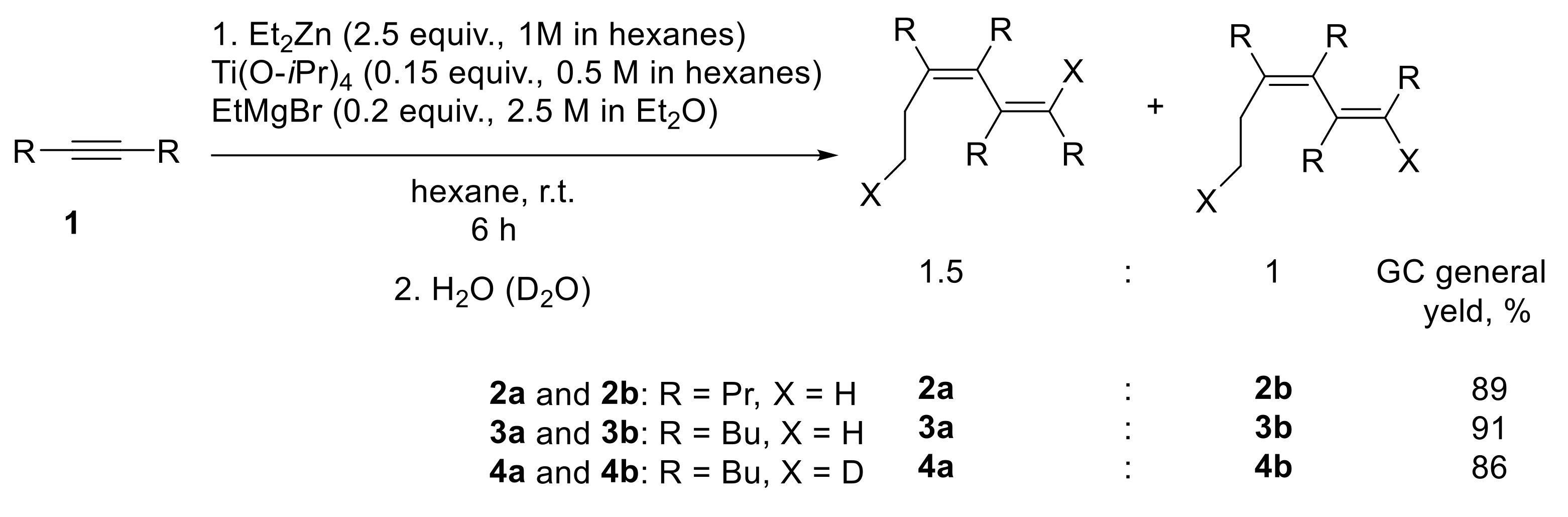
2. Results and Discussion
We found that the reaction of dialkyl-substituted alkynes 1 (5-decyne, 4-octyne, 3-hexyne) with 2.5 equivalents of Et
2Zn (1 M in hexanes) in the presence of 15 mol.% Ti(O-
iPr)
4, (0.3 M in hexanes) and 20 mol.% EtMgBr (2.5 M in Et
2O) in hexane solution at room temperature for 6 h, after deuterolysis or hydrolysis, gave stereoisomeric hexa-1,3-diene derivatives 2,3,4 in a ratio of ~1.5:1 (
Scheme 1). The structure of the resulting compounds was established using 1D- and 2D-NMR spectroscopy of the products of their deuterolysis 4 and hydrolysis 2,3. It should be emphasized that a hexane–diethyl ether (in an approximate volume ratio of ~30:1) solvent system was used, since the Grignard reagent used in the reaction was a 2.5 M solution in Et
2O. It was found that the reaction proceeded equally effectively in a solution of a mixture of methylene chloride with diethyl ether (hexane was replaced by methylene chloride). According to [
4], the reaction of 5-decyne with Et
2Zn in the diethyl ether—hexane solvent system in the presence of catalytic amounts of Ti(O-
iPr)
4 and EtMgBr followed the classical route of the carbozincation reaction and led to the formation of the product of 2-zincoethylzincation. However, the reaction of 5-decyne with a reaction system consisting of 2.5 equiv. of Et
2Zn, 10 mol.% Ti (O-
iPr)
4, and 20 mol.% EtMgBr in a solution of a mixture of diethyl ether—hexane (approximate volume ratio 1:1) at 23 °C proceeded slowly. The formation of (Z)-5-ethyl-5-decene with a yield of 64% was observed only after 4 days, provided that an additional portion of Ti(O-
iPr)
4 in the amount of 10 mol% was added to the reaction system after 24 h.
