Abstract
Due to their unique properties, polythiophene and other conductive polymers have become the subject of intensive research and are promising substrate materials for innovative and trendsetting applications. To this day, boron trifluoride diethyl etherate (BFEE) is the preferred solvent for the electropolymerization of thiophene, although it does not allow for reproducible film qualities due to its decomposition under ambient conditions. We therefore want to equip the reader with a starter kit for the electropolymerization of high-quality polythiophene films from stable solvents and a simple yet efficient method to remove the deposited films from the electrodes for their reuse. By drying the working solution prior to its utilization, and by adding a Lewis acid catalyst, films that display enhanced electron transfer and a smooth surface topography can be obtained, which can both be beneficial for the analytic performance of a subsequently built biosensor.
1. Introduction
Conductive polymers constitute an innovative and valuable substrate material for various electrochemical sensors. They can be electropolymerized and deposited on electrodes to form thin films that grant a number of advantages: They enhance electron transfer between the working electrode and the redox mediator [1], while they can reduce electrode poisoning [2] and fouling, which would significantly affect the analytical characteristics of electrochemical sensors [3]. Furthermore, by using monomers with a variety of functional groups, they can be co-deposited with another compound of interest, or easily be modified subsequent to electropolymerization.
Polythiophene and its derivatives have gained increasing attention due to their structural versatility as well as environmental stability, which exceeds that of polypyrrole and polyaniline [4]. Especially on gold, polythiophene demonstrates highly stable absorption, which is due to its numerous covalent-like binding sites of sulfur onto gold. The removal of polythiophene and other conductive polymers therefore presents a challenging task and is oftentimes highlighted as a significant drawback in their utilization [5].
In this communication, we report on our recent discovery of a Lewis acid catalysis that allows for milder electropolymerization conditions in stable solvents. This way, high-quality polythiophene films can be obtained in a reproducible manner. Furthermore, we found that drying of the working solution is necessary and present a simple method for this purpose. The starter kit is rounded off by our protocol [6] for the fast and gentle removal of polythiophene and other conductive polymer films from gold electrodes.
2. Materials and Methods
2.1. Electrodes and Equipment
Screen-printed thick-film electrode chips (SPE) with an integrated gold working electrode, silver reference electrode and platinum counter electrode (model DRP-250AT) were obtained from Metrohm DropSens (Llanera, Spain). For electropolymerization, the integrated silver electrode was used. For characterization, an external Ag/AgCl reference electrode (Sensortechnik Meinsberg, Waldheim, Germany) was used.
Electrochemical procedures were performed using the SP-300 potentiostat/galvanostat with an impedance analyzer (Bio-Logic Science Instruments SAS, Claix, France). Impedance measurement was carried out with a sinusoidal 7.07 mV rms excitation voltage around the DC potential of 0 V in the frequency range of 100 kHz to 10 Hz. The measurement buffer contained potassium ferricyanide and potassium ferrocyanide, 2 mM each. Impedance spectra were fitted with the Randles–Ershler equivalent circuit [7,8] with a constant phase element instead of a capacitor.
Films were characterized by scanning electron microscopy (SEM, ZEISS EVO LS10, Carl Zeiss AG, Jena, Germany), energy-dispersive X-ray spectroscopy (EDX, Bruker, Microanalysis GmbH, Berlin, Germany), consisting of the detector XFlash® 5030 T 127 eV and the signal processing unit XFlash® SVE III, and white light interferometry (WLI, ContourGT-X 3D Optical Profiler, Bruker Nano GmbH, Berlin, Germany) with SPIPTM 6.6.5 image processing software (Image Metrology A/S, Hørsholm, Denmark).
2.2. Electrode Cleaning
The optimized cleaning procedure for polythiophene removal was as follows: polythiophene-coated electrodes were incubated in 2 M sodium perchlorate in acetonitrile, and a constant potential of 2.4 V was applied for one minute. The films were removed by rinsing or wiping. The clean working electrodes were then characterized by electrochemical impedance spectroscopy. Before the next electropolymerization, the silver reference electrodes were regenerated by treatment with a Q-tip that was immersed in 30 mg/mL aqueous thiourea until the shiny silver surface was restored.
