Abstract
In general, epilepsy is considered to be one of most prevalent neurological disorders and frequently appears as sudden seizures resulting in injuries, accidents, sudden unexpected death, etc. Also, it is reported that around 60 million people across the globe are experiencing various seizures due to epilepsy. So, there is demand for ambulatory seizure detection devices to prevent such accidents and to improve the quality of life for epilepsy patients. In this work, an intelligent Internet of Medical Things (IoMT)-based wearable device is designed and developed to monitor seizures in epilepsy patients. Due to the lack of an accelerometer dataset for epileptic seizures, the proposed device was developed, and a dataset mimicking seizure-like activities was generated. Further, the proposed device utilizes an MPU6500-based inertial measurement unit (IMU) which is integrated into an ESP32 microcontroller board. The ESP32 has a built-in wireless fidelity (WiFi) + Bluetooth (BLE) un that supports MicroPython v1.22.1 programming. Also, the machine learning algorithms such as Decision Trees (DT), Support Vector Machines (SVM), and Random Forest (RF) were programmed using MicroPython v1.22.1 programming and deployed on a tiny edge computing device to monitor the activity of the epileptic patients. All the adopted machine learning algorithms were compared in terms of performance metrics such as accuracy, precision, recall, false positive rate (FPR), etc., and the efficacy of the device was analysed. Results demonstrate that the proposed device is capable of identifying the activities of individuals, which is highly useful for epilepsy patients to monitor epileptic seizures. Furthermore, the proposed device was deployed with an RF algorithm since it exhibits an accuracy of 95% which is better compared to other machine learning algorithms. Also, the proposed device is simple and cost-effective and, in the event of a seizure event, can alert caretakers of epilepsy patients with an FPR of less than 4%.
1. Introduction
Generally, seizures are immediate, uncontrollable and abnormal electrical activity in the brain. Seizures affect the normal activities of a person at the motor, emotional, behavioural and conscious level. Depending on the neurogenic activity in the brain’s hemispheres, seizures can be further classified into two types, namely focal and generalised seizures. The epileptic seizure is a neurological disorder caused by the anomalous electrical discharge or hyperactivity of neurons. Additionally, the seizure is a transient phase, while epilepsy is a chronic disorder and can be fatal at times. According to the World Health Organisation (WHO), epileptic seizures are of concern as a major health issue [1].
Focal seizures are partial seizures that occur on a specific side of the cerebral hemisphere and later progress to the other side. Furthermore, the focal seizure affects single limbs and further affects limbs on both sides (both arms and legs). Generalised seizures occur due to the simultaneous generation of irregular electrical signals from both sides of the cerebral hemisphere. Also, the generalised seizure can be either a motor or non-motor seizure depending on the behaviour and activity during the seizure attack [2].
Seizures can occur during sleep. Sleep and seizures have a bidirectional relationship wherein disturbances in sleep patterns can aggravate seizure susceptibility while seizures can significantly disrupt normal sleep activity. The relation between sleep apnoea and epilepsy provides techniques to treat seizures [3]. Sleeping disorders or sleep deprivation are most common in people with epilepsy. Sleep disorders may aggravate the existing problems and seizures of epileptic people. This can be controlled by anti-seizure treatments [4]. Detecting seizures during sleep is a challenging task.
Neuroimaging plays a vital role in the characterization and analysis of seizures. Additionally, it enables the visualization of structural and functional brain alterations associated with the activity, especially in epilepsy. The various methods include electroencephalogram (EEG), computed tomography (CT) scan, magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), positron emission tomography (PET), and genetic testing, which provides critical insights about the seizure onset zones for the diagnosis of epileptic seizures [5]. EEG is the most reliable method indicating the unusual patterns of brain signals. Further, observation of the EEG signals helps in the identification of the type of seizure. Neuroimaging aids in the computation of seizures. Additionally, the continuous electromagnetic fields of the brain are evaluated by the magnetoencephalography (MEG), which measures the magnetic fields induced by neuronal activity [6]. Furthermore, MEG enables the characterization of brain activity with millisecond temporal resolution.