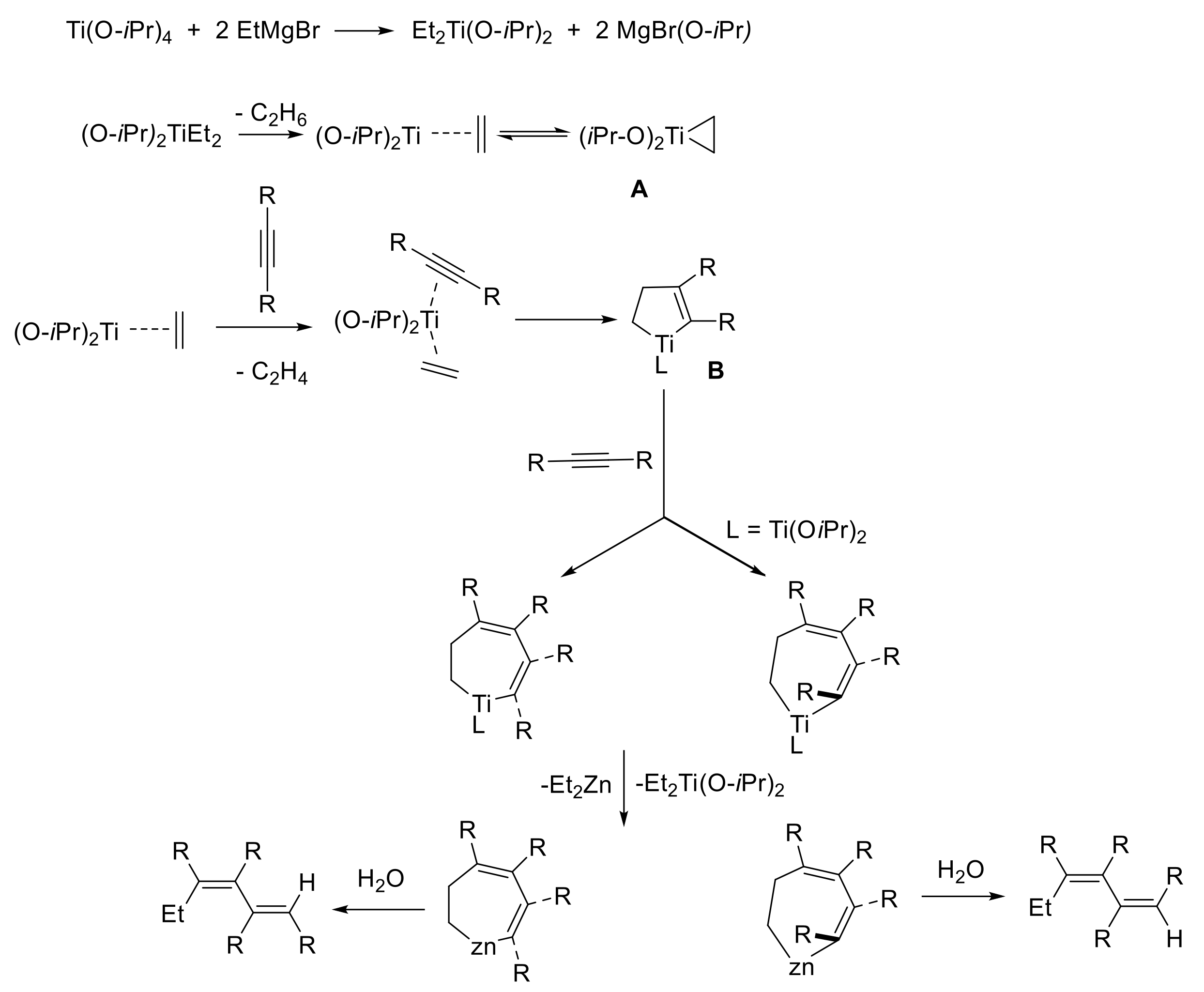
We believe that the formation of the tetra-alkylhexa-1,3-diene derivatives in the studied reaction occurred as follows. According to
Scheme 2, a rapid ligand exchange between titanium (IV) isopropoxide and ethylmagnesium bromide gave an unstable diethyltitanium compound, which was further converted into a titanacyclopropane intermediate (titanium (II)-ethylene complex). Kulinkovich was the first to suggest the generation of a titanacyclopropane intermediate upon the interaction of Grignard reagents with titanium (IV) alkoxides [
5]. According to
Scheme 2, the further insertion of the triple bond of alkyne at the Ti-C bond of the titanacyclopropane intermediate
A led to the formation of the titanium-cyclopentene intermediate
B. As a result of the deuterolysis of the carbometallation reaction of 5-decyne, we obtained a mixture of two dideuterated compounds: (5
E, 7
E)-6,7-dibutyl-5-(ethyl-2-
d)dodeca-5,7-diene-8-
d 4a and (5
E, 7
Z)-6,7-dibutyl-5-ethyldodeca-5,7-diene
4b. Based on the observed regiochemistry of the reaction, we assumed that the formation of the diene occurred as a result of the insertion of the triple bond of the second acetylene molecule at the Ti-C(sp
2) bond of the titanium cyclopentene intermediate
B. We believe that the metal–carbon bond of intermediate
B, where the titanium atom was bonded to the more nucleophilic sp
2-hybridized carbon atom, was more reactive towards the second alkyne molecule. The process of insertion of the second molecule of the acetylene substrate proceeded non-stereoselectively, which led to the formation of the second stereoisomer: (5
E, 7
Z)-6,7-dibutyl-5-ethyldodeca-5,7-diene. Further
trans-metalation of tetralkyltitanacyclohepta-2,4-diene and subsequent hydrolysis led to the formation of a mixture of stereoisomers: (5
E, 7
E)-6,7-dibutyl-5-ethyldodeca-5,7-diene and (5
E, 7
Z)-6,7-dibutyl-5-ethyldodeca-5,7-diene in a 1.5:1 ratio.