2.3. Electropolymerization
For investigation of film properties, 100 mM thiophene was electropolymerized in acetonitrile with 500 mM KPF6 as a supporting electrolyte/counter ion in the current range of 1–5 mA (=8 to 40 mA/cm2) for 1 min. The concentration of the catalyst ZnF2—if added—was 6.4 mM (saturated solution).
3. Results and Discussion
3.1. Choice of Solvent and Drying of Working Solution for Efficient and Reproducible Film Synthesis
For the electropolymerization of thiophene, a variety of solvents can be utilized that include acetonitrile, benzonitrile, nitrobenzene, propylene carbonate, dimethyl sulphate, diethyl sulphate, and dichloromethane [9,10,11,12]. In order for the medium to conduct an electric current, a supporting electrolyte has to be added. Typically, perchlorates, hexafluorophosphates, and tetrafluoroborates are utilized. They additionally act as counter anions that diffuse into the forming films to compensate for the positive charges in the polymer backbone.
The above-listed salts are quite hygroscopic, which we found to be problematic, since even minor amounts of water in the working solution negatively affect the electropolymerization of thiophene. We investigated thiophene polymerization in acetonitrile with KPF6 as a supporting electrolyte and found that films decreased in thickness and conductivity when water was added to the dried working solution. The charge transfer resistance of the films increased with increasing water content to values >1000 Ω for the addition of 500 mM water, as can be seen in Figure 1A. Furthermore, as the EDX of the obtained films showed, they not only decreased in thickness—demonstrated by the decreasing amounts of carbon and sulfur and the increasing amount of gold—but also showed an increasing oxygen content (see Figure 1B). This points at the incorporation of carbonyl groups [10], which would interrupt the movement of charge carriers, which accounts for the polymers’ conductivity. We found that drying the solution of the counter ion/solvent over a molecular sieve for at least a week constitutes a simple yet effective method to remove water and allow for successful electropolymerization.
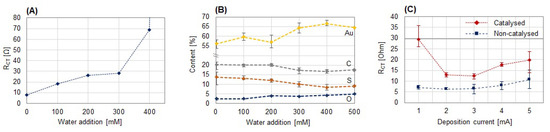
Figure 1.
(A) Charge transfer resistance RCT from EIS of polythiophene films synthesized with different concentrations of water added to the dried working solution. (B) Elemental composition of the same films. (C) Charge transfer resistance RCT of films deposited at different currents from dried working solutions. Here, 100 mM thiophene was polymerized in acetonitrile for 1 min with 500 mM KPF6 as the supporting electrolyte/counter ion. Experiments were performed in triplicate.
The above-listed solvents eligible for thiophene electropolymerization have multiple properties in common: aprotic character, high dielectric constant, low nucleophilicity, and, unfortunately, a high initial oxidation potential of ≥1.6 V. At this potential, polythiophene, however, already over-oxidizes, which reduces the film conductivity. This issue, suitably termed the “polythiophene paradox”, therefore describes the fact that polythiophene is damaged at potentials where it is formed [13,14]. The solvent boron trifluoride diethyl etherate (BFEE) seems to provide a remedy for this issue, since it grants milder polymerization conditions so that thiophene already oxidizes at 1.0 V [15]. However, film qualities cannot easily be reproduced when polymerized in BFEE, due to the multiple decomposition reactions of the solvent under ambient conditions.
3.2. Electropolymerization of High-Quality Polythiophene Films by Lewis Acid Catalysts
We recently discovered that the mild polymerization conditions in BFEE are granted by the catalytic effect of boron trifluoride—a strong Lewis acid—that facilitates monomer oxidation [16]. We were able to identify fluorine-based Lewis acids as a suitable class of catalysts that allow the synthesis of high-quality polythiophene films from stable solvents in a reproducible manner. Fluorine-based acids combine high acidic strength with electrochemical stability at the potentials that are necessary for thiophene oxidation.
