Application of the Reduced Graphene Oxide–Multiwalled Carbon Nanotubes Composite for Development of the Electrochemical Aptasensor for Oxytetracycline Detection †
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the Biosensor and Electrochemical Measurements
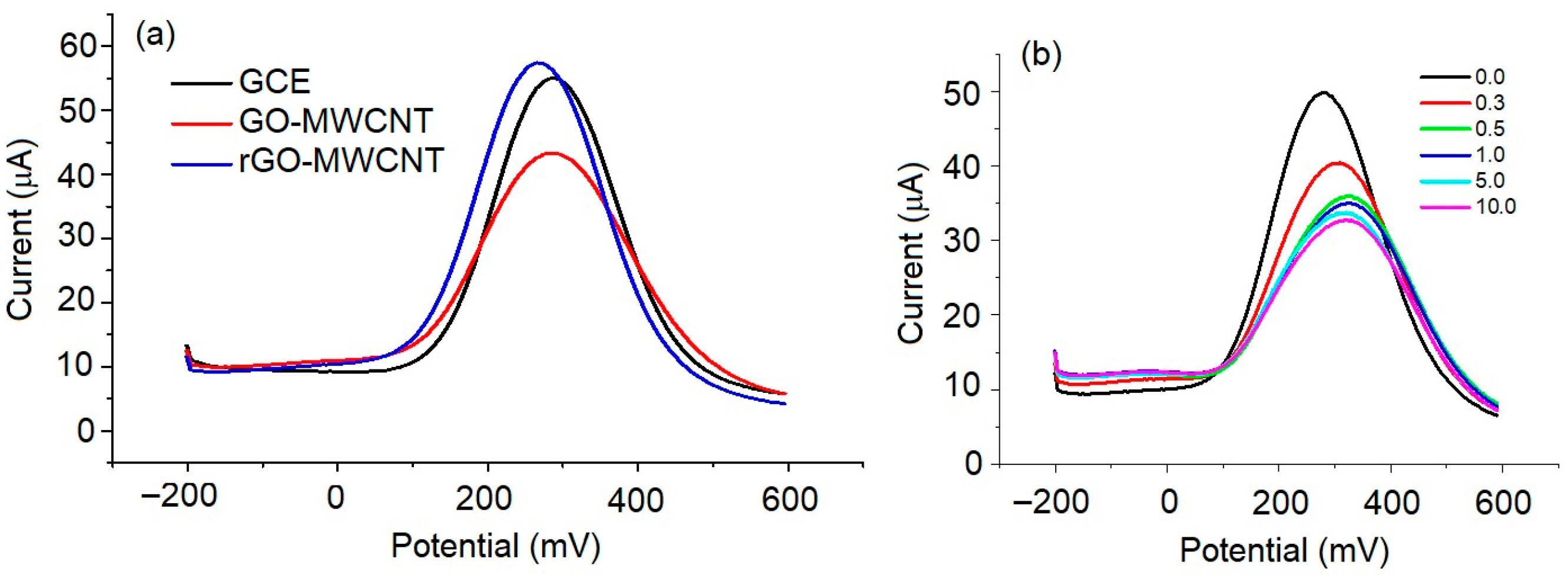
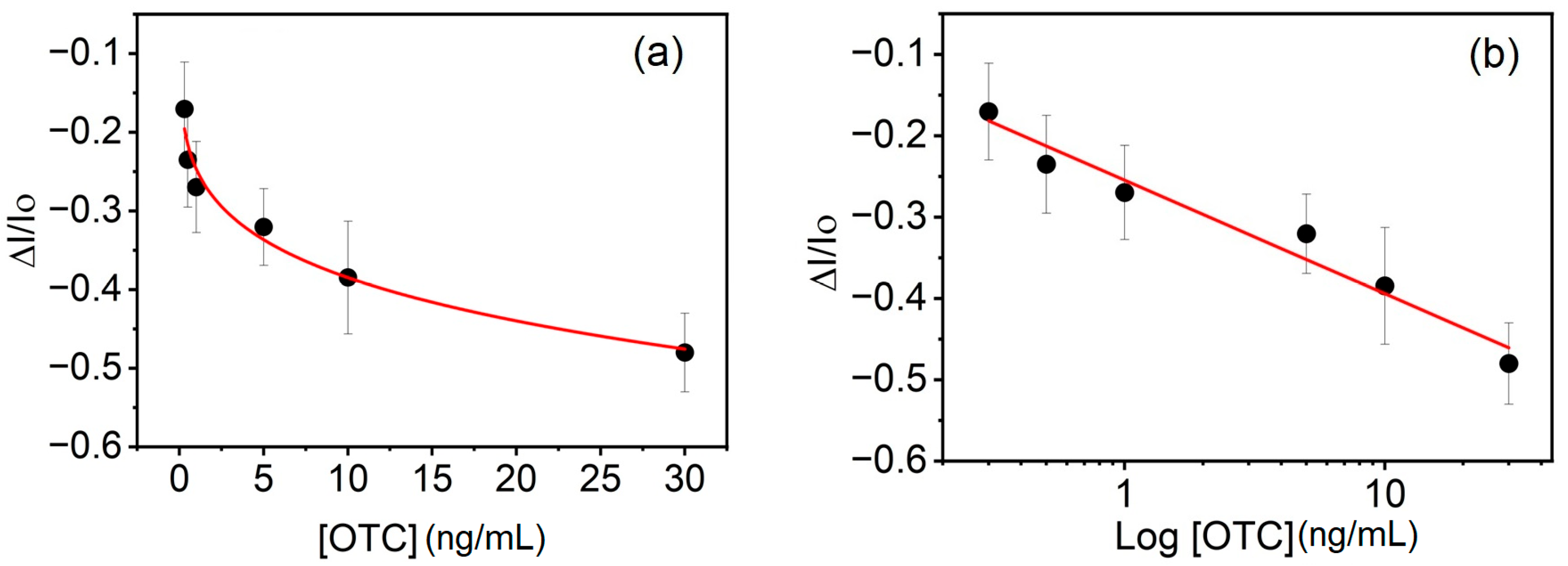
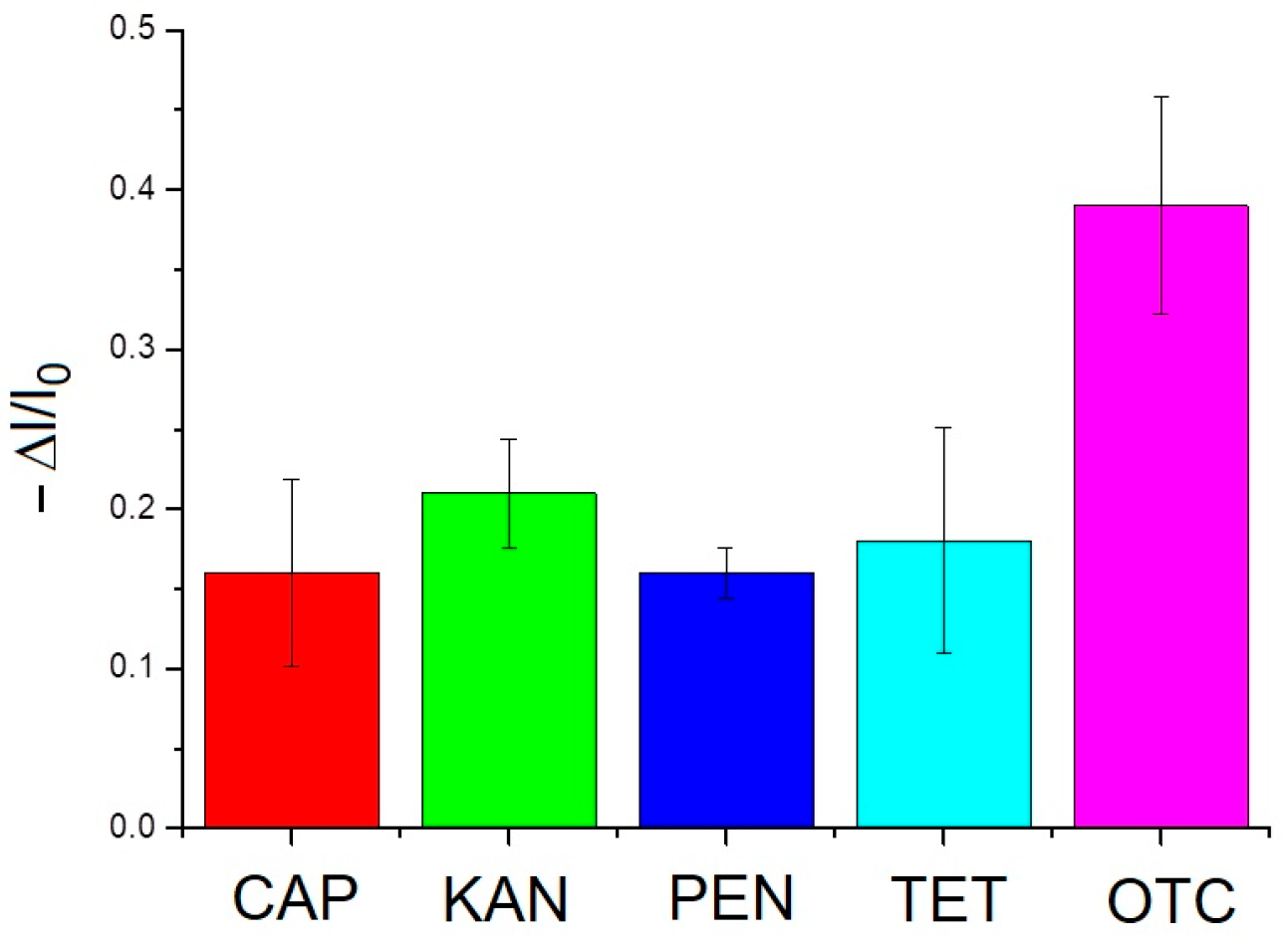
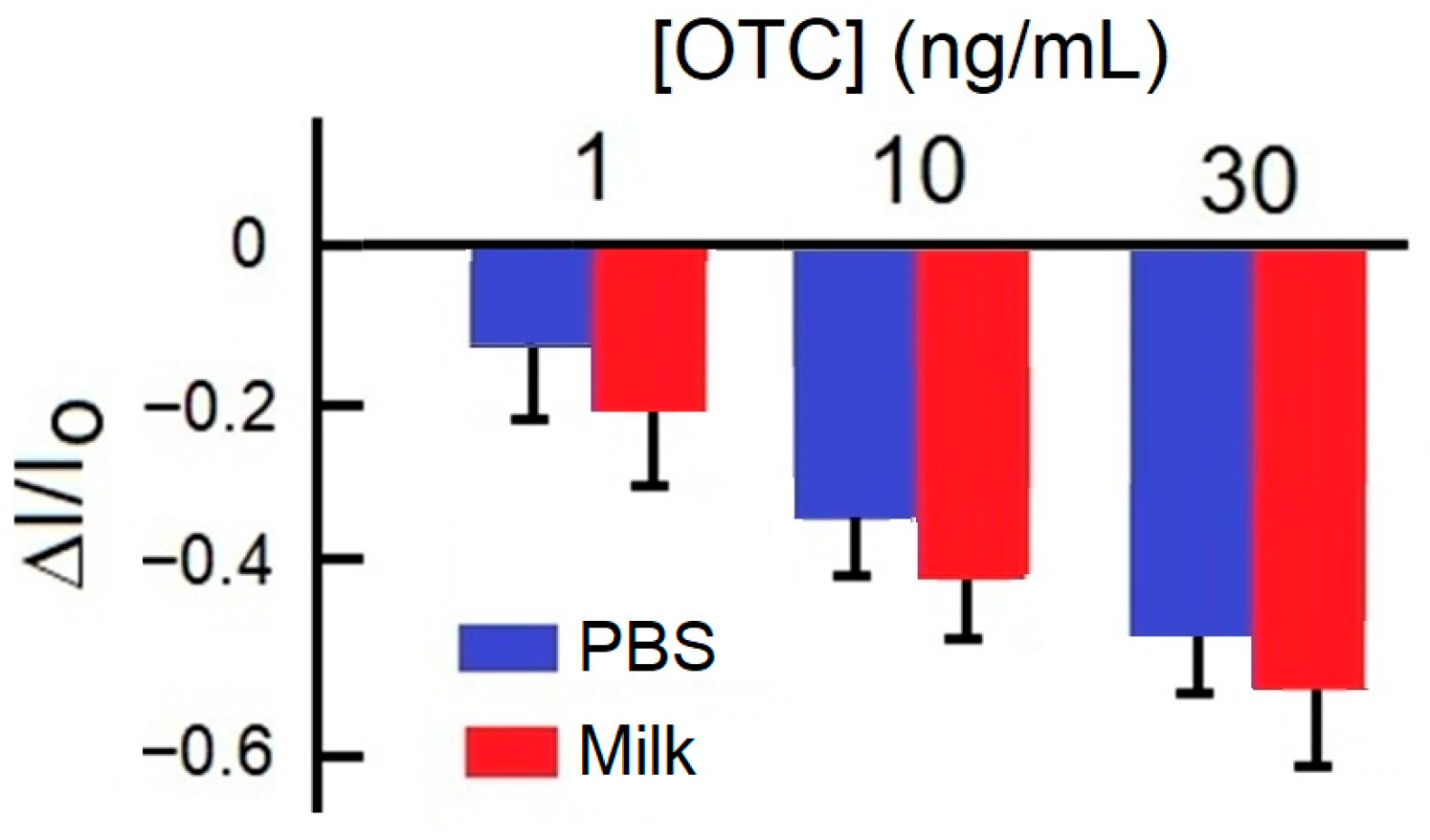
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Commission Regulation (EU). No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union. 2010, 15, 1–72. [Google Scholar]
- Akbarzadeh, S.; Khajehsharifi, H.; Hajihosseini, S. Detection of oxytetracycline using an electrochemical label-free aptamer-based biosensor. Biosensors 2022, 12, 468. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, D.; Morsi, R.; Usman, M.; Meetani, M.A. Recent advances in the chromatographic analysis of emerging pollutants in dairy milk: A review (2018–2023). Molecules 2024, 29, 1296. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; Wai, H.K.F.; Wu, P.; Lai, S.W.; Chan, O.S.K.; Tun, H.M. A universal LC-MS/MS method for simultaneous detection of antibiotic residues in animal and environmental samples. Antibiotics 2022, 11, 845. [Google Scholar] [CrossRef]
- Wang, B.; Xie, K.; Lee, K. Veterinary drug residues in animal-derived foods: Sample preparation and analytical methods. Foods 2021, 10, 555. