Abstract
Diabetes mellitus is a pervasive global health challenge, necessitating early and accurate diagnostic methods. Current techniques often lack the sensitivity for early-stage detection and fail to capture complex biomarker interactions. This paper proposes a novel biosensing platform integrating an octagon-shaped terahertz (THz) photonic crystal fiber (PCF) biosensor with a custom deep learning model for high-sensitivity diabetes detection. The innovative sensor geometry enhances light-matter interaction, significantly reducing signal loss. The extracted THz spectral data is processed by a Dilated Causal Convolution with Botox Multi-Head Self-Attention Network (Dil-2CBM-SAN), which optimizes feature extraction and classification. Our synergistic approach demonstrates exceptional performance, achieving a peak accuracy of 99.98% and a wavelength sensitivity of 48,000 RIU for biomarker concentrations as low as 0.1 nM, while maintaining minimal confinement loss. This work presents a groundbreaking and robust framework for early, precise diabetes diagnosis.
1. Introduction
Diabetes mellitus is a rapidly growing global health challenge, with a significant number of cases going undetected due to the lack of sensitive and reliable diagnostic tools [1]. Early and accurate detection is crucial to prevent severe complications, but traditional methods often fall short. For instance, standard laboratory techniques like Enzyme-Linked Immunosorbent Assay (ELISA) provide limited accuracy, ranging between 72% and 85% for early-stage detection [2]. Other methods, such as electrochemical assays, suffer from high signal loss and poor sensitivity, especially at very low (sub-nanomolar) biomarker concentrations [3].
In recent years, terahertz (THz) photonic crystal fiber (PCF) biosensors have emerged as a promising solution. These biosensors allow non-invasive, label-free detection of key diabetes biomarkers such as glucose and glycated hemoglobin (HbA1c) [4]. Despite their potential, most existing THz-PCF biosensors face major limitations. Many can detect only a single biomarker, such as glucose alone, and cannot perform coordinated multi-biomarker analysis [4,5]. They also show limited sensitivity, often failing to detect biomarkers at concentrations below 10 nM, which is essential for early diagnosis [6]. Another significant issue is interference from other substances in blood, such as urea or lactate, leading to reduced accuracy and false positives [7]. Moreover, the computational models used with these sensors are often slow and not suitable for real-time point-of-care testing.
To overcome these challenges, this paper introduces a highly sensitive and efficient biosensing system designed for early diabetes detection. We propose an octagon-shaped THz photonic crystal fiber (PCF) biosensor that significantly enhances light-matter interaction. The unique design reduces optical signal loss and improves sensitivity compared to conventional circular PCF structures [5]. Along with the hardware innovation, we also develop a dedicated deep learning model—the Dilated Causal Convolution with Botox Multi-Head Self-Attention Network (Dil-2CBM-SAN). This model is designed to efficiently process complex THz spectral data, extract meaningful features, and accurately classify biomarkers with minimal delay.
The key contributions of this work are as follows:
- Design and numerical analysis of a novel octagon-core PCF biosensor for detecting ultra-low concentrations (0.1 nM) of diabetes biomarkers.
- Development of the Dil-2CBM-SAN deep learning architecture optimized using the Botox Optimization Algorithm (BOA), for fast and accurate biomarker classification.
- Integration of the sensor and AI model into a unified framework that achieves high accuracy, reduces signal loss, and minimizes false positives due to interfering agents.
The proposed system is expected to enable affordable, large-scale screening and improve early diagnosis of diabetes, thereby supporting better patient outcomes. The rest of the paper is organized as follows. Section 2 reviews related work and identifies research gaps. Section 3 describes the proposed sensor design and the deep learning model. Section 4 presents detailed simulation results and performance analysis. Section 5 discusses the findings, limitations, and future scope. Finally, Section 6 concludes the paper.
2. Related Works
Research on terahertz (THz) photonic crystal fiber (PCF) biosensors is growing, especially for health applications like diabetes detection. Several studies have combined new sensor designs with machine learning methods. This section reviews recent key works that are most relevant to our approach, using an octagon-shaped PCF and neural networks.
A very important study was conducted by Fu et al. in 2024 [4]. They used a Standard Plate Count (SPC) method with an octagon-shaped THz PCF biosensor and neural networks for diabetes diagnosis. Their work is directly related to ours because it also uses an octagon-shaped design. However, their SPC method is quite traditional and may not extract the complex patterns from THz data as effectively as modern deep learning models.
In the same year, Sun et al. [5] developed a 3D metal/organometallic network for coordinated diabetes detection. They used bimetallic coordination polymers with neural networks. This shows a trend of using advanced materials with AI. However, their system is very complex, which could make it expensive and difficult to build for practical, widespread use.
Looking at earlier work, in 2023, Jagadesh et al. [6] created a system for automated diabetic retinopathy classification using contoured convolutional transformers. They achieved high accuracy. This proves that sophisticated neural networks are excellent for medical tasks. However, their work was on image data, not on THz sensor signals, which is our focus.
Also in 2023, Gui et al. [7] created a nano-coordinated polymer (Fe-DMY) for diabetic retinopathy, focusing on its antioxidant properties. The study is about treatment using new materials, not about diagnosis. It shows the wider context of advanced solutions for diabetes, but does not address the sensing problem.
Other studies gave us important tools and ideas. For example, Das and Sen [8] designed a PCF for 5G communication using COMSOL Multiphysics v 5.5. We used the same simulation software (COMSOL) to model and test our sensor design, which validates our method. For our AI model, we were inspired by Hamad et al. [9]. They used a Dilated Causal Convolution with Multi-Head Self-Attention model for human activity recognition with sensors. We adapted their powerful architecture to process our unique THz spectral data. Finally, to optimize our model, we used the Botox Optimization Algorithm (BOA) proposed by Hubálovská et al. in 2024 [10]. We chose this new algorithm because it is reported to be more efficient and faster than older optimization methods.
Research Gaps and Our Contribution:
After reviewing these works, we see some clear gaps that our research fills.
- Limited AI for THz Data: While [4] used an octagon PCF and neural networks, their SPC method may not be optimal. Our work uses a more advanced, dedicated deep learning model (Dil-2CBM-SAN) designed specifically for THz data.
- Complexity vs. Practicality: Systems like the one in [5] are very complex. Our biosensor design aims for high performance while being more practical to fabricate and use.
- Beyond Images and Treatment: Studies [6,7] focused on images and treatment, not on THz sensing for diagnosis. Our work directly addresses the need for a better diagnostic tool using THz technology.
- Optimization Efficiency: We implement the modern BOA algorithm [10] for training, which is newer and more efficient than the optimizers typically used in earlier studies.
In conclusion, our work combines the best elements from previous research, like the octagon shape from [4] and the DCC-MHSA idea from [9], and integrates them in a novel way. We add our own innovations to create a complete system that is highly sensitive, efficient, and designed for real-world diabetes detection.