Recent advancements in wearable technologies have explored new possibilities for non-invasive and continuous monitoring of neurological conditions in real-time applications. The conducting polymer hydrogels engineered through supramolecular strategies have enabled highly flexible and biocompatible wearable sensors for physiological monitoring, as discussed by [7]. Similarly, the development of multidimensional motion sensors has advanced AI-enhanced environments in dynamic settings, namely virtual reality (VR) sports [8]. Furthermore, Wu et al., 2023 [9] demonstrated innovations in nanostructured conductive polypyrrole in the integration of antibacterial and high-performance features in flexible wearable devices.
Although continuous monitoring with an EEG setup is not possible in real time, several wearables for seizure detection have been developed. Such devices demonstrate that continuous seizure monitoring outside clinical settings is feasible; however, many existing devices have limitations such as limited accuracy, discomfort on prolonged usage and reliance on a single bio-signal modality. Within this context, the proposed accelerometer-driven hardware–software co-design advances seizure detection. Also, the proposed work employs an electrode-free, accelerometer-based wearable for continuous monitoring of sleep postures and seizure-like activities. By integrating machine learning algorithms, the system not only detects seizure activity with high accuracy but also classifies various sleeping postures, thereby providing dual functionality in a single platform. Finally, the user-friendly hardware with robust machine learning analytics enhances comfort, scalability, etc., providing a practical solution for home-based, privacy-sensitive monitoring of epileptic seizures.
2. Literature Survey
Seizures occur non-randomly, following circadian rhythms and other factors such as seizure location, hormonal influences, etc. Amengual-Gual et al., (2019) have analysed seizure timing patterns and their distribution across circadian cycles, providing deep insights into the occurrence of seizures in patients with epilepsy [10]. Kusmakar et al., (2018) have demonstrated the automated detection of generalised tonic-clonic seizures (GTCS) by utilising a wearable accelerometer device along with a machine learning algorithm, namely support vector data description (SVDD). Further, the authors have stated that the proposed method includes full-body convulsions to classify from different conditions, namely complex partial seizures (CPS) and psychogenic non-epileptic seizures (PNES) [11].
Davidson et al., (2022) have proposed a method to enhance the classification of epileptic seizures from EEG data with the use of machine learning methods, namely Naive Bayes Algorithm and Genetic Algorithm, for better seizure detection [12]. Additionally, Roy et al., (2020) have conducted a comparative analysis of machine learning algorithms in classifying types of seizures [13].
Yu liu et al., (2020) [14] have taken a unique approach in creating an efficient model using Convolutional Neural Network (CNN), and in order to improve the accuracy, multiple bio-signals were utilized. However, the authors have faced challenges, namely the requirement for a large dataset to train the modal and signal noise [14]. Moreover, an innovative and unique approach was proposed by Zablar et al., (2024) [15] to self-report the seizures with a mobile application diary which makes the process user-friendly. Further, the authors have demonstrated that the proposed approach has high precision in the patient’s self-reporting of seizure occurrence, with higher accuracy [15]. Komal et al., (2024) have reviewed remote seizure detection devices including mobile applications, wearables and biosensors along with their reliability and accuracy [16]. Yadav et al., (2008) have proposed a dual-stage classification system to monitor epilepsy long-term in order to classify the data as non-seizure or seizure [17].
3. Methodology
In this work, an intelligent Internet of Medical Things (IoMT)-based wearable device is designed and developed to monitor seizures in epilepsy patients during sleep. A seizure event during sleep can be unnoticed and lead to severe causalities. Therefore, it is essential to propose alternative systems for the detection of epileptic seizures that do not rely on conventional EEG electrodes. Such electrode-free approaches are often based on wearable motion sensors, and the accelerometer sensor is one such electrode-less device which can be used for the detection of epileptic seizures. Due to the lack of an accelerometer dataset for epileptic seizures, the proposed device was developed, and a dataset mimicking seizure-like activities was generated. The proposed device was worn on the shin, specifically the tibial area of the lower leg. Furthermore, the placement area was selected based on prior findings that convulsive seizure events typically produce high-amplitude or rhythmic movements in the lower limbs, especially during tonic-clonic seizures. Also, compared to the wrist or waist, shin placement provides good and more consistent accelerometric signals during leg-dominated seizure activity and also minimizes motion artifacts due to unrelated hand movements. A group of volunteers were asked to perform seizure-mimicking activities with the supervision of a physician under controlled conditions. The seizure-mimicking dataset was developed by instructing healthy adult volunteers to perform predefined motor actions which closely resemble common seizure semiologies based on the literature and neurologist guidance. In this regard, seizures such as tonic-clonic movements, myoclonic jerks and hypermotor patterns were mimicked under controlled conditions. Figure 1 shows a hardware block diagram of the proposed wearable device. Further, the proposed device utilizes a sensor module, IoMT controller, battery, battery recharging module, enclosure and strap.