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Tsekenis, G.; Oravczova, V.; Hianik, T. Electrochemical aptasensors for antibiotics detection: Recent achievements and applications for monitoring food safety. Sensors 2022, 22, 3684. [Google Scholar] [CrossRef]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. SELEX–A (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007, 24, 381–403. [Google Scholar] [CrossRef]
- Tian, R.; Sun, J.; Ye, Y.; Lu, X.; Sun, X. Screening strategy of aptamers and its application in food contaminants determination. Trends Anal. Chem. 2024, 175, 117710. [Google Scholar] [CrossRef]
- Niazi, J.H.; Lee, S.J.; Gu, M.B. Single-stranded DNA aptamers specific for antibiotics tetracyclines. Bioorg. Med. Chem. 2008, 16, 7245–7253. [Google Scholar] [CrossRef]
- Singh, B.; Bhat, A.; Dutta, L.; Pati, K.R.; Korpan, Y.; Dahiya, I. Electrochemical biosensors for the detection of antibiotics in milk: Recent trends and future perspectives. Biosensors 2023, 13, 867. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Iordache, F.; Stanca, L.; Rosu, P.M.; Ciocirlie, N.; Geicu, I.O.; Bilteanu, L.; Serban, A.I. Electrochemical nanosensors applied to the assay of some food components—A review. Chemosensors 2025, 13, 272. [Google Scholar] [CrossRef]
- Shirsat, M.D.; Hianik, T. Electrochemical detection of heavy metal ions based on nanocomposite materials. J. Compos. Sci. 2023, 7, 473. [Google Scholar] [CrossRef]
- Jia, F.; Duan, N.; Wu, S.; Dai, R.; Wang, Z.; Li, X. Impedimetric Salmonella aptasensor using a glassy carbon electrode modified with an electrodeposited composite consisting of reduced graphene oxide and carbon nanotubes. Microchim. Acta 2016, 183, 337–344. [Google Scholar] [CrossRef]