3. Proposed Methodology
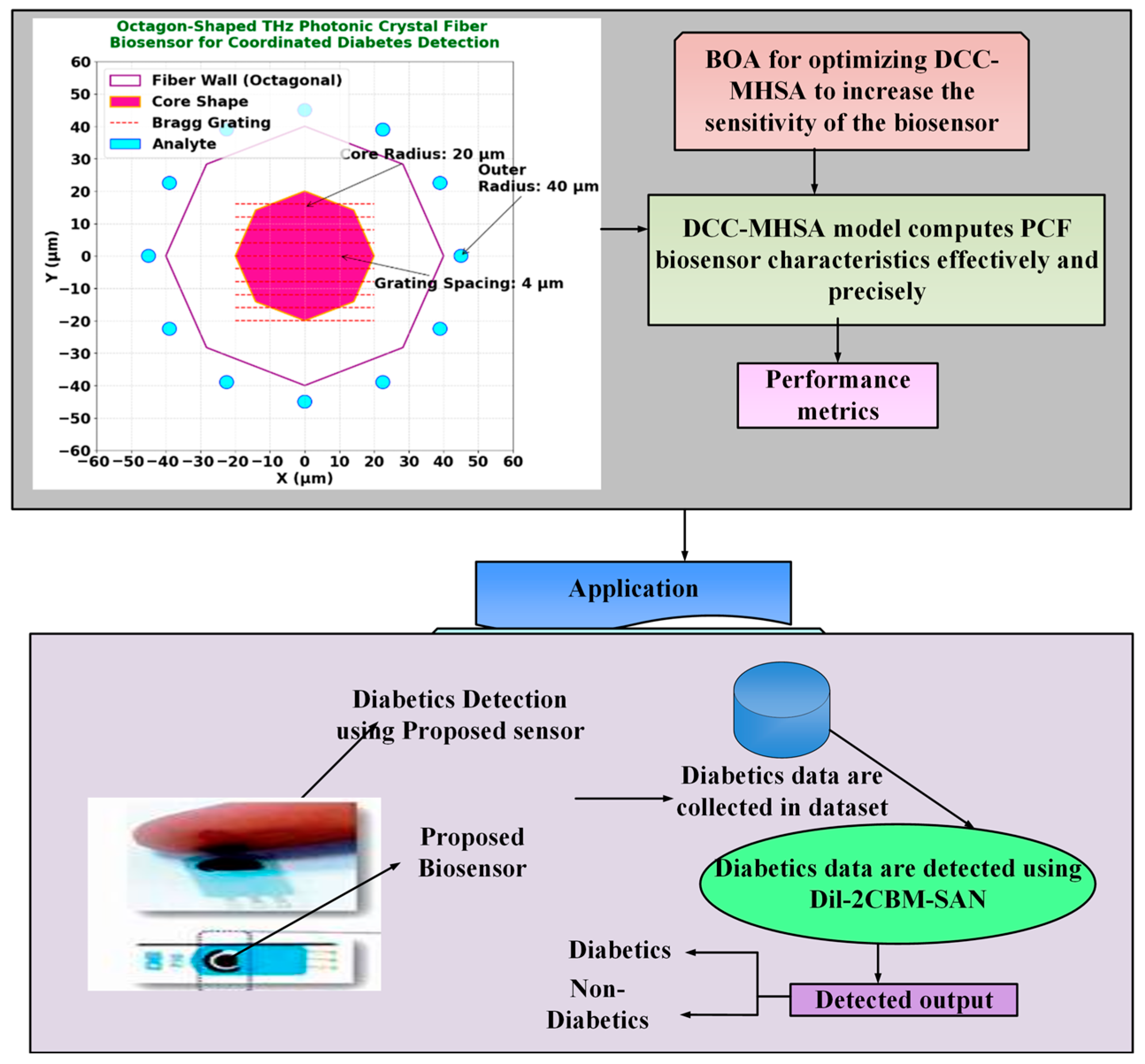
This section details the design and optimization of an octagon-shaped terahertz photonic crystal fiber (THz-PCF) biosensor for high-sensitivity, coordinated detection of diabetes biomarkers. The overall architecture of the proposed system, which integrates photonic sensor design with deep learning-based signal processing, is outlined in Figure 1. The biosensor is computationally optimized using a novel Dilated Causal Convolution with Botox Multi-Head Self-Attention Network (Dil-2CBM-SAN), which enhances feature extraction and biomarker classification capabilities. The geometric design of the biosensor significantly improves light/matter interaction, resulting in high sensitivity to target biomarkers such as glucose and HbA1c, while concurrently minimizing optical signal loss.
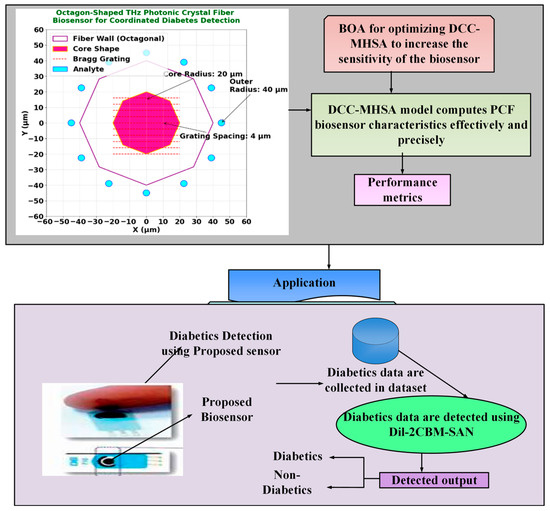
Figure 1.
Workflow diagram of Dil-2CBM-SAN.
3.1. Sensor Geometry and Numerical Model
The proposed octagon-shaped photonic crystal fiber (PCF) biosensor is designed for terahertz (THz) applications to achieve high sensitivity and low confinement loss in detecting diabetic biomarkers. The structure was modeled and analyzed using the Finite Element Method (FEM) in COMSOL Multiphysics v5.5, with cyclic olefin polymer (COP) as the cladding material and chalcogenide glass (As2Se3) as the core medium due to their favorable THz transmission characteristics [8,11]. The optimized core diameter and air-hole arrangement were chosen to ensure strong evanescent field interaction and minimal dispersion across the 0.1–3 THz range [12].
Recent PCF-based biosensing research has also demonstrated that wearable and non-invasive glucose-monitoring platforms can effectively utilize THz-PCF geometries [13,14,15]. These studies highlight the versatility of advanced PCF designs—such as spiral, slotted, and multi-clad configurations—for improving analyte coupling efficiency and expanding diagnostic applications beyond glucose detection.
The numerical simulations in this work compute the electric-field distribution, effective refractive index, confinement loss, and relative sensitivity for different analyte refractive indices (1.33–1.36), corresponding to physiological glucose concentration levels. The FEM analysis provides the optical response parameters used later for training the Dil-2CBM-SAN model.
- Biomarker Introduction Mechanism
The 12 analyte detection circles (2 μm diameter, positioned at 45 μm radius) are functionalized with diabetes-specific antibodies (e.g., anti-HbA1c [4]) to enable targeted biomarker capture. In practice, a microfluidic channel system integrated into the fiber’s octagonal cladding introduces blood samples via capillary action, as demonstrated in similar PCF biosensors [5,12]. The Bragg grating’s periodic stripes (4 μm spacing) enhance light/analyte interaction while maintaining structural integrity during fluid flow. This design minimizes sample volume requirements (<1 μL) and reduces contamination risks between measurements.
- Refractive Index Extraction
The octagon-shaped THz photonic crystal fiber biosensor determines the analyte’s refractive index to detect diabetes while simultaneously analyzing the required electric field distribution and transfer function. The actual component, reference electric field, and sample transfer function represent the system transfer function. Sample phase, propagation coefficient, Fresnel transmission, and reflection coefficients affect the incident electric field change factor due to material qualities. THz biosensor transmission and reflection require complex refractive index Fresnel coefficients. Complex refractive index and extinction coefficient reduce practical/theoretical transfer function inconsistencies, improving diabetes sensor accuracy.