By exemplarily utilizing zinc fluoride in acetonitrile, we were able to synthesize polythiophene films under milder conditions with improved properties. Films were electropolymerized at currents ranging from 1 to 5 mA, which equal current densities of 8–40 mA/cm2 for electrodes of 4 mm diameter. When the catalyst ZnF2 was utilized, the potential measured during electropolymerization was lower, emphasizing the milder polymerization conditions. The films were deposited in triplicate and subsequently characterized by electrochemical impedance spectroscopy. The obtained RCT are pictured in Figure 1C. In a broad range of deposition currents, films of reproducible and low charge transfer resistance could be obtained. Such films enhance the electron transfer, since their RCT is lower than the RCT of a typical uncoated electrode (pictured by a grey line), which is beneficial for the analytic performance of the biosensor.
From catalyzed electropolymerization, resulting RCT values were marginally higher compared to films from non-catalyzed polymerization. RCT characterizes the conductivity perpendicular to the film, which is known to be 10.000-times lower than along the polymer backbone in the film plane [17]. Due to the milder polymerization conditions, the polymers deposit in a more ordered manner on the electrode, which plausibly increases the RCT. This observation points at the improved conductivity in the film plane, which is desirable for applications other than electrode coatings.
In addition to enhanced electron transfer, a smooth surface is advantageous for the construction of electrochemical biosensors. We observed that this could be achieved by the utilization of ZnF2. The calculated surface area ratio from white light interferometry is pictured in Figure 2D. With the help of the catalyst, films resulting from synthesis at 1–3 mA were only marginally rougher (11.6–18.9%) than the uncoated electrode (5.2%), while films from non-catalyzed synthesis were significantly rougher (65.0–96.7%). A visualization of the surface topography of films deposited with 3 mA is presented in Figure 2A. The 3D white light images are accompanied by corresponding SEM images (see Figure 2B,C). Without the use of the catalyst, films were produced whose surface was of hyphae or root-like structure, while films from catalyzed polymerization showed a globular surface structure. Such smooth film surfaces minimize the steric hindrance of subsequently immobilized bioreceptors and therefore should grant improved target binding.
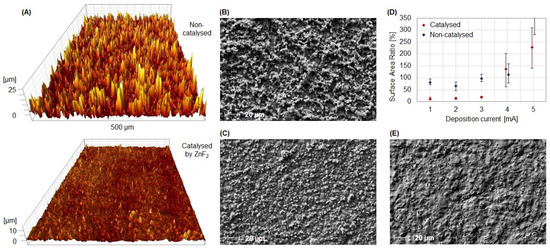
Figure 2.
Surface topography of electropolymerized polythiophene films. (A) Three-dimensional white light images of films deposited with 3 mA with and without the catalyst ZnF2. (B,C) Complementary SEM images of the same films. (D) Surface area ratio of films deposited with currents ranging from 1 to 5 mA. (E) SEM image of a clean, uncoated electrode.
3.3. Removal of Strongly Adhering Conductive Polymer Films from Gold Electrodes
Our developed protocol for polymer film removal alters the internal tension profile of the compound material CP/gold to flake the films off the electrode [6]. By the application of a high potential, the films are significantly oxidized, which introduces new positive charges into the polymer backbone. Oxidation can be investigated by cyclic voltammetry (see Figure 3A): if polythiophene films are scanned to an increasingly high potential, two oxidation peaks can be measured. The first corresponds to reversible oxidation (doping), while the second is caused by irreversible over-oxidation.
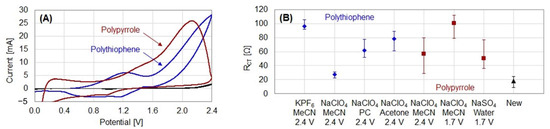
Figure 3.
(A) Oxidation of polythiophene and polypyrrole during cyclic voltammetry in 2 M NaClO4 in acetonitrile with a scan rate of 500 mV/s. In black, CV of cleaning solution. (B) Removal of polymer films with different counter ions (500 mM) and solvents (MeCN: acetonitrile, PC: propylene carbonate). Three films were removed with each cleaning solution for 1 min. The distribution of RCT of five new electrodes is included. Reprinted from [6], Copyright (2021), with permission from Elsevier.
Driven by the newly formed positive charges in the polymer, counter ions from the cleaning solution diffuse into the film. This is reported to lead to film swelling [18,19]. The film expansion introduces a shear stress to the interphase CP/gold, which breaks the bonds that account for the highly stable absorption of the polymer films. As a result, the films can easily be washed or wiped off the electrodes.