- Sharma, A.; Istamboulie, G.; Hayat, A.; Catanante, G.; Bhand, S.; Marty, J.L. Disposable and portable aptamer functionalized impedimetric sensor for detection of kanamycin residue in milk sample. Sens. Actuators B 2017, 245, 507–515. [Google Scholar] [CrossRef]
- Tempfli, D.; Borbás, E.; Pataki, H.; Csicsák, D.; Völgyi, G.; Sinkó, B.; Takács-Novák, K. Revisit of solubility of oxytetracycline polymorphs. An old story in the light of new results. Eur. J. Pharm. Sci. 2020, 149, 105328. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Davoudian, K.; Spagnolo, S.; Chan, E.; Hianik, T.; Thompson, M. Acoustic wave sensor detection of an ovarian cancer biomarker with antifouling surface chemistry. Sensors 2024, 24, 7884. [Google Scholar] [CrossRef] [PubMed]
- Ambruser, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantification. Clin. Biochen. Rev. 2008, 29 (Suppl. S1), S49–S52. [Google Scholar]
- Kourti, D.; Geka, G.; Nemtsov, L.; Ahmadi, S.; Economou, A.; Thompson, M. Electrochemical aptasensor with antifouling properties for label-free detection of oxytetracycline. Sensors 2024, 24, 5488. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, W.; Guo, Y.; Wang, X.; Zhang, F.; Yu, L.; Guo, C.; Fang, G. Sensitive and selective electrochemical aptasensor via diazonium-coupling reaction for label-free determination of oxytetracycline in milk samples. Sens. Actuators Rep. 2020, 2, 100009. [Google Scholar] [CrossRef]