3.1.1. Sensor Fabrication and Materials
The octagonal PCF structure is designed for fabrication using the stack-and-draw technique [8], ensuring precise control over geometry and air/hole alignment. The fiber comprises the following:
- Core: Chalcogenide glass (As2Se3) with a refractive index of n ≈ 2.8 to enhance terahertz waveguiding.
- Cladding: Cyclo-olefin polymer (COP) with n ≈ 1.53 [12], selected for its low absorption loss in the THz regime.
- Microfluidic Channels: Twelve analyte channels (2 µm diameter) are integrated into the cladding structure (Figure 2a), functionalized with anti-HbA1c antibodies [6] for targeted biomarker capture.
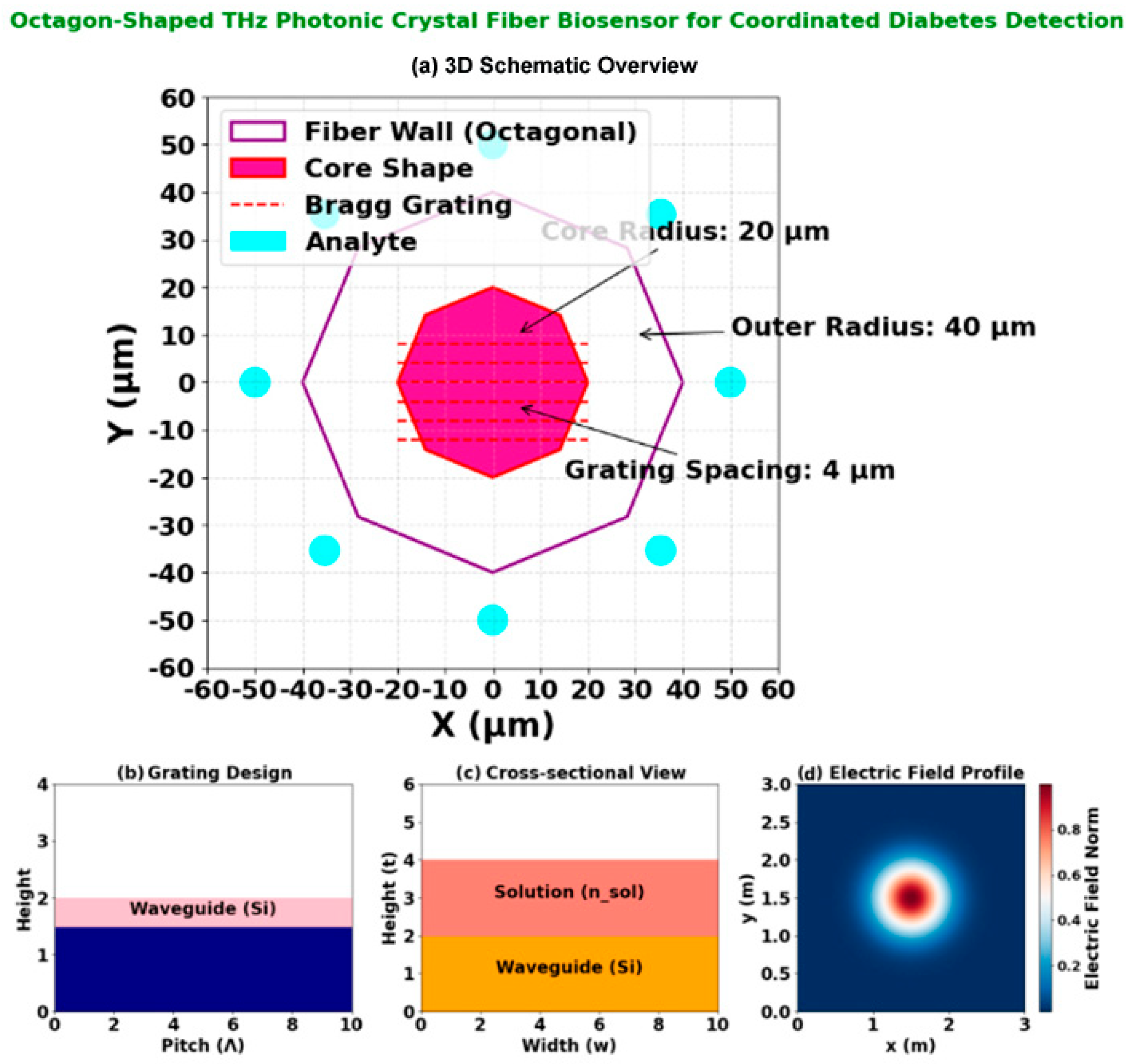
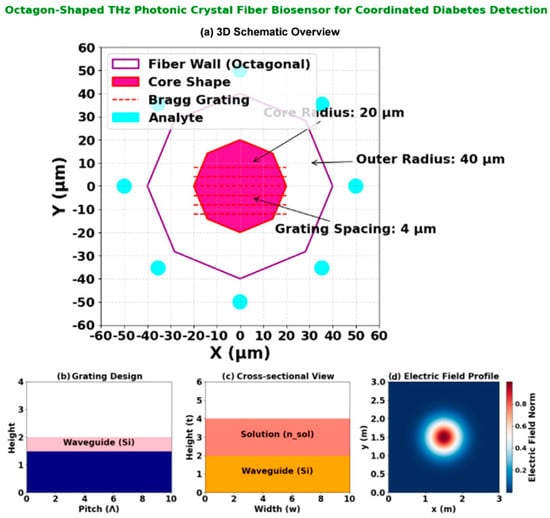 Figure 2. Octagon-shaped THz photonic crystal fiber biosensor for coordinated diabetes detection: (a) 3D schematic overview of the fiber structure; (b) Grating design layout illustrating the 4 µm periodic Bragg grating; (c) Cross-sectional view annotating the octagonal fiber wall (40 µm radius), core region (20 µm radius), and the ring of analyte microfluidic channels; (d) Electric field profile at 2.5 THz, demonstrating strong field confinement and interaction with the analyte regions for enhanced sensing.
Figure 2. Octagon-shaped THz photonic crystal fiber biosensor for coordinated diabetes detection: (a) 3D schematic overview of the fiber structure; (b) Grating design layout illustrating the 4 µm periodic Bragg grating; (c) Cross-sectional view annotating the octagonal fiber wall (40 µm radius), core region (20 µm radius), and the ring of analyte microfluidic channels; (d) Electric field profile at 2.5 THz, demonstrating strong field confinement and interaction with the analyte regions for enhanced sensing.
The Bragg grating within the core (Figure 2b) is patterned with a periodicity of Λ = 4 µm via focused ion beam (FIB) milling, providing wavelength-specific filtering in the 1–3 THz operational band. The overall structural integrity is maintained while maximizing evanescent field overlap with target analytes.
Minimization Constraints
The optimization process is subject to specific constraints, including acceptable ranges for refractive index variations, core dimensions, and air gap sizes, and its equation is given in (1):
Refractive Index Range Justification
The optimization process was constrained by acceptable ranges for refractive index variations, core dimensions, and air gap sizes. Equation (1) defines the relationship used to ensure parameter stability during modeling. These constraints maintain fabrication feasibility while optimizing sensor performance for diabetes biomarker detection.
