Abstract
The condensation products of cyanothioacetamide with aldehydes—(E)-arylmethylenecyanothioacetamides—have proven to be readily available and multifunctional starting reagents in the chemistry of S,N-containing compounds. We decided to study the interaction of formaldehyde with thioamides as a possible way to obtain N-(hydroxymethylene)thioamides, which are promising thioamidoalkylating agents and new ligands for complexation. It was found that the reaction of thioamides and formaldehyde proceeds easily when the reagents are heated in the absence of catalysts in an aqueous-alcohol medium, and leads, with good yields, to the expected N-(hydroxymethylene)thioamides. The structure of N-(hydroxymethylene)thioamides 2 was confirmed by IR and NMR spectroscopy data.
1. Introduction
We decided to study the reaction of formaldehyde with thioamides as a possible way to obtain N-(methylol)thioacrylamides—promising thioamidoalkylating agents and new ligands for complexation and possible intermediates in the synthesis of condensed heterocycles of the 1,3,5-thiadiazine series [1,2,3]. 2-Cyanothioacrylamides 1 (Scheme 1), derived from easily available cyanothioacetamide, have proven themselves to be convenient reagents for the construction of various S,N-heterocycles [4,5,6,7].
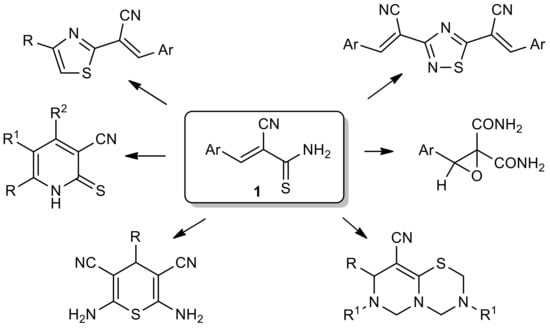
Scheme 1.
The diversity of compounds prepared from thioacrylamides 1.
2. Results and Discussion
We found that unsaturated thioamides 1 easily react with an excess of aq. 37% formaldehyde when slightly heated in EtOH in the absence of any catalysts, with the formation of N-hydroxymethylation products 2 (Scheme 2). N-(Hydroxymethyl)thioamides 2 have a slightly more intense coloration than starting compounds 1. We suggest that moderate yields (46–60%) are due to better solubility in comparison with the starting thioamides 1 in an aqueous alcohol medium, as well as due to the side reactions of hydrolysis (retro-Knoevenagel) of starting acrylthioamides 1. The addition of both basic (K2CO3) and acidic (aq. HCl) catalysts leads to resinification of the reaction mass and does not affect the process. The structure of N-(methylol)thioamides 2 is confirmed by IR and NMR spectroscopy data.
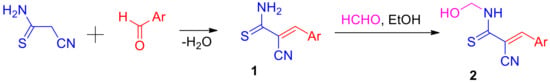
Scheme 2.
Synthesis and N-hydroxymethylation of thioacrylamides 1. Ar = substituted phenyl, 2-thienyl, 2-furyl.
Next, the condensation product of cyanothioacetamide with isatin—thioacrylamide 3—reacts with the excess of aqueous formalin on heating in EtOH to form product 4 (Scheme 3):
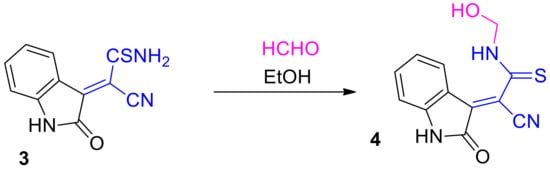
Scheme 3.
N-hydroxymethylation of thioacrylamides 3.
3. Experimental Section
Method for the Synthesis of N-(methylol)thioamides 2
Arylmethylidentianothioacetamide 1 (1.0 mmol) was placed in a beaker, then 96% ethanol (3–5 mL) and aq. 37% HCHO (5 mL) were added. To dissolve thioamide completely, the mixture was slightly warmed. The reaction mixture was stirred at room temperature for 1–2 h, during which the product precipitated. Over time, the mixture turned a brighter color compared to the starting solutions of thioacrylamides 1. The suspension was kept for 24 hours at 20–25 °C, and the solid product was filtered off. To remove impurities, the precipitate was recrystallized from acetone or alcohol. The yields ranged from 40 to 60%.