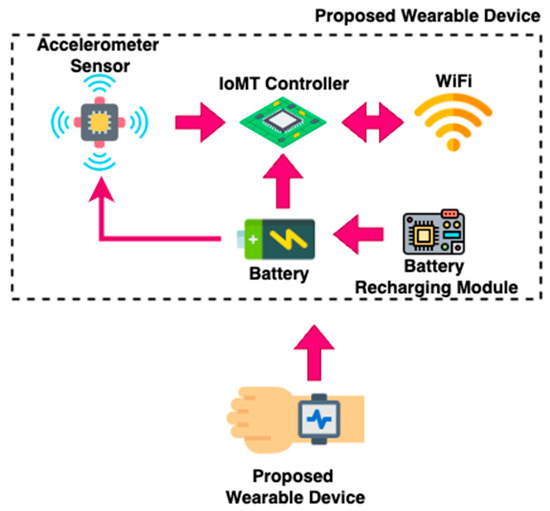
Figure 1.
Proposed Wearable Device.
3.1. Sensor Module
In this work, an ST Microelectronics LSM9DS1 inertial measurement unit was utilized to measure 3-axis accelerometer values. Further, the sensor module was capable of measuring 3-axis accelerometer values, gyro values and magnetometer values with 9 degrees of freedom (DOF). The sensor module could be connected to a 3.3 Volt power source available on the controller board, and the output of the sensor module was connected to the controller board using the Inter-Integrated Circuit (I2C) protocol.
3.2. IoMT Controller
In this work, an ESP32 Cam-based controller was used as the IoMT controller. Further, the controller board had a low-power 32-bit central processing unit which could operated at up to 160 MHz clock speed. Also, the controller had built-in SRAM with capacity of 520 KB and external 4MPSRAM. Additionally, the WiFi and Bluetooth modules were integrated into the utilized controller, making it suitable for IoT applications. The machine learning algorithm was deployed to the utilized IoMT controller using MicroPython v1.22.1 programming, and the controller received sensor accelerometer values to predict seizures.
3.3. Battery
In this work, a 3.7 Volt 1500 mAH Lithium Polymer battery was used to power various components, namely the sensor module and controller board. Also, the battery utilized was the rechargeable type, and appropriate recharging arrangements were customized.
3.4. Battery Recharging Module
The battery recharging module was a type of charging board for a single-cell 3.7 V Lithium Polymer battery. Also, the TP4056-based module-based charging board was very simple, compact and easy to handle with short-circuit protection features.
3.5. Enclosure and Strap
An enclosure is used to cover the electronic components of the proposed wearable device, and a strap is used to affix the proposed device to epileptic patients.