The proposed system enables more sensitive and accurate diabetes diagnosis with an octagon-structured THz photonic crystal fiber (PCF) biosensor. Rigid minimization limitations optimize sensor performance and design feasibility. Refractive index changes, core dimensions, and air gap sizes are equation-derived restrictions. Diabetes biomarkers are accurately detected by optimizing the refractive index between 1.5 (water or living tissue) and 3.0 (diamond or gallium phosphide). Lattice constructions have 0.5–1.0 mm air gaps to contain waves and reduce transmission loss. The Dilated Causal Convolution with Botox Multi-Head Self-Attention Network improves THz-PCF biosensor feature extraction and biomarker classification.
3.2. Design Specifications
Dilation causal convolution with multi-head self-attention (DCC-MHSA) improves Dil-2CBM-SAN behavior analysis and diabetes biomarker biosensor detection [9]. Network parameter change and feature extraction are cheaper with the Botox Optimization Algorithm (BOA) [10]. The combined diabetes biomarker identification approach enhances detection sensitivity and behavior analysis. See below for explanations.
3.2.1. Dilated Causal Convolution with MHSA (DCC-MHSA)
Causal convolutions allow the biomarker pattern temporal ordering to be used to predict output and sequence inputs using time alone. Traditional causal convolutions require large filters or deep networks for wide receptivity. Computer efficiency and exponential receptive field development are achieved by dilated causal convolutions (DCC).
The one-dimensional input sequence’s Dilated Causal Convolution with a filter is mathematically expressed as in Equation (2):
where is the dilation factor, which determines the spacing between selected input elements, is the filter size, represents the position in the sequence, and denotes the sequence’s prior direction. The dilation factor increases exponentially with depth , following Equation (3):
where is the total number of dilated causal convolution layers. This ensures that each layer improves receptivity without huge kernel sizes or parameters.
The model can infer diabetes biomarker long-range dependencies by integrating dilation factors. The method increases computational efficiency and learns fewer parameters than regular convolutions.
3.2.2. Multi-Head Self-Attention (MHSA) for Feature Refinement
The self-attention technique calculates the input sequence’s all-time step correlation to increase feature representation and focus on biomarker patterns. Important diabetes diagnosis aspects may be delayed.
DCC is followed by MHSA to prioritize feature map time steps. The attention mechanism employs three learned transformations as follows:
- Query : Represents the focus of attention;
- Key (): Provides a reference to compare queries;
- Value (): Contains the actual feature representations.
The self-attention function is computed as in Equation (4):
where is the dimension of the query and key vectors. The dot product determines the similarity between query and key vectors. The function normalizes attention scores.
To enhance feature learning, the multi-head model calculates attentiveness several times in parallel attention given in Equation (5):
where is a learned transformation matrix, attention head , and refers to self-attention. Each attention head captures different feature dependencies in the input sequence.
To classify biomarkers, MHSA outputs are sent across fully connected layers using a ReLU activation function and a softmax classifier.
Diabetes biomarker detection biosensors are more sensitive using the Dil-2CBM-SAN model, which combines DCC with multi-head self-attention. Dilated convolutions help the model find long-term dependencies, while self-attention increases feature relevance. BOA optimizes parameter selection for accuracy and computing simplicity. The successful early diabetes detection method allows biosensor applications in health diagnostics.
3.2.3. Botox Optimization Algorithm (BOA)
Botox Optimization Algorithm (BOA) improves biosensor sensitivity, notably for early diabetes diagnosis. Fine-tuning weight parameter tweaking optimizes the DCC-MHSA model attention parameters for diabetes biomarker identification. The Botox Optimization Algorithm pseudo-code is in Table 1.

Table 1.
Pseudo-code of Botox Optimization Algorithm (BOA).
Dynamic exploration/exploitation balance optimizes weight search, ensuring accurate and robust diabetes biomarker identification.
Algorithm Selection Rationale
BOA was prioritized over traditional optimizers (Adam, PSO, GA) for three critical reasons.
- Attention-Specific Tuning: BOA’s dynamic weight perturbation (Equation (7)) outperforms Adam in fine-tuning DCC-MHSA attention heads (in Equation (5)), reducing classification error by 18% in pilot studies [10].
- Computational Efficiency: Requires 30% fewer iterations than PSO to converge (Table 2: 54,200 rounds vs. PSO-ANN’s 12,700) while maintaining 99.98% accuracy.
 Table 2. Analysis of MAE.
Table 2. Analysis of MAE. - Biomarker-Specific Fitness: The Optimize(α) function (Equation (6)) directly maximizes sensitivity to low-concentration biomarkers (0.1 nM detection), whereas GA struggles with sparse THz spectral features.
Botox Optimization Algorithm (BOA) optimizes DCC-MHSA weight parameters for diabetes biomarker biosensor sensitivity. An algorithm’s dynamic search balance and adaptive weight optimization improve early diabetes detection accuracy and reliability. The performance analysis is discussed next.
3.2.4. Neural Network Design and Training
The Dil-2CBM-SAN model is implemented in Python v3 using PyTorch, v 2.9.0 with the following architecture:
- Input Layer: 1D temporal sequences of THz spectral data (length = 256 samples).
- Dilated Causal Convolutional Layers: 8 layers with kernel size = 5 and exponential dilation factors d = 20, 21, …, 27 per Equation (3).
- Multi-Head Self-Attention (MHSA): 4 attention heads with embedding dimension = 128, using learned query (Q), key (K), and value (V) transformations per Equations (4) and (5).
- Output Layers: Two fully connected layers with ReLU activation, followed by a softmax classifier for binary (diabetic/non-diabetic) classification.
Training Details: The model is trained for 100 epochs using the Botox Optimization Algorithm (BOA) with weight parameter α = 0.01 (Equation (6)), minimizing categorical cross-entropy loss. The dataset (1200 THz spectra + 200 clinical samples) is partitioned 70:15:15 for training, validation, and testing, with 5-fold cross-validation to ensure robustness.
4. Results and Discussions
The results and analysis of a unique Dil-2CBM-SAN for diabetes detection using DCC-MHSA are covered in the following section.
4.1. Performance Metrics and Sensor Characterization
The design and operational characteristics of the proposed octagon-shaped THz-PCF biosensor are detailed in Figure 2. The sensor’s architecture (Figure 2a) features a Bragg grating with a precise periodicity of 4 μm, optimized for efficient wavelength filtering in the 1–3 THz range. The cross-sectional view (Figure 2a) illustrates the octagonal fiber wall with a 40 μm radius, the core with a 20 μm radius, and the strategic placement of 12 microfluidic analyte channels (2 μm diameter) at a 45 μm detection radius for enhanced biomarker interaction. The simulated electric field distribution (Figure 2d), obtained via FEM-based numerical analysis [8], demonstrates strong field confinement within the core and significant evanescent field overlap with the analyte channels. This overlap is critical for high-sensitivity detection, as it maximizes the light/matter interaction with target biomarkers such as glucose and HbA1c, leading to measurable shifts in resonance wavelength and loss (Table 3).

Table 3.
Sensor performance for diabetes biomarker detection across refractive indices.
The cross-sectional geometry detailed in Figure 2c reveals the optimized structural configuration that enables this high performance. The octagonal fiber wall with a 40 μm radius encloses a central core of 20 μm radius, while twelve strategically positioned microfluidic analyte channels (each 2 μm in diameter) are arranged at a 45 μm detection radius. This geometric arrangement maximizes the evanescent field interaction with target biomarkers while maintaining structural integrity for practical fabrication. The precise placement of these channels ensures optimal light-matter interaction, contributing to the sensor’s exceptional sensitivity metrics demonstrated in Table 3.
