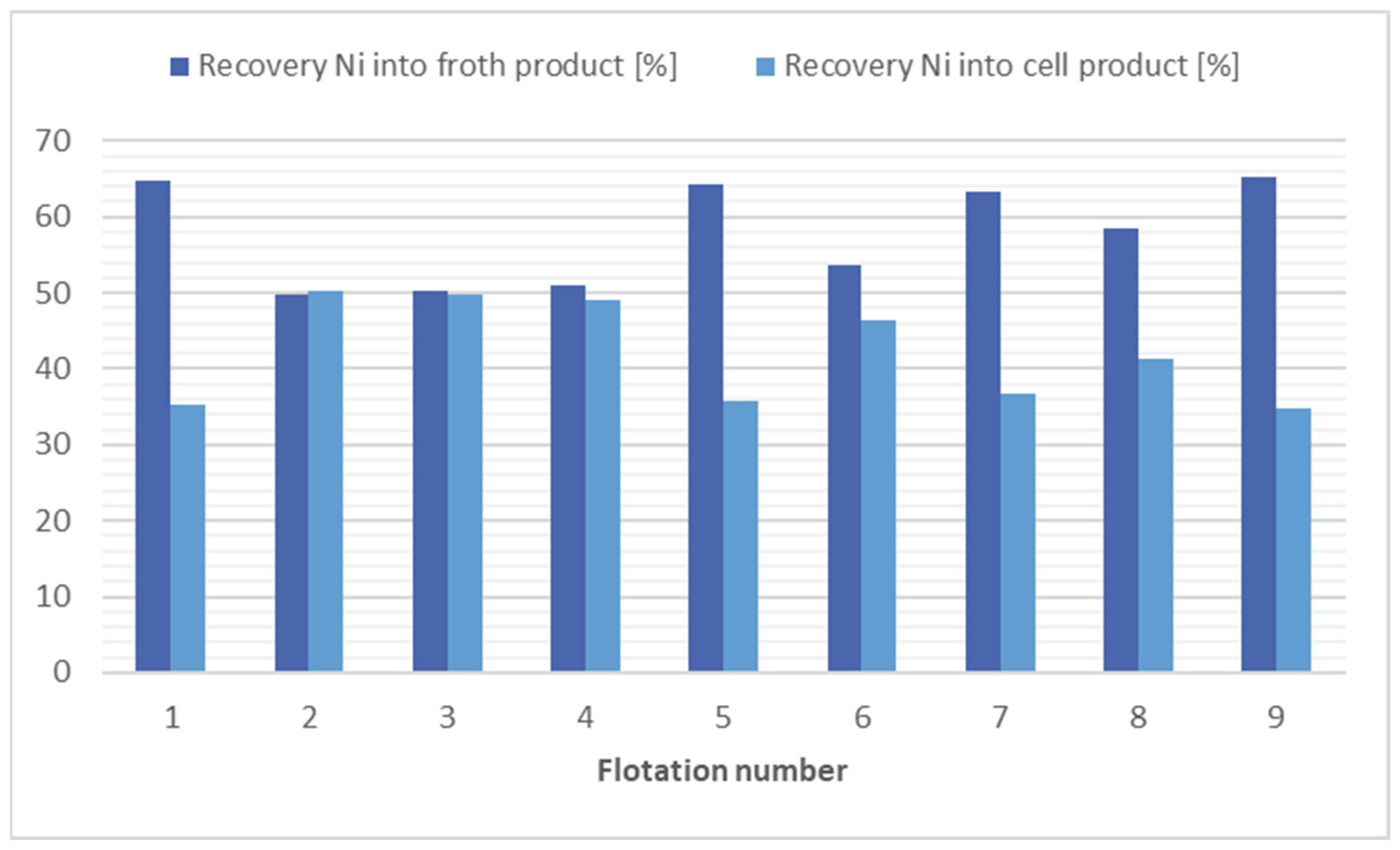
The findings of the flotation process for nickel separation suggest that the most efficacious methods appear to be the application of kerosene (4000 g/t), flotation 1, and the application of potassium ethyl xanthogenate (240 g/t), flotation 7. Taking into consideration the economic and environmental factors, it is evident that flotation 8 offers a relatively efficient solution. This approach involves the absence of a collector and yet achieves a substantial reduction in nickel concentration, with reduced levels (1.13 times). The results of the flotation tests for nickel separation are shown in Figure 3.