(E)-N-(Hydroxymethyl)-3-(4-chlorophenyl)-2-cyanoprop-2-enethioamide (2 (Ar = 4-ClC6H4). Yield 46%, orange powder. IR spectrum, ν, cm–1: 3390, 3325 (O–H, N–H), 2222 (C≡N). 1H NMR spectrum (200 MHz, DMSO-d6), δ, ppm: 5.00–5.06 m (2H, CH2), 6.32 t (1H, OH, 3J = 7.1 Hz), 7.64 d (2H, H3,5 Ar, 3J = 8.6 Hz), 7.90 s (1H, CH=), 7.92 d (2H, H2,6 Ar, 3J = 8.6 Hz), 10.90–10.92 m (1H, NH). 1H NMR spectrum (400 MHz, CDCl3), δ, ppm: 4.29 t (1H, OH, 3J = 8.4 Hz), 5.22–5.26 m (2H, CH2), 7.49 d (2H, H3,5 Ar, 3J = 8.6 Hz), 7.93 d (2H, H2,6 Ar, 3J = 8.6 Hz), 8.59–8.62 m (1H, NH), 8.72 s (1H, CH=). Mass spectrum, m/z (Irel, %): 235.3 [M − H2O + H]+, 253.1 [M + H]+, 331.1 [M + DMSO + H]+, 487.3 [2M − H2O +H]+, 522.3 [2M + NH4]+. Found, %: C 52.26; H 3.63; N 11.05. C11H9ClN2OS. Calculated, %: C 52.28; H 3.59; N 11.08. Mcalc 252.72.
(E)-N-(Hydroxymethyl)-3-(furan-2-yl)-2-cyanoprop-2-enethioamide (2, Ar = 2-furyl). Yield 54%, orange–brown powder. IR spectrum, ν, cm–1: 3420, 3210 (O–H, N–H), 2215 (C≡N). 1H NMR spectrum (200 MHz, DMSO-d6), δ, ppm: 5.00–5.04 m (2H, CH2), 6.20 t (1H, OH, 3J = 7.2 Hz), 6.83–6.84 m (1H, H4 furyl), 7.41 d (1H, H3 furyl, 3J = 3.7 Hz), 7.90 s (1H, CH=), 8.13–8.14 m (1H, H5 furyl), 10.63–10.64 m (1H, NH). Mass spectrum, m/z (Irel, %): 209.1 [M + H]+, 269.3 [M − H2O + DMSO + H]+, 287.1 [M + DMSO + H]+, 399.1 [2M − H2O + H]+, 434.4 [2M + NH4]+, 607.5 [3M − H2O + H]+. Found, %: C 51.87; H 3.93; N 13.46. C9H8N2O2S. Calculated, %: C 51.91; H 3.87; N 13.45. Mcalc 208.24.
Author Contributions
Conceptualization, V.V.D.; methodology, V.V.D.; investigation and analysis, A.G.L. and P.G.D.; writing—original draft preparation, A.G.L. and P.G.D.; writing—review and editing, V.V.D.; supervision, V.V.D.; funding acquisition, V.V.D. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out with the financial support of the Kuban Science Foundation, scientific project No. MFI-20.1-26/20.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, C.Y.; Chang, H.T.; Hu, C. Complexation reactions in a heterogeneous system. Inorg. Chim. Acta 1990, 172, 151–158. [Google Scholar]
- Liu, C.Y.; Hu, C.C.; Yeh, K.Y.; Chen, M.J. Synthesis of chelating resins and its application in ligand exchange chromatography. Fresenius J. Anal. Chem. 1991, 339, 877–881. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolusko, S.G.; Chigorina, E.A. Hydroxymethylation of 3-Aryl-2-cyanoprop-2-enethioamides. Russ. J. Gen. Chem. 2020, 90, 1411–1417. [Google Scholar] [CrossRef]
- Abdel-Galil, F.M.; Sherif, S.M.; Elnagdi, M.H. Utility of cyanoacetamide and cyanothioacetamide in heterocyclic synthesis. Heterocycles 1986, 24, 2023–2048. [Google Scholar]
- Liebscher, J.; Abegaz, B.; Knoll, A. The chemistry of 3-aminothioacrylamides; part ii: 3-Aminothioacrylamides as useful synthons in organic synthesis. Phosphorus Sulfur Relat. Elem. 1988, 35, 5–34. [Google Scholar] [CrossRef]
- Litvinov, V.P. Cyanoacetamides and their thio-and selenocarbonyl analogues as promising reagents for fine organic synthesis. Russ. Chem. Rev. 1999, 68, 737–763. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Dyachenko, I.V.; Nenajdenko, V.G. Cyanothioacetamide: A polyfunctional reagent with broad synthetic utility. Russ. Chem. Rev. 2018, 87, 1. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).