3.6. Proposed IoMT Device
Figure 2 shows the proposed methodology for the development of the wearable device to detect epileptic seizures. The proposed methodology consists of two different phases, namely a training phase and testing phase for the development of the wearable device to detect epileptic seizures. The training phase includes accelerometer signal acquisition, pre-processing, feature extraction and training of artificial intelligence (AI)/machine learning models. Firstly, an accelerometer sensor inside a smartphone is utilized to record accelerometer values for seizure-like activity. As real epileptic seizure data collection is clinically constrained and ethically challenging, seizure-mimicking activities were performed under controlled conditions with the supervision of a physician. These activities, along with regular sleep postures, were recorded using the smartphone’s embedded accelerometer and gyroscope sensor. Additionally, the 3-axial linear acceleration and gyroscope signals were captured at a constant rate of 50 Hz. The acquired signals primarily represent time-series data, which are characterized by fluctuations in body movement patterns associated with both normal sleep postures and seizure-mimicking activities. Once the seizure-like dataset was created, the acquired signals were pre-processed using noise filters and then sampled with fixed-width sliding windows of 2.56 s. An overlap of 50% was applied, providing 128 readings/window. Raw signals are highly susceptible to noise, drift and missing values, which makes the preprocessing crucial before feeding the signals into the feature extraction process. Therefore, a Butterworth low-pass filter was employed to separate gravitational components and body acceleration. Finally, the filtered signals were fed into the feature extraction process. The features such as variance, skewness, maximum fractal length, spectral entropy, mean frequency and median frequency were extracted from both recorded normal and epileptic signals. Further, the various AI models such as decision trees, SVM and RF were trained using the acquired normal and epileptic signals.
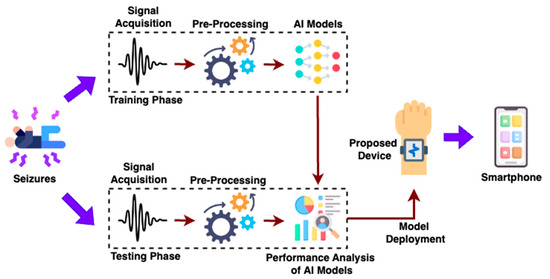
Figure 2.
Proposed Methodology for Development of the Wearable Device.
Once the machine learning models were trained, they were tested. In the testing phase, the adopted machine learning models were compared in terms of performance metrics such as accuracy, precision, recall and false alarm rate (FAR), and the efficacy of each model was analysed. By analysing the performance parameters of the various different machine learning models, the best model was selected and deployed on the proposed wearable hardware device. The proposed device can be affixed with the help of a strap to epileptic patients and is capable of identifying epileptic seizures during sleep. Also, patient caretakers can be notified if the patients/individuals undergo epileptic seizures since the proposed device is equipped with WiFi network connectivity.
4. Results and Discussion
In this work, a smartphone, namely an Apple iPhone 11, was utilized to acquire accelerometer signals from normal and seizure-mimicking activities. Furthermore, the mobile application recorded the accelerometer signals and stored them in a comma-separated value (.csv) file. A total of 300 accelerometer signals were collected, comprising 150 normal and 150 seizure-mimicking activities taken with the help of the inbuilt accelerometer of the smartphone. Out of these, 240 (80%) of the signals were taken for training machine learning models such as decision trees, random forest and support vector machine. Additionally, the remaining 60 signals (20%), namely 30 normal and 30 seizure-mimicking samples, were utilized for testing, ensuring an unbiased evaluation of model performance. All the acquired signals were pre-processed, and the extraction of features was performed to train and test the machine learning models. Evaluation metrics such as accuracy, precision, recall and FPR were derived using Equations (1)–(4), respectively:
where TP, TN, FP and FN denote True Positives, True Negatives, False Positives and False Negatives, respectively. The accuracy measures the proportion of correctly classified postures out of all postures; precision is the proportion of true positive predictions among all predicted positives; recall measures the proportion of true positives correctly identified out of all actual positives; and finally, FPR is the proportion of actual negative classes incorrectly classified as positive. Table 1 presents the performance metrics of various machine learning models such as decision trees, random forest and support vector machine.

Table 1.
Performance metrices of various machine learning models.
In this work, k-fold cross-validation with k = 5 was set to evaluate the performance of all models such as decision trees, random forest and support vector machine. Furthermore, this method was chosen to ensure robust and unbiased performance metrics by testing the model on multiple subsets of the data. Also, each fold utilized a different part of the data for validation, while training the model on the remaining data provided a more generalized performance estimate. The comparative results in terms of performance metrics of various machine learning models such as decision trees, random forest and support vector machine are illustrated in Figure 3. Further, the performance metrics such as accuracy, precision, recall and FPR were derived for all three different machine learning models.