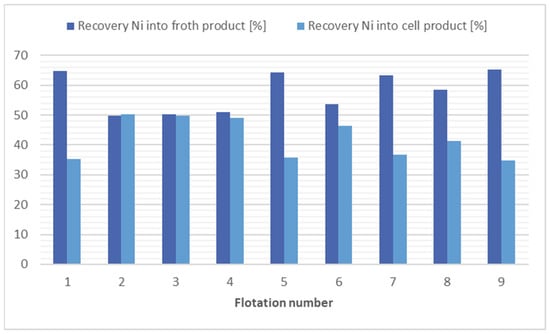
Figure 3.
Comparison of flotation results for nickel separation.
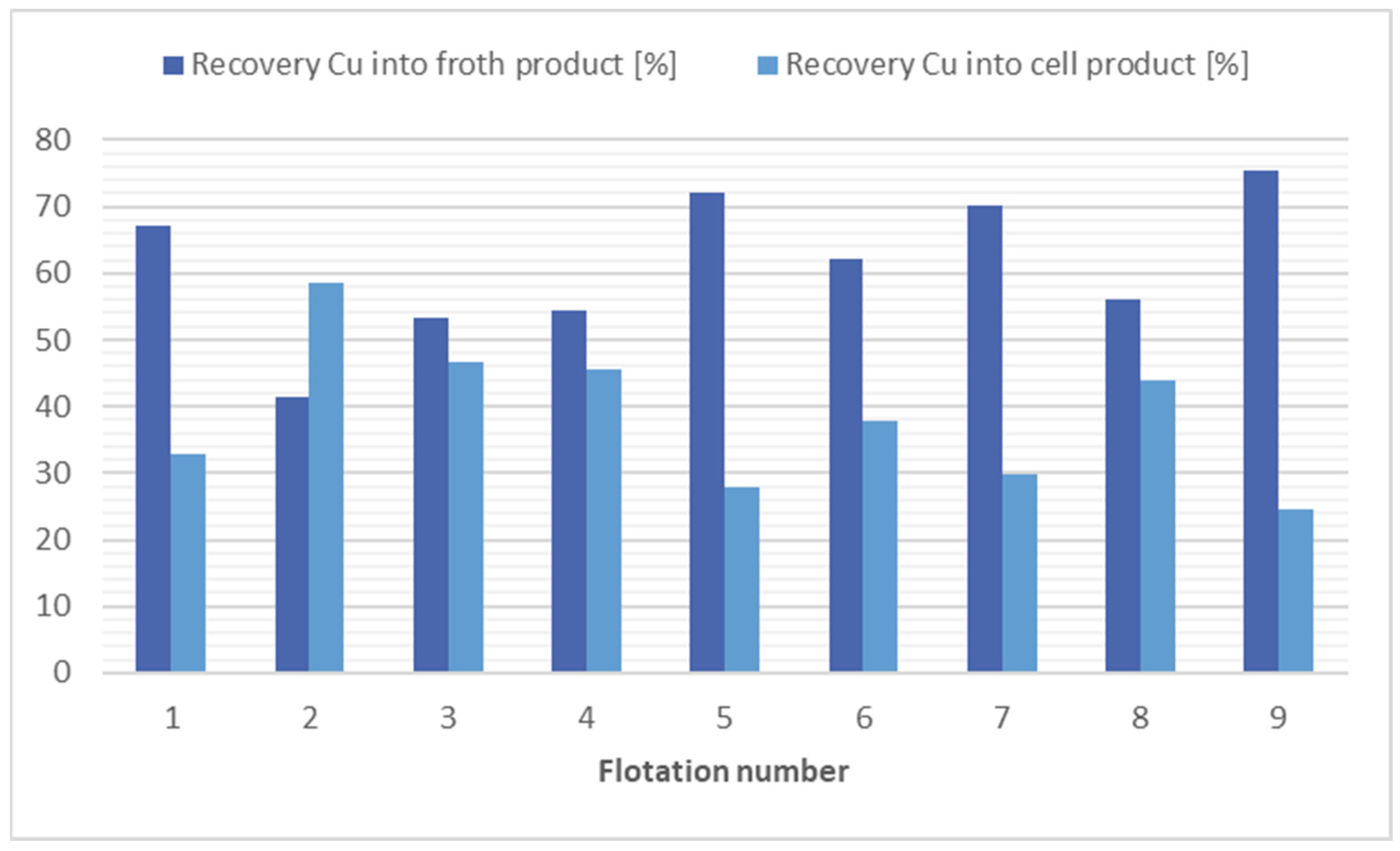
The analyses of the flotation tests of soil samples show that the collector combination of kerosene (12,000 g/t) and potassium ethyl xanthogenate (200 g/t) were most effective at separating copper from the soil, flotation 9. The flotation 9 achieved a 1.8 times reduction in Cu concentration in the cell product at a Cu recovery of 43.92%. On the other hand, flotation 7 is more economically and ecologically advantageous, where