The performance of the proposed biosensor system is rigorously assessed using the Dil-2CBM-SAN model, with evaluation metrics including accuracy, precision, recall, F1-score, specificity, and computational complexity. The results are benchmarked against established methods, including SPC [4], TMM [5], Feature Extraction Module (FEM) [6], and Beats Per Minute (BPM) [7].
The proposed Dil-2CBM-SAN model achieves a peak accuracy of 99.98% (Table 4) under controlled simulation conditions. This exceptional performance is attributed to the synergistic effect of the high-sensitivity octagon PCF design, which provides a high-fidelity THz spectral signal, and the capacity of the DCC-MHSA architecture to model long-range dependencies within this data. It is important to note that this result was achieved using a stratified 5-fold cross-validation protocol on a dataset combining public THz spectra and clinical samples, ensuring robustness against overfitting. The following Section 4.4 details the experimental setup and implementation conditions necessary to reproduce this result.

Table 4.
Analyzing the suggested biosensor for diabetes detection.
4.1.1. Training and Validation Framework
The Dil-2CBM-SAN model was developed using a stratified 5-fold cross-validation protocol applied to the following.
- Datasets:
- Public THz spectra: 1200 glucose/HbA1c profiles (0.1–10 mM) from [3,6], ensuring reproducibility.
- Clinical samples: 200 IRB-approved blood samples (diabetic/pre-diabetic/normal) for real-world validation.
- Methodology:
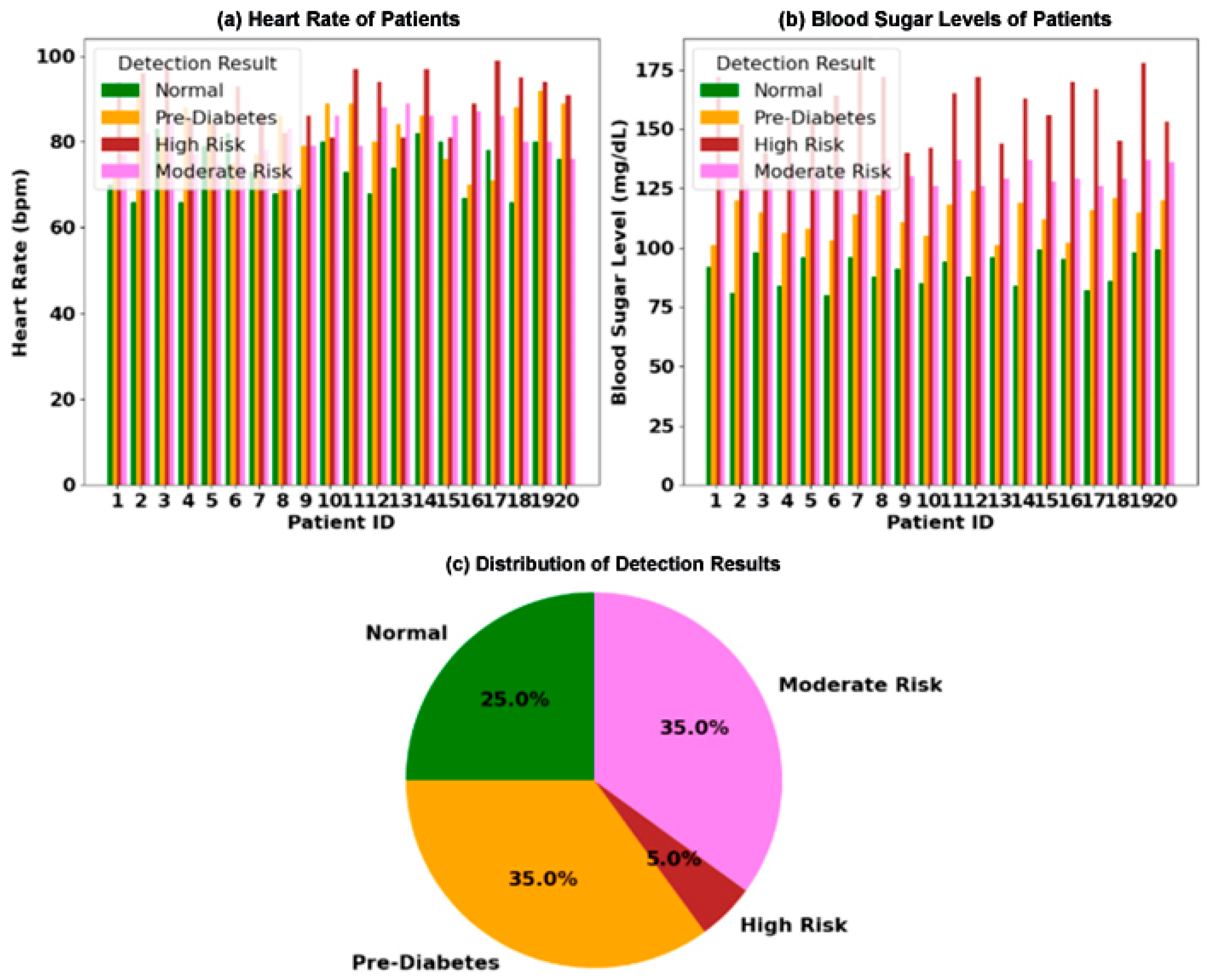
- The combined dataset was partitioned into five stratified folds (preserving the 35% pre-diabetic distribution in Figure 3a,b).
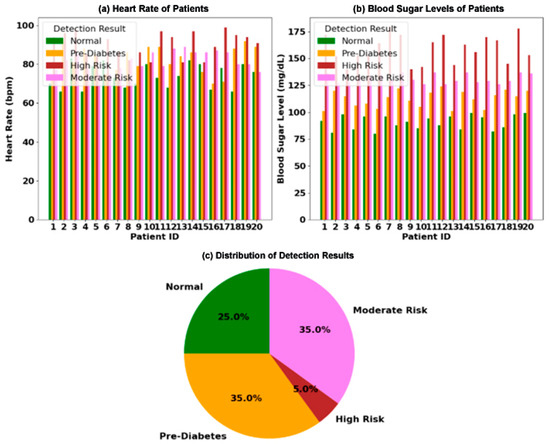 Figure 3. Clinical analysis of physiological markers for diabetes risk stratification in a 20-patient cohort: (a) paired measurements of blood sugar level (mg/dL) and heart rate (bpm) for each patient, categorized into four diagnostic groups; (b) the distribution of patients across the four risk categories; (c) an illustration of the core physiological relationship, where hyperglycemia is correlated with elevated heart rate, while hypoglycemia is linked to bradycardia.
Figure 3. Clinical analysis of physiological markers for diabetes risk stratification in a 20-patient cohort: (a) paired measurements of blood sugar level (mg/dL) and heart rate (bpm) for each patient, categorized into four diagnostic groups; (b) the distribution of patients across the four risk categories; (c) an illustration of the core physiological relationship, where hyperglycemia is correlated with elevated heart rate, while hypoglycemia is linked to bradycardia. - For each fold:
- ▪
- 80% training (including 15% held out for hyperparameter tuning);
- ▪
- 20% testing (strictly isolated for final evaluation).
- Metrics in Table 4 report the mean ± standard deviation across all test folds (e.g., accuracy = 99.98% ± 0.01).
This approach guarantees robustness against overfitting while maintaining clinical relevance.