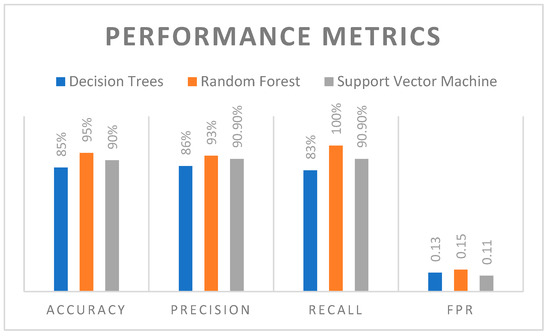
Figure 3.
Comparison of Various Machine Learning Models.
Through comparison, it was observed that the RF machine learning algorithm outperformed the other models with an accuracy of 95%, which indicates its superior generalization capability. However, the SVM followed closely with an accuracy of 90%, and the decision trees trailed with an accuracy of 85% due to their tendency to overfit and higher variance. Furthermore, in terms of precision, RF achieved the highest score of 93%, showing its robustness in classifying epileptic seizures with minimal false positive rates. Also, the SVM achieved precision of 90.9%, while decision trees achieved 86%.
From the recall analysis, the RF had a recall of 100%, which highlights its ability to detect actual seizure events without any FPR. However, the RF model was tuned using a grid search to optimize hyperparameters such as the number of trees and the maximum depth of each tree in order to avoid overfitting. Cross-validation was employed to reduce the overfitting issues since the model was validated on different folds of the data. Moreover, it was ensured that the model was not simply memorizing the training set but improved with new data. Additionally, the SVM achieved recall of 90.9%, and the recall of decision trees was comparatively lower at 83%, which indicates possible missed classifications. The FPR was lowest in SVM, at around 0.11, followed by decision trees (0.13), and the highest in RF (0.15). However, RF was most sensitive with high recall; its slightly higher FPR demonstrated the trade-off between specificity and sensitivity.
The results demonstrate that RF provided the most balanced and accurate classification for the detection of epileptic seizures from various sleep postures due to its ensemble nature and capability to reduce overfitting. Also, the performance of SVM was consistently good, with higher reliability and minimal FPR. Moreover, the decision trees were simple and interpretable but comparatively weaker, especially in recall and accuracy. The RF machine learning algorithm has been deployed in the proposed IoMT-based wearable device.
5. Conclusions
In this work, a cost-effective and intelligent IoMT-based wearable device was designed and developed for the monitoring of neurological disorders, especially seizures of epilepsy patients. Addressing the lack of real-world accelerometer data for seizures, a representative dataset was generated to train and evaluate the proposed system. Further, three different benchmark machine learning algorithms, namely decision trees, random forest and support vector machine, were utilized, and the performance of all three algorithms was compared with the help of performance metrics. The integration of the IMU with the ESP32 microcontroller enabled on-chip processing using a lightweight machine learning algorithm which eliminated the need for external servers. Results demonstrate that the performance of random forest was better, demonstrating its superiority among the other utilized benchmark models. The Python programming language, specifically MicroPython v1.22.1 programming, was used to code the RF algorithm in the developed IoMT device. The results affirm the feasibility and practicality of the proposed framework in continuous seizure monitoring for epilepsy patients. In the near future, it is planned to develop a mobile application to display the status of monitored patients using open-source software. A continuous beep sound will be generated in the mobile application to alert caretakers to patient seizure events.