For the removal of polythiophene, we found the optimum potential to be 2.4 V. By the application of lower potentials, the films did not come off as easily, while higher potentials damaged the utilized electrodes. Applying the potential for 1 min is sufficient. Since the procedure is based on a physical process, various solvents and counter ions can be used. They need to be electrochemically stable at the applied potential. The utilization of solvents in which the monomers are soluble should be beneficial for the swelling process due to superior film wettability. We successfully removed polythiophene films with various solvents and counter ions and characterized the electrodes by electrochemical impedance spectroscopy (see Figure 3B). Sodium perchlorate in acetonitrile was found to be the best cleaning solution, as the cleaned electrodes showed the lowest RCT. By increasing the NaClO4 concentration from 500 mM to 2 M, we could further decrease the RCT. In total, 26 films were removed with each solution: with 500 mM NaClO4, the resulting RCT was 13.8–91.8 Ω, while it could be decreased to 15.4–47.8 Ω by utilizing 2 M NaClO4.
The presented protocol can also be employed for the removal of other conductive polymers, which we exemplarily investigated with polypyrrole. Here, a potential of 1.7 V can be applied to still allow for easy and efficient film removal. Pyrrole oxidizes at significantly lower potentials than thiophene (≈1.2 V [20]), which supposedly is the reason for the lower potential necessary for cleaning. At potentials not greater than 1.7 V, water can be used as a solvent. By employing aqueous 500 mM NaSO4, an even lower RCT resulted compared to the employment of 500 mM NaClO4 in acetonitrile.
The presented protocol therefore offers the great advantage of using inexpensive and harmless aqueous solutions of sulfates, and presumably nitrates and phosphates, to remove polypyrrole from electrodes. Similarly, other conductive polymers that can be electropolymerized at low potentials, such as aniline (≈0.9 V [21]), azulene (≈1.0 V [20]), or carbazole (≈1.2 V [22]), should also be easily removed. Following the presented procedure, the utilized electrode chips could easily be reused up to fifteen times.
4. Conclusions
With this communication, we wish to equip the reader with all the information we perceive necessary for the electropolymerization of high-quality polythiophene films. We found that the working solutions have to be water-free to allow for efficient electropolymerization, which can effortlessly be achieved by storage over a molecular sieve. We furthermore communicate our recently discovered Lewis acid catalysis, which allows for mild polymerization conditions and reproducible film synthesis from stable solvents. Fluorine-based catalysts, such as AlF3, TiF4, ZnF2, ZrF4, InF3, LaF3, CeF3, NdF3, and GdF3, represent ideal candidates, since they possess the required acidic strength and are electrochemically stable. The obtained films enhance the electron transfer and are of smooth surface topography, which are both beneficial for the analytic performance of the biosensor.
For electrode regeneration, we developed a fast but gentle method with which the films can be easily removed from the electrodes. The protocol is versatile, can be applied to a series of solvents, counter ions, and other conductive polymers, and furthermore allows the electrodes to be reused up to fifteen times.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ecsa-8-11292/s1. Poster from the conference.