a similar result was achieved (1.7 times reduction at a Cu recovery of 48.77%), but only with the addition of potassium ethyl xanthogenate at 240 g/t. Furthermore, in flotation 8, where no collector was used, a 1.13 times reduction in copper concentration was achieved with a cell product mass yield of 50.59%. The results of the flotation tests for copper separation are shown in Figure 4.
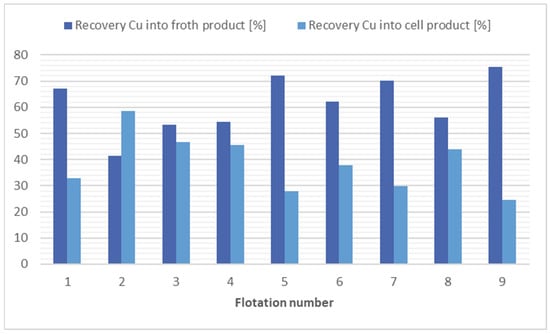
Figure 4.
Comparison of flotation results in copper separation.
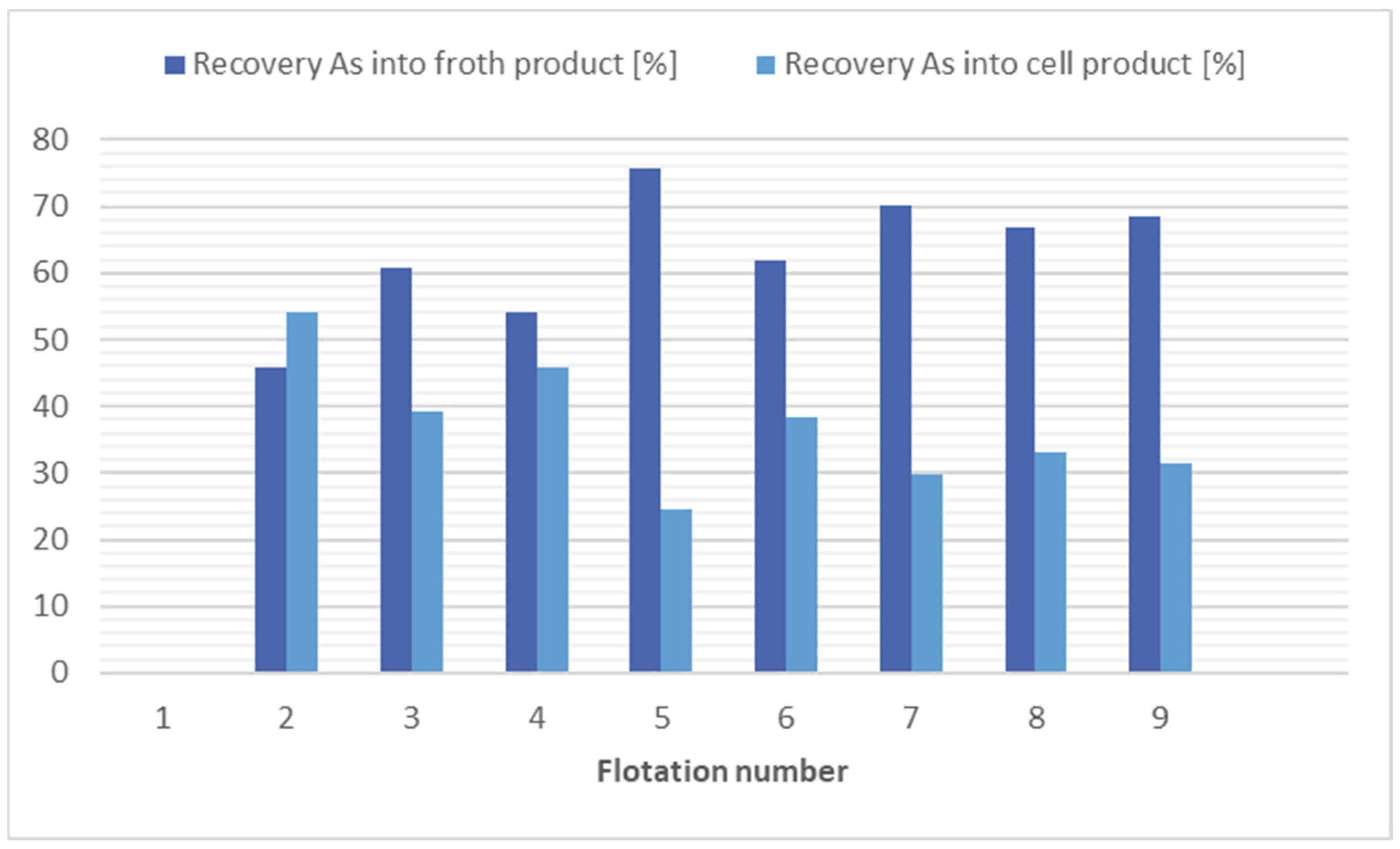
Based on the results of the flotations for arsenic separation, it appears that the most efficient is the use of kerosene (12,000 g/t) with potassium ethyl xanthogenate (200 g/t), flotation 9. With consideration of economic and ecological factors, it appears that flotation 8 is the best choice, where no collector was applied and still a significant reduction in arsenic recovery (1.5 times) was achieved. The results of the flotation tests for arsenic separation are shown in Figure 5.
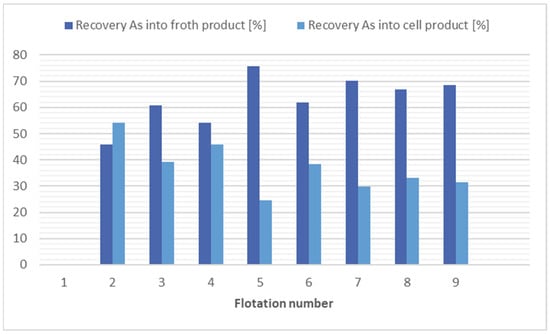
Figure 5.
Comparison of flotation results for arsenic separation.