4. Experimental Part
The reagents were obtained from Sigma-Aldrich or Acros. Hexane and dichloromethane were distilled over P2O5. Diethyl ether was dried over sodium. Nuclear magnetic resonance spectroscopy was performed on a Brucker Avance 500. The 1H NMR spectra were recorded at 500 MHz and 13C-{1H} NMR spectra at 100 MHz in CDCl3. The chemical shifts are reported in ppm relative to tetramethylsilane (TMS) as the internal standard. Elemental analysis was performed using a Carlo-Erba CHN 1106 elemental analyzer. Mass spectra were obtained on a Finnigan 4021 instrument.
The typical procedure for (5E,7E)-6,7-dibutyl-5-ethyldodeca-5,7-diene (3a) and (5E,7Z)-6,7-dibutyl-5-ethyldodeca-5,7-diene (3b) was completed as follows. To a solution of 276 mg of dec-5-yne (2 mmol) and Et2Zn (1 M in hexanes, 5 mL, 5 mmol) in hexane (6 mL), Ti(O-iPr)4 (0.5 M in hexanes, 0.6 mL, 0.3 mmol) was added. Ethylmagnesiurn bromide (2.5 M in Et2O, 0.16 mL, 0.4 mmol) was then added and the reaction mixture rapidly turned black. After 18 h at 23 °C, the reaction mixture was diluted with Et2O (5 mL), and 25 wt% KOH solution (3 mL) was added dropwise while the reaction flask was cooled in an ice bath. The aqueous layer was extracted with diethyl ether (3 × 5 mL). The combined organic layers were washed with brine (10 mL) and dried over anhydrous CaCl2. The reaction mixture was filtered through a filter paper and concentrated in vacuo to give the crude product as a yellow oil. The residue was distilled through a micro column at 1 mmHg to afford 3a and 3b (559 mg, 91%) as a colorless oil. b.p. 159–161 °C. 1H NMR (500 MHz, CDCl3): δ = 0.86–0.99 (m, 15H), 1.28–1.43 (m, 8H), 1.99–2.08 (m, 18H), 4.99–5.03 (q, J = 14 Hz, J = 7.05 Hz, 18H). 13C NMR (500 MHz, CDCl3): δ = 13.58 (1C), 13.92, 13.89* (1C), 14.04 (1C), 14.54, 14.34* (1C), 14.65, 14,63* (1C), 21.38 (1C), 21.83, 21.77* (1C), 22.08 (1C), 22.68 (1C), 23.26, 23.24* (C(1)), 25.64 (1C), 29.85 (1C), 29.89 (1C), 31.89 (1C), 32.04 (C1), 32.13 (1C), 32.26, 32.22* (1C), 34.89 (1C), 127.44, 127.27* (1C), 136.05, 135.75* (1C), 137.71, 137,48* (1C), 140.06, 139.94 (1C). MS (EI): m/z, % = 204 (15) [M+], 161 (11), 147 (13), 117 (18), 105 (100). Anal. calcd for C22H42, (%): C, 86.19; H, 13.81; Found, %: C, 86.25; H, 13.77. (5E,7E)-6,7-dibutyl-5-(ethyl-2-d)dodeca-5,7-diene-8-d (4a) and (5E,7Z)-6,7-dibutyl-5-(ethyl-2-d)dodeca-5,7-diene-8-d (4b). Using the procedure described above, 276 mg of dec-5-yne (2 mmol) and D2O (instead of H2O) gave a crude product that was distilled through a micro column at 1,2 mmHg to afford 4a and 4b (531 mg, 86%) as a colorless oil. b.p. 160–162 °C (1,2 mmHg). 1H NMR (500MHz, CDCl3): δ = 0.89–0.94 (m, 6H), 0.97–1.02 (m, 2H), 1.31–1.36 (m, 8H), 1.99–2.04 (m, 6H). 13C NMR (500MHz, CDCl3): δ = 12.49–12.95 (1C), 14.06 (2C), 22.44 (1C), 22.87 (1C), 27.26, 27,37* (1C), 29.42, 29.49* (1C), 29.91 (1C), 30.79 (1C), 32.42 (1C), 122.89–123.44 (t, J = 22Hz, 1C), 140.96, 141.05* (1C) (GAM-155-2). Anal. calcd for C22H40D2, (%): C, 85.63; Found, %: C, 85.71.