Author Contributions
Conceptualization, investigation, validation, visualization, writing—original draft preparation, writing—review and editing, F.V.O.; writing—review and editing, D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Free State of Thuringia (Thuringian Ministry for Economic Affairs, Science and Digital Society) and the Federal Ministry of Economic Affairs and Energy within the framework of the AiF-ZIM-program (grant No. KK5019401JO0).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Nicole Hauptmann and Jörg Schemberg from our institute for their kind feedback.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wong, A.; Santos, A.M.; Fatibello-Filho, O. Simultaneous determination of paracetamol and levofloxacin using a glassy carbon electrode modified with carbon black, silver nanoparticles and PEDOT:PSS film. Sens. Actuators B Chem. 2018, 255, 2264–2273. [Google Scholar] [CrossRef]
- Zanardi, C.; Terzi, F.; Seeber, R. Polythiophenes and polythiophene-based composites in amperometric sensing. Anal. Bioanal. Chem. 2013, 405, 509–531. [Google Scholar] [CrossRef]
- Oberhaus, F.V.; Frense, D.; Beckmann, D. Immobilization Techniques for Aptamers on Gold Electrodes for the Electrochemical Detection of Proteins: A Review. Biosensors 2020, 10, 45. [Google Scholar] [CrossRef]
- Moon, J.M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef]
- Bobade, R.S. Polythiophene composites: A review of selected applications. J. Polym. Eng. 2011, 31, 209–215. [Google Scholar] [CrossRef]
- Oberhaus, F.V.; Frense, D. Fast, simple, and gentle method for removal of polythiophene and other conductive polymer films from gold electrodes. J. Electroanal. Chem. 2021, 895, 115466. [Google Scholar] [CrossRef]
- Randles, J.E.B. Kinetics of rapid electrode reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Ershler, B. Investigation of electrode reactions by the method of charging-curves and with the aid of alternating currents. Discuss. Faraday Soc. 1947, 1, 269–277. [Google Scholar] [CrossRef]
- Aeiyach, S.; Bazzaoui, E.A.; Lacaze, P.-C. Electropolymerization of thiophene on oxidizable metals in organic media. J. Electroanal. Chem. 1997, 434, 153–162. [Google Scholar] [CrossRef]
- Roncali, J. Conjugated poly(thiophenes): Synthesis, functionalization, and applications. Chem. Rev. 1992, 92, 711–738. [Google Scholar] [CrossRef]
- Sato, M.-a.; Tanaka, S.; Kaeriyama, K. Electrochemical preparation of conducting poly(3-methylthiophene): Comparison with polythiophene and poly(3-ethylthiophene). Synth. Met. 1986, 14, 279–288. [Google Scholar] [CrossRef]
- Hotta, S.; Hosaka, T.; Shimotsuma, W. Electrochemically prepared polythienylene films. Synth. Met. 1983, 6, 69–71. [Google Scholar] [CrossRef]
- Gratzl, M.; Hsu, D.F.; Riley, A.M.; Janata, J. Electrochemically deposited polythiophene. 1. Ohmic drop compensation and the polythiophene paradox. J. Phys. Chem. 1990, 94, 5973–5981. [Google Scholar] [CrossRef]
- Krische, B.; Zagorska, M. The polythiophene paradox. Synth. Met. 1989, 28, 263–268. [Google Scholar] [CrossRef]
- Shi, G.; Jin, S.; Xue, G.; Li, C. A conducting polymer film stronger than aluminum. Science 1995, 267, 994–996. [Google Scholar] [CrossRef] [PubMed]
- Oberhaus, F.V.; Frense, D. Catalysing electropolymerization: High-quality polythiophene films for electrochemical sensors by the utilization of fluorine based Lewis acid catalysts. Electrochim. Acta 2022, 402. [Google Scholar] [CrossRef]
- Jin, S.; Xue, G. Interaction between Thiophene and Solvated Lewis Acids and the Low-Potential Electrochemical Deposition of a Highly Anisotropic Conducting Polythiophene Film. Macromolecules 1997, 30, 5753–5757. [Google Scholar] [CrossRef]
- Lizarraga, L.; Andrade, E.M.; Molina, F.V. Swelling and volume changes of polyaniline upon redox switching. J. Electroanal. Chem. 2004, 561, 127–135. [Google Scholar] [CrossRef]
- Pei, Q.; Inganaes, O. Electrochemical applications of the bending beam method. 1. Mass transport and volume changes in polypyrrole during redox. J. Phys. Chem. 1992, 96, 10507–10514. [Google Scholar] [CrossRef]
- Waltman, R.J.; Bargon, J. Electrically conducting polymers: A review of the electropolymerization reaction, of the effects of chemical structure on polymer film properties, and of applications towards technology. Can. J. Chem. 1986, 64, 76–95. [Google Scholar] [CrossRef] [Green Version]
- Gvozdenović, M.M.; Jugović, B.; Stevanović, J.S.; Grgur, B. Electrochemical synthesis of electroconducting polymers. Hem. Ind. 2014, 68, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Karon, K.; Lapkowski, M. Carbazole electrochemistry: A short review. J. Solid State Electrochem. 2015, 19, 2601–2610. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).