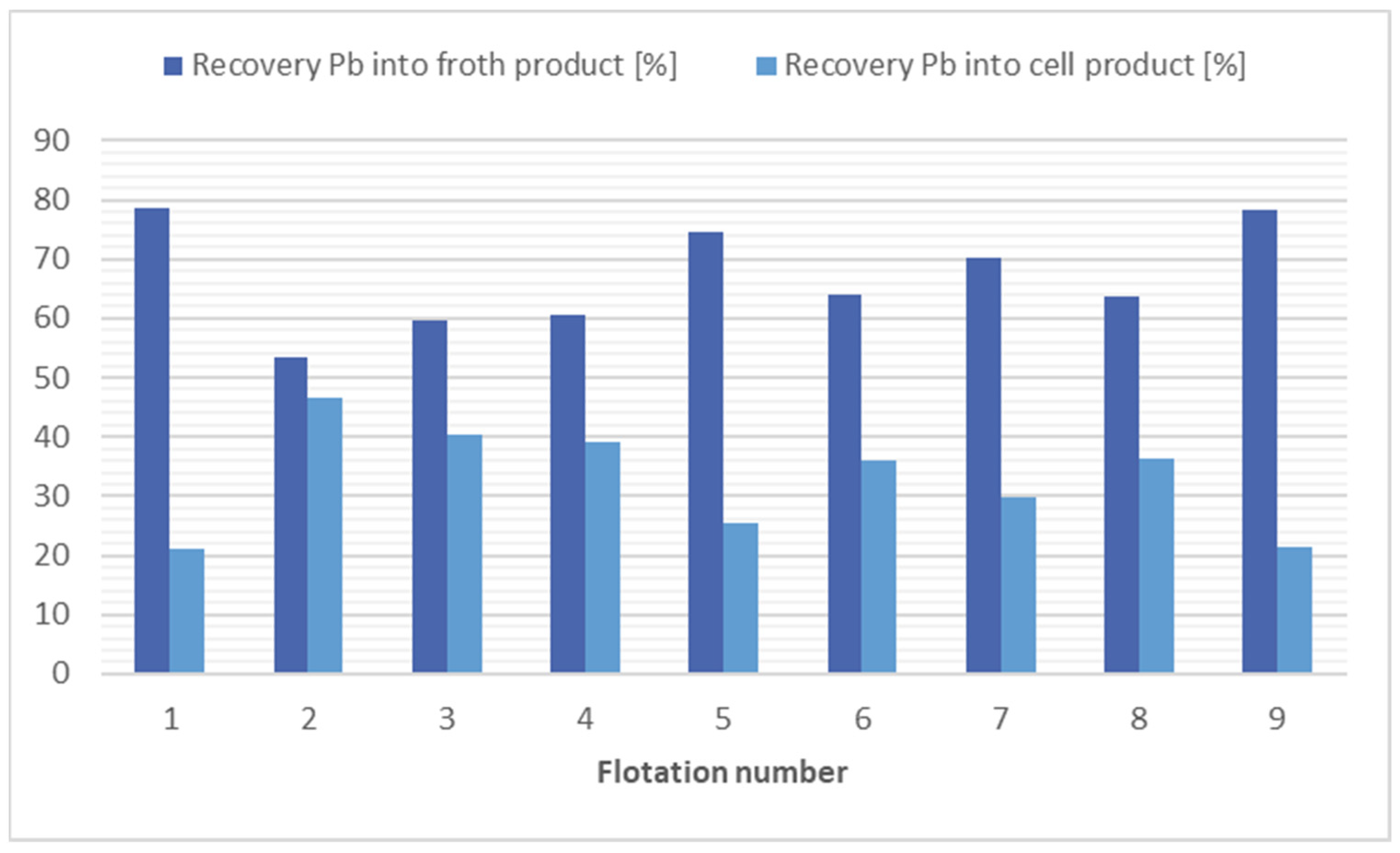
The greatest reductions in lead concentrations occurred in flotation 1 using kerosene at 4000 g/t and in flotation 9 using potassium ethyl xanthogenate (200 g/t) in combination with kerosene (12,000 g/t). In these flotations, the Pb concentration in the cell product was almost doubled; however, the mass yield was around 42–44%. Good results were also obtained in the flotation without collector application, flotation 8, due to the higher mass yield of the cell product (50.59%) and from an economic and environmental point of view. The results of the flotation tests for lead separation are shown in Figure 6.