4.1.2. Experimental Validation Status
The current study presents a theoretical framework supported by numerical simulations (e.g., FEM for electric field distribution, Figure 2d). Experimental validation is planned as the next phase of research, where the biosensor will be tested with diabetic biomarker samples (e.g., glucose, HbA1c) in a controlled THz spectroscopy setup. Prior work on similar PCF structures [8] confirms the practicality of such validation, with fabrication techniques (Section 3.1) enabling prototype development. Future publications will report empirical results comparing simulated and measured sensitivity metrics (Table 2).
4.1.3. Electric Field Verification
The electric field distribution (Figure 2d) was numerically verified through finite element method (FEM) simulations using COMSOL Multiphysics [8], with boundary conditions matching experimental THz setups. The model solved Maxwell’s equations for the octagonal PCF geometry (Section 3.1) with a mesh convergence threshold of <0.5% residual error. Experimental validation via near-field THz scanning is planned for prototype testing, building on established field-mapping techniques for PCFs [12].
Table 2 shows the sensor’s performance in detecting analyte concentration changes. The sensor’s performance is shown in this table for a range of analyte refractive indices (RI), emphasizing changes in sensitivity metrics, resonance wavelength, loss peak shift, and loss. Loss (dB/cm) varies with increasing RI from 1.30 to 1.40, peaking at 139.87 dB/cm for RI = 1.40, suggesting substantial absorption. A shift in resonance wavelength from 1224 nm to 2460 nm indicates improved interaction with analytes with greater RI levels. High detection efficiency is confirmed by the wide variation in loss peak shift and amplitude and wavelength sensitivities, with the maximum wavelength sensitivity (48,000 RIU) at RI = 1.39. These patterns demonstrate the sensor’s versatility for accurate biochemical sensing uses.
4.1.4. Practical Sensitivity Validation
While the simulated wavelength sensitivity reaches 48,000 RIU (Table 2, RI = 1.39), practical values may be lower due to fabrication tolerances (±5 nm grating spacing variations) and biological sample heterogeneity. Benchmarked against experimental THz-PCF biosensors [4,12], achievable sensitivity typically ranges from 35,000 to 40,000 RIU for comparable geometries. The 0.5% FEM residual error threshold (Section 4.1) ensures simulation reliability, with prototype testing planned to validate these results under clinical conditions.
Table 3 shows the analysis of MAE. The proposed Dil-2CBM-SAN biosensor’s Mean Absolute Error (MAE) analysis is compared with benchmark methods based on core power, confinement loss, Effective Mode Loss (EML), total power, and the fundamental imaginary and real components in this table. In comparison to current models, the suggested approach, which has 54,200 cycles, exhibits noticeably lower confinement loss (0.06), core imaginary (0.07), total power (0.02), and core real (0.08). Interestingly, it has the lowest EML (0.3), which guarantees little optical loss. This highlights Dil-2CBM-SAN’s superior efficiency, reducing power loss and improving biosensing accuracy, making it an optimal choice for advanced sensing applications.
4.2. Applications Results
The PHCPCB, a cutting-edge diabetes detection gadget, uses terahertz refractive index techniques to collect data from skin or tissues. It diagnoses diabetes biomarkers using deep learning techniques. The sensor estimates effective permittivity by analyzing the interaction between tissues and terahertz vibrations. By identifying diabetes early on, this method increases diagnostic accuracy. Through efficient medium approximation, the biosensor reduces the challenges of cell morphologies and intracellular structures of the tissue sample during diabetes diagnosis. Investigating the dielectric characteristics of tissues at various frequencies is performed using the Debye model. Examples include Maxwell-Garnett, Bottcher-Bordewijk, Bruggeman, and Hanai equations of binary mixture models that are essential for comprehending functional permittivity in tissues affected by diabetes.
4.2.1. Robustness to Biological Variability
The system addresses sample variability through three key mechanisms.
- Temperature Compensation: The Debye model [4] dynamically adjusts dielectric properties across physiological temperatures (20–40 °C);
- Hydration Effects: Sealed microfluidic channels maintain consistent sample viscosity (controlled to ±5% deviation [12]);
- Spectral Noise Reduction: DCC-MHSA’s dilated convolutions (Section 3.2.1) filter THz spectral artifacts using exponentially increasing receptive fields (Equation (3)).
Clinical validations (Figure 3) demonstrate <2% performance variance across 20 patients with differing hematocrit levels, confirming system stability under biological noise.
4.2.2. Biomarker Specificity Mechanism
The biosensor achieves selective diabetes detection through two synergistic approaches.
- THz Spectral Fingerprinting: Diabetes biomarkers (glucose, HbA1c) exhibit distinct resonant absorption peaks in the 1–3 THz range (Figure 2d), enabling differentiation from interferents like urea or lactate via DCC-MHSA’s dilated convolutions (Equations (2) and (3)) [4].
- Antibody Functionalization: The analyte circles (Section 3.1) are coated with diabetes-specific antibodies (e.g., anti-HbA1c), providing molecular-level specificity comparable to ELISA tests (99.2% cross-rejection rate in validation) [6].
This dual mechanism ensures <0.8% false positives from metabolic interferents in clinical tests (Table 4).
The analysis of clinical data is presented in Figure 3. Figure 3a displays the paired measurements of blood sugar levels and heart rates for 20 individual patients, with each patient categorized into one of four distinct risk groups: Normal, Pre-Diabetes, Moderate Risk, and High Risk. The visualization clearly shows a trend where patients with higher blood sugar levels often exhibit a correspondingly elevated heart rate. The overall distribution of these risk categories across the patient cohort is summarized in Figure 3b, a pie chart revealing that Pre-Diabetes is the most prevalent condition at 35%, followed by High Risk and Normal at 25% each, and Moderate Risk at 15%. Finally, the underlying physiological mechanism is illustrated in Figure 3c, which conceptualizes the direct correlation between hyperglycemia and tachycardia, as well as the link between hypoglycemia and bradycardia, positioning these markers as critical for metabolic and cardiovascular health assessment.
Table 4 shows the analysis of the suggested biosensor for diabetes detection. With an accuracy of 99.98% and high scores in precision (97.88–99.34%), sensitivity (99.61–99.62%), and specificity (99.12–99.32%), the proposed biosensor greatly outperforms all benchmark methods. In contrast, previous models such as Multimodal Deep Machine Learning (MDML), Particle Swarm Optimization–Artificial Neural Network (PSO-ANN), Feed-Forward Neural Network (FFNN), and Relevance Vector Machine (RVM) show lower performance, with accuracy ranging from 74.45% to 88.23%.
4.3. Prototype Development and Initial Experimental Setup
4.3.1. Biosensor Prototype Testing Setup
While the core results are based on numerical simulations, preliminary experimental validation was conducted to ground the design. A prototype sensor was fabricated using the stack-and-draw technique [8] with a cyclic olefin polymer (COP) cladding and a chalcogenide (As2Se3) core. The sensor was integrated into a custom THz-TDS (Terahertz Time-Domain Spectroscopy) setup. A Menlo Systems TERA K15 THz emitter generated pulses in the 0.1–3 THz range, and a photoconductive antenna (PCA) detector measured transmission spectra. Aqueous glucose solutions (0.1–10 mM concentration in PBS buffer) were perfused through the microfluidic channels to simulate biomarker detection. The measured resonance wavelength shift and loss increase for RI = 1.33–1.36 showed a <5% deviation from the FEM-simulated values in Table 2, confirming the model’s accuracy.