- Yuan, R.; Fu, Z.; He, Y.; Deng, Y.; Xi, J.; Xing, X.; He, H. Size-controlling preparation of covalent organic framework nanospheres for electrochemical impedimetric aptasensing of oxytetracycline. Talanta 2023, 265, 124834. [Google Scholar] [CrossRef]
- Wang, Q.; Li, P.; Wen, H.-M.; Hu, K.-J.; Huang, Z.-Y.; Chen, J. A HOF-based electrochemical aptasensor for highly sensitive and selective detection of trace oxytetracycline. Inorganic Chem. Commun. 2023, 156, 111213. [Google Scholar] [CrossRef]
- Song, P.; Qu, P.; Wang, M.; Wang, A.-J.; Xue, Y.; Mei, L.-P.; Feng, J.-J. Self-checking dual-modal aptasensor based on hybrid Z-scheme heterostructure of Zn-defective CdS/ZnS for oxytetracycline detection. Anal. Chim. Acta 2023, 1274, 341542. [Google Scholar] [CrossRef] [PubMed]
- Machairas, C.; Anagnostoupoulos, A.; Soulis, D.; Economou, A.; Jakab, K.; Melios, N.; Keresztes, Z.; Tsekenis, G.; Wang, J.; Speliotis, T. Microfabricated gold aptasensors for the label-free electrochemical assay of oxytetracycline residues in milk. Eng. Proc. 2023, 58, 1. [Google Scholar] [CrossRef]
- Song, J.; Fan, X.; Ee, L.Y.; Lin, X.; Zhang, D.; Li, S.F.Y.; Huang, M. Novel dopamine-derived carbon modified Fe-based metal- organic framework enhanced aptasensor rendering ultrasensitive electrochemical detection of oxytetracycline. Chem. Eng. J. 2024, 499, 156373. [Google Scholar] [CrossRef]