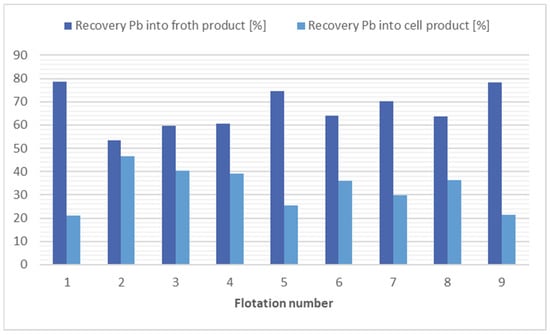
Figure 6.
Comparison of flotation results in lead separation.
4. Discussion
Flotation tests demonstrated that similar results were achieved with the non-polar collector (kerosene) as with the polar collector (potassium ethyl xanthogenate), which as an anionic active collector is designed specifically for metal flotation. It is likely that the flotation using kerosene resulted in flotation of the hydrophobic particles to which the respective metals were bound. The greatest reduction in metal recovery was achieved when the lowest kerosene dosage was 4000 g/t, flotation 1. On the contrary, the lowest recovery reductions were found using a kerosene dosage of 8000 g/t, flotation 2. The application of potassium ethyl xanthogenate at the lowest dosage of 160 g/t, flotation 5, was also found to be effective. In almost all cases, the results were similar to the application with 4000 g/t kerosene, flotation 1. Significant reductions in metal recovery in the cell products of the individual flotations were also achieved with the combination of kerosene (12,000 g/t) and potassium ethyl xanthogenate (200 g/t), flotation 9. As demonstrated in the study by Dermont et al. [4], the effectiveness of the collector in soil flotation was thus verified. Furthermore, the research in this work also confirmed the possibility of applying flotation without the use of a collector. The results were very satisfactory when all flotation tests were compared and were close to the values that were achieved with the application of collector. These results show significant advantages in terms of cost savings for the collector, higher mass yields of cell products (e.g., future soils to be used directly in remediation operations), and the ecological aspect of not further burdening an already pollutant-laden soil with flotation reagents.
The purpose and benefit of flotation is its speed and effectiveness. It is likely that collective flotation would take place, so flotations 7, 8, and 9 were selected as the optimal ones after evaluating the results.
5. Conclusions
In the context of flotation experiments, this study examined the impact of kerosene and potassium ethyl xanthogenate on process efficiency. Non-collector flotation was also employed, achieving highly satisfactory results. The flotation process exhibited a marked advantage in terms of cost savings, enhanced mass yield of cell product, and a reduced environmental impact. In conclusion, flotation of soils can provide a very suitable alternative in decontamination technologies.
Author Contributions
Conceptualization, R.K. and I.S.; methodology, I.S.; software, M.U.; validation, R.K., I.S. and A.P.; formal analysis, M.S.; investigation, M.U.; resources, I.S.; data curation, M.S.; writing—original draft preparation, I.S.; writing—review and editing, R.K.; visualization, R.K.; supervision, A.P.; project administration, M.U.; funding acquisition, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grant of SGS No. SP2022/40, Faculty of Mining and Geology, VSB—Technical University of Ostrava, Czech Republic.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the published results can be found in the dissertation on the topic of The use of flotation and phytoremediation in the treatment of anthropogenically impacted soils. Author Ivona Sobková, VSB-Technical University of Ostrava, 2022. Available online: http://hdl.handle.net/10084/151395 (accessed on 2 June 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ČSN ISO | Czech technical standard based on the international ISO standard |
| RD | Ore mines |
| VŠB-TUO | VSB-Technical University of Ostrava |
| WD XRF | Wavelength Dispersive X-ray Fluorescence |
References
- Rejšek, K.; Vácha, R. Nauka o Půdě; Agriprint: Olomouc, Czech Republic, 2018. [Google Scholar]
- EU. Tematická Strategie Pro Ochranu Půdy. Eur-Lex.Europa.Eu. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52006DC0231 (accessed on 22 November 2020).
- ČSN ISO 11464; Soil Quality—Preparation of Samples for Physico-Chemical Analyses. Czech Standards Institute: Prague, Czech Republic, 2011.
- Dermont, G. Remediation of metal-contaminated urban soil using flotation technique. Sci. Total Environ. 2010, 408, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).