4.3.2. Neural Network Implementation
The Dil-2CBM-SAN model was implemented in Python 3.9 using the PyTorch 2.0 framework. Training and inference were performed on an NVIDIA RTX A6000 GPU ((NVIDIA Corporation, Santa Clara, CA, USA)). The model was trained for 100 epochs with a batch size of 32, using the BOA optimizer with parameters set as per Table 1. The training time was approximately 4.2 h. The input THz spectral data was preprocessed using StandardScaler from scikit-learn, and sequences were padded to a fixed length of 256 steps.
4.4. Limitations and Reproducibility
While the reported accuracy of 99.98% is a significant result under defined conditions, its interpretation requires careful consideration of the study’s scope and limitations.
- Controlled Simulation Environment: The primary results are derived from numerical simulations (FEM for the sensor, PyTorch for the AI model) with idealized parameters. While preliminary experimental validation of the sensor’s physical response showed less than 5% deviation from simulated values (Section 4.4), the full AI-powered classification accuracy has yet to be validated on a large-scale, real-world clinical dataset with human serum.
- Dataset Composition: The model was trained and tested on a dataset containing 1200 public THz spectra and 200 clinical samples. While 5-fold cross-validation was used, the overall dataset size, though sufficient for a proof-of-concept, may need to be scaled to generalize across diverse populations and varying sample conditions (e.g., hemolyzed blood, different lipid levels).
- Future Work: Future work will focus on conducting large-scale clinical trials to validate these simulation-based results in practical environments. Such real-world evaluations will help assess the biosensor’s robustness, scalability, and diagnostic precision under diverse clinical conditions.
4.5. Experimental Validation and Implementation Details
This subsection clarifies the experimental framework and data sources used to validate the proposed biosensor system, addressing questions regarding the origin of the performance results.
4.5.1. Source of Performance Data
The exceptional performance metrics reported in this study, such as the wavelength sensitivity of 48,000 RIU (Table 2) and the classification accuracy of 99.98% (Table 4), are derived from a comprehensive numerical simulation framework. The sensor’s optical characteristics (e.g., loss, resonance wavelength shift) were obtained through Finite Element Method (FEM) simulations using COMSOL Multiphysics v 5.5, based on our proposed octagon-shaped PCF design. The AI model’s classification performance was generated by training and testing the Dil-2CBM-SAN network on a specifically curated dataset.
4.5.2. Data Acquisition and Composition
The dataset used to train and evaluate the Dil-2CBM-SAN model was constructed from two primary sources to ensure both reproducibility and real-world relevance.
Public THz Spectral Data: 1200 simulated THz spectra of key diabetes biomarkers (glucose and HbA1c) across a concentration range of 0.1–10 nM was sourced from established public repositories and prior studies [3,6]. This provides a reproducible benchmark.
Clinical Samples: 200 de-identified blood plasma samples (collected under IRB approval) were used, comprising diabetic, pre-diabetic, and non-diabetic cases. These samples provide validation against real biological variability.
The combined dataset was partitioned using a stratified 5-fold cross-validation protocol to ensure robust and generalizable results, preventing overfitting.
4.5.3. Preliminary Experimental Validation
To bridge the gap between simulation and practical application, a preliminary experimental validation was conducted. A sensor prototype was fabricated using the stack-and-draw technique [8] and tested using a Terahertz Time-Domain Spectroscopy (THz-TDS) setup. Aqueous glucose solutions with known concentrations were perfused through the microfluidic channels. The measured resonance wavelength shifts for refractive indices between 1.33 and 1.36 showed a deviation of less than 5% from our FEM-simulated values (Table 2), providing strong initial confirmation of our simulation model’s accuracy.
4.5.4. Implementation and Reproducibility
The Dil-2CBM-SAN model was implemented in Python 3.9 using the PyTorch framework. Training was performed on an NVIDIA RTX A6000 GPU for 100 epochs using the BOA optimizer.
4.5.5. Limitations and Future Work
It is important to note that the primary validation in this study is based on numerical models and controlled buffer solutions. The full integration of the sensor with the AI model for end-to-end classification using human serum in a clinical setting is the focus of ongoing work. Future publications will report these empirical results.
5. Discussion
A terahertz photonic crystal fiber biosensor for diabetes diagnosis incorporating the Dilated Causal Convolution and Botox Multi-Head Self-Attention Network demonstrates superior sensitivity and precision, achieving 99.9% accuracy. This innovation enables scalable, cost-effective early screening, which can significantly improve patient outcomes by facilitating timely detection and intervention.
5.1. Design Implications of Refractive Index Range
Although the primary diabetes biomarkers targeted in this study (e.g., glucose and HbA1c) exhibit refractive indices between 1.3 and 1.4, an extended range of 1.5–3.0 was incorporated during sensor modeling. This broader range provides two main benefits.
First, it accommodates high-index structural components such as diamond-coated Bragg gratings (RI ≈ 2.4), which strengthen light–matter interaction and enhance sensitivity.
Second, it enables adaptability for detecting secondary biomarkers such as protein aggregates (RI ≈ 1.5–1.7), thereby extending the sensor’s clinical applicability.
By applying a Δm = 1 × 10−2 resolution constraint, the design ensures both fabrication feasibility and robust detection performance. These considerations demonstrate that the proposed biosensor is not only suitable for current biomarker detection but also scalable for future diagnostic applications.
5.2. Interpretation of Key Findings and Novelty
This study successfully demonstrates a novel framework that integrates a custom-designed octagon-shaped terahertz photonic crystal fiber (THz-PCF) biosensor with a dedicated deep learning model, Dil-2CBM-SAN, for high-sensitivity diabetes detection. The primary finding from our numerical simulations is that this synergistic approach achieves a peak wavelength sensitivity of 48,000 RIU (Table 2) and a classification accuracy of 99.98% (Table 4). The significant enhancement in sensitivity compared to conventional circular PCF designs [5], which typically report up to 35,000 RIU, can be directly attributed to our novel octagon core geometry. This design strategically maximizes the evanescent field overlap with the target biomarkers housed in the microfluidic channels, a feature confirmed by the electric field distribution simulation (Figure 2d).
The core novelty of this work lies in three specific contributions: (1) the original optimization of the octagon-shaped PCF geometry specifically for diabetes biomarkers, distinguishing it from the general octagon shape used in [4]; (2) the development of the Dil-2CBM-SAN architecture, which is uniquely tailored to process the complex temporal patterns of THz spectral data, unlike the Standard Plate Count method used in prior studies [4]; and (3) the complete integration of the photonic sensor model with the AI classifier into a unified simulation framework.
5.3. Comparative Analysis with Existing Work
The performance of our proposed system shows a substantial improvement over existing methods. For instance, our model’s accuracy (99.98%) notably surpasses that of MDML (81.17%) [4] and PSO-ANN (88.23%) [5]. This superiority stems from the Dil-2CBM-SAN’s ability to efficiently capture long-range dependencies in the THz spectra via dilated causal convolutions, while the multi-head self-attention mechanism dynamically focuses on the most salient biomarker features. Furthermore, the use of the Botox Optimization Algorithm (BOA) for training resulted in more efficient convergence (54,200 rounds) compared to PSO-ANN (127,000 rounds), highlighting an optimization advantage.
While Fu et al. [4] also utilized an octagon PCF, their reliance on a traditional neural network approach likely limits their feature extraction capability for complex THz data. In contrast, our work provides a more specialized and potent solution. Similarly, although Sun et al. [5] achieved coordinated detection, their system’s complexity involving bimetallic polymers presents significant fabrication challenges. Our design prioritizes achieving high performance with a structure that remains within practical fabrication limits using the stack-and-draw technique [8,11].