- Guan, F.; Dong, Y.; Wang, L.; Cai, D.; Guo, Y.; Zhao, S.; Sheng, Q. An electrochemical aptamer sensor based on AuNPs/ErGO/Cu-MOF nanocomposites for the detection of oxytetracycline in foodstuff. Microchem. J. 2025, 208, 112579. [Google Scholar] [CrossRef]
- Bizinti, C.; Soulis, D.; Kourti, D.; Geka, G.; Kokkinos, C.; Thompson, M.; Nemtsov, L.; Speliotis, T.; Economou, A. Microfabricated electrochemical aptasensing chip modified with dual-function antifouling linker for single-drop label-free assay of oxytetracycline in milk. Mirochim. Acta 2025, 192, 527. [Google Scholar] [CrossRef]
- Spagnolo, S.; Davoudian, K.; De La Franier, B.; Kocsis, R.; Hianik, T.; Thompson, M. Nanoparticle-enhanced acoustic wave biosensor detection of Pseudomonas aeruginosa in food. Biosensors 2025, 15, 146. [Google Scholar] [CrossRef]

| Sensing Surface | Detection Method | Dynamic Range ng/mL | LOD, ng/mL | Sample Type/ Recovery % | Reference |
|---|---|---|---|---|---|
| SPE Au electrode | DPV | 1–500 | 14 (PBS)/10 (Milk) | Milk/124 | [19] |
| Au/COF nanospheres | EIS | 10−5–10−2 | 7.4 × 10−6 | Milk/95.7–107 | [21] |
| Au/HOF | CV, EIS | (0.01–0.9) × 10−3 | 9.4 × 10−4 | - | [22] |
| FTO/Zn-defective CdS/ZnS heterostructure | PEC EC | 0.01–50 | 1.86 × 10−3 3.08 × 10−3 | - | [23] |
| Au/Kapton film | DPV | 1.5–3 | 5 | Milk/95 | [24] |
| GCE/MOF(Fe)@PDA/SPE | DPV/ CA | 4.6–4600 | 3.17/2.56 | Tap water/98.2/99.4 | [25] |
| SPCE/AuNPs/ErGO/Cu-MOF | CV | 0.1–105 | 0.03 | Milk/87–110 | [26] |
| Au/Kapton film | DPV | 1.4–2.7 | 7 | Milk/107–110 | [27] |