Author Contributions
A.R. and N.V. conceptualized the idea for this manuscript. A.R. provided the resources. A.R. designed and developed the hardware for data acquisition. A.R. and N.V. carried out the investigation and curation of the acquired data. A.R. and N.V. validated the data and results acquired. A.R. prepared the original draft. A.R. and N.V. reviewed and edited the original draft. N.V. supervised and administered the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Giourou, E.; Stavropoulou-Deli, A.; Giannakopoulou, A.; Kostopoulos, G.K.; Koutroumanidis, M. Introduction to epilepsy and related brain disorders. In Cyberphysical Systems for Epilepsy and Related Brain Disorders: Multi-Parametric Monitoring and Analysis for Diagnosis and Optimal Disease Management; Springer International Publishing: Cham, Switzerland, 2015; pp. 11–38. [Google Scholar]
- Sarmast, S.T.; Abdullahi, A.M.; Jahan, N. Current classification of seizures and epilepsies: Scope, limitations and recommendations for future action. Cureus 2020, 12, e10549. [Google Scholar] [CrossRef] [PubMed]
- Roliz, A.H.; Kothare, S. The interaction between sleep and epilepsy. Curr. Neurol. Neurosci. Rep. 2022, 22, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Nobili, L.; de Weerd, A.; Rubboli, G.; Beniczky, S.; Derry, C.; Eriksson, S.; Halasz, P.; Högl, B.; Santamaria, J.; Khatami, R.; et al. Standard procedures for the diagnostic pathway of sleep-related epilepsies and comorbid sleep disorders: An EAN, ESRS and ILAE-Europe consensus review. Eur. J. Neurol. 2021, 28, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Anwar, H.; Khan, Q.U.; Nadeem, N.; Pervaiz, I.; Ali, M.; Cheema, F.F. Epileptic seizures. Discoveries 2020, 8, e110. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Carmant, L. Seizures and epilepsy: An overview for neuroscientists. Cold Spring Harb. Perspect. Med. 2015, 5, a022426. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ou, Q.; Dong, C.; Zhou, J.; Hu, H.; Li, C.; Huang, Z. Conducting polymer hydrogels based on supramolecular strategies for wearable sensors. Exploration 2024, 4, 20220167. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.H.; Zhang, Z.; An, K.; He, T.; Sun, Z.; Pu, X.; Lee, C. A wearable multidimensional motion sensor for AI-enhanced VR sports. Research 2023, 6, 0154. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xiao, D.; Liu, P.; Liao, Q.; Ruan, Q.; Huang, C.; Liu, L.; Li, D.; Zhang, X.; Chu, P.K. Nanostructured conductive polypyrrole for antibacterial components in flexible wearable devices. Research 2023, 6, 0074. [Google Scholar] [CrossRef] [PubMed]
- Amengual-Gual, M.; Fernández, I.S.; Loddenkemper, T. Patterns of epileptic seizure occurrence. Brain Res. 2019, 1703, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kusmakar, S.; Karmakar, C.K.; Yan, B.; O’Brien, T.J.; Muthuganapathy, R.; Palaniswami, M. Automated detection of convulsive seizures using a wearable accelerometer device. IEEE Trans. Biomed. Eng. 2018, 66, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; McCallan, N.; Ng, K.Y.; Biglarbeigi, P.; Finlay, D.; Lan, B.L.; McLaughlin, J. Epileptic seizure classification using combined labels and a genetic algorithm. In Proceedings of the 2022 IEEE 21st Mediterranean Electrotechnical Conference (MELECON), Palermo, Italy, 14–16 June 2022; pp. 430–435. [Google Scholar] [CrossRef]
- Roy, S.; Asif, U.; Tang, J.; Harrer, S. Seizure type classification using EEG signals and machine learning: Setting a benchmark. In Proceedings of the 2020 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 5 December 2020; pp. 1–6. [Google Scholar]
- Liu, Y.; Sivathamboo, S.; Goodin, P.; Bonnington, P.; Kwan, P.; Kuhlmann, L.; O’Brein, T.; Perucca, P.; Ge, Z. Epileptic seizure detection using convolutional neural network: A multi-biosignal study. In Proceedings of the Australasian Computer Science Week Multiconference, Victoria, Australia, 3–7 February 2020; pp. 1–8. [Google Scholar]
- Zabler, N.; Swinnen, L.; Biondi, A.; Novitskaya, Y.; Schütz, E.; Epitashvili, N.; Dümpelmann, M.; Richardson, M.P.; Van Paesschen, W.; Hirsch, M.; et al. High precision in epileptic seizure self-reporting with an app diary. Sci. Rep. 2024, 14, 15823. [Google Scholar] [CrossRef] [PubMed]
- Komal, K.; Cleary, F.; Wells, J.S.G.; Bennett, L. A systematic review of the literature reporting on remote monitoring epileptic seizure detection devices. Epilepsy Res. 2024, 201, 107334. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Agarwal, R.; Swamy, M.N.S. A novel dual-stage classifier for automatic detection of epileptic seizures. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–24 August 2008; pp. 911–914. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).