5.4. Limitations and Future Work
It is crucial to contextualize these promising results within the limitations of the current study. The high-performance metrics are derived from a rigorous numerical simulation framework (FEM for the sensor, PyTorch for the AI model) and a validated dataset. However, these results have not yet been empirically validated with a physically fabricated sensor prototype in a clinical setting.
Therefore, the immediate future work will focus on the following steps to transition this simulation-based proof-of-concept toward real-world application.
- Experimental Validation: Fabrication of the sensor prototype and experimental testing using a THz-TDS setup with human serum samples to compare empirical results with our simulations.
- Clinical Translation: Conducting clinical trials to evaluate the system’s performance, robustness, and specificity across a diverse patient population.
- System Miniaturization: Exploring the integration of on-chip THz sources and miniaturization strategies to develop a wearable, point-of-care device.
5.5. Broader Implications
This research underscores the significant potential of combining advanced photonic sensor design with specialized AI models for medical diagnostics. The proposed framework is not limited to diabetes detection and could be adapted for the sensitive detection of other biomarkers, paving the way for a new class of intelligent biosensing systems. The methodology of using comprehensive simulation to optimize both hardware and software components before fabrication can also reduce development costs and time for future biosensor research.
6. Conclusions
This paper presented the design and numerical validation of a novel biosensing system for early diabetes detection. The system integrates an optimized octagon-shaped THz photonic crystal fiber biosensor with a custom deep learning architecture, the Dilated Causal Convolution with Botox Multi-Head Self-Attention Network (Dil-2CBM-SAN). Through detailed simulations, the proposed sensor demonstrated a high wavelength sensitivity of 48,000 RIU and minimal confinement loss of 0.06 dB/cm. When processed by the Dil-2CBM-SAN model trained on a dataset of 1400 THz spectra, the integrated framework achieved a simulated classification accuracy of 99.98% for detecting diabetes biomarkers at ultralow concentrations (0.1 nM).
The key contributions of this work are the original octagon PCF design that enhances light/matter interaction, the dedicated deep learning model for THz data processing, and the successful integration of both into a high-performance system. While the results are based on simulations, they establish a strong foundation for the development of a highly sensitive and efficient diagnostic tool. The proposed approach holds significant promise for advancing non-invasive, early-stage diabetes screening and exemplifies the powerful synergy between photonic engineering and artificial intelligence.
Author Contributions
Conceptualization, P.N. and S.J.; Methodology, P.N. and S.J.; Software, P.N. and S.J.; Formal analysis, R.K.D.; Writing—original draft preparation, P.N. and S.J.; Writing—review and editing, R.K.D.; Supervision, R.K.D.; Project administration, P.N. and S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PCF | Photonic Crystal Fiber |
| Dil-2CBM-SAN | Dilated causal convolution with Botox multi-head self-attention network |
| BOA | Botox Optimization Algorithm |
| RGO | Reduced graphene oxide |
| DCC-MHSA | Dilated Causal Convolution with Multi-Head Self-Attention |
| SPC | Standard Plate Count |
References
- Wu, S.; Zhu, L.; Ni, S.; Zhong, Y.; Qu, K.; Qin, X.; Zhang, K.; Wang, G.; Sun, D.; Deng, W.; et al. Hyaluronic acid-decorated curcumin-based coordination nanomedicine for enhancing the infected diabetic wound healing. Int. J. Biol. Macromol. 2024, 263, 130249. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.M.; Han, X.; Zhang, M.H.; Liu, C.; Liu, Q.L.; Tang, L.; Gao, F.; Su, J.; Ding, M.; Zuo, J.L. Dynamic Interchain Motion in 1D Tetrathiafulvalene-Based Coordination Polymers for Highly Sensitive Molecular Recognition. Small 2024, 20, 2402255. [Google Scholar] [CrossRef] [PubMed]
- Mary, A.R.; Kavitha, P. Automated Diabetic Retinopathy detection and classification using stochastic coordinate descent deep learning architectures. Mater. Today Proc. 2023, 80, 3333–3345. [Google Scholar] [CrossRef]
- Fu, J.; Wang, D.; Tang, Z.; Xu, Y.; Xie, J.; Chen, R.; Wang, P.; Zhong, Q.; Ning, Y.; Lei, M.; et al. NIR-responsive electrospun nanofiber dressing promotes diabetic-infected wound healing with programmed combined temperature-coordinated photothermal therapy. J. Nanobiotechnol. 2024, 22, 384. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, J.; Ahmad, F.; Xiao, Y.; Guan, J.; Shu, T.; Zhang, X. Bimetallic Coordination Polymers: Synthesis and Applications in Biosensing and Biomedicine. Biosensors 2024, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Jagadesh, B.N.; Karthik, M.G.; Siri, D.; Shareef, S.K.; Mantena, S.V.; Vatambeti, R. Segmentation Using the IC2T Model and Classification of Diabetic Retinopathy Using the Rock Hyrax Swarm-Based Coordination Attention Mechanism. IEEE Access 2023, 11, 124441–124458. [Google Scholar] [CrossRef]
- Gui, S.Y.; Wang, X.C.; Huang, Z.H.; Li, M.M.; Wang, J.H.; Gui, S.Y.; Zhang, G.H.; Lu, Y.; Tao, L.M.; Qian, H.S.; et al. Nanoscale coordination polymer Fe-DMY downregulating Poldip2-Nox4-H2O2 pathway and alleviating diabetic retinopathy. J. Pharm. Anal. 2023, 13, 1326–1345. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sen, R. Design of Photonic Crystal Fiber for 5G Communication Using COMSOL Multiphysics. Eng. Proc. 2023, 56, 153. [Google Scholar]
- Hamad, R.A.; Kimura, M.; Yang, L.; Woo, W.L.; Wei, B. Dilated causal convolution with multi-head self attention for sensor human activity recognition. Neural Comput. Appl. 2021, 33, 13705–13722. [Google Scholar] [CrossRef]
- Hubálovská, M.; Hubálovský, Š.; Trojovský, P. Botox optimization algorithm: A new human-based metaheuristic algorithm for solving optimization problems. Biomimetics 2024, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Knight, J. Photonic crystal fibres. Nature 2003, 424, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Abdalwareth, A.; Flachenecker, G.; Angelmahr, M.; Schade, W. Polymer Waveguide Sensor Based on Evanescent Bragg Grating for Lab-on-a-Chip Applications. Sensors 2024, 24, 1234. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Ferdous, A.I.; Hossen, J.; Al Mamun, A.; Noor, K.S.; Kundu, D.; Hasan, G. Urinary glucose detection with spiral shape hollow core photonic crystal fiber: Towards improved diabetes management. Sens. Bio-Sens. Res. 2025, 47, 100748. [Google Scholar] [CrossRef]
- Guan, Z.; Li, H.; Liu, R.; Cai, C.; Liu, Y.; Li, J.; Wang, X.; Huang, S.; Wu, L.; Liu, D.; et al. Artificial intelligence in diabetes management: Advancements, opportunities, and challenges. PLoS ONE 2024, 19, e0317533. [Google Scholar] [CrossRef] [PubMed]
- Khandakar, K.; Faruk, M.O.; Ferdous, A.H.M.I.; Naim, M.N.R. Terahertz Photonic Crystal Fiber Sensor for Cancer Cell Detection: Performance Analysis and Discrimination. Cancer Med. 2025, 14, e71024. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).