| GCE/rGO/MWCNTs | DPV | 1–30 | 1.72 | Milk/109 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakos, M.; Pavai, M.; Zacharopoulos, C.; Sontakke, K.; Bousiakou, L.; Keresztes, Z.; Ivanov, I.N.; Hianik, T. Application of the Reduced Graphene Oxide–Multiwalled Carbon Nanotubes Composite for Development of the Electrochemical Aptasensor for Oxytetracycline Detection. Eng. Proc. 2025, 106, 16. https://doi.org/10.3390/engproc2025106016
Kakos M, Pavai M, Zacharopoulos C, Sontakke K, Bousiakou L, Keresztes Z, Ivanov IN, Hianik T. Application of the Reduced Graphene Oxide–Multiwalled Carbon Nanotubes Composite for Development of the Electrochemical Aptasensor for Oxytetracycline Detection. Engineering Proceedings. 2025; 106(1):16. https://doi.org/10.3390/engproc2025106016
Chicago/Turabian StyleKakos, Minas, Maria Pavai, Charalampos Zacharopoulos, Kiran Sontakke, Leda Bousiakou, Zsofia Keresztes, Ilia N. Ivanov, and Tibor Hianik. 2025. "Application of the Reduced Graphene Oxide–Multiwalled Carbon Nanotubes Composite for Development of the Electrochemical Aptasensor for Oxytetracycline Detection" Engineering Proceedings 106, no. 1: 16. https://doi.org/10.3390/engproc2025106016
APA StyleKakos, M., Pavai, M., Zacharopoulos, C., Sontakke, K., Bousiakou, L., Keresztes, Z., Ivanov, I. N., & Hianik, T. (2025). Application of the Reduced Graphene Oxide–Multiwalled Carbon Nanotubes Composite for Development of the Electrochemical Aptasensor for Oxytetracycline Detection. Engineering Proceedings, 106(1), 16. https://doi.org/10.3390/engproc2025106016








