Drug delivery systems are intensively studied for a wide range of biomedical applications [1,2,3]. A special class of materials is related to porous materials, which have the ability to host and release biological active agents (BAAs). The release of biological active agents can be tuned according to needs. Mesoporous silica has a history of about 30 years and can be used for the release of a wide range of BAAs. The release is dependent on the size of the pores and can be further tuned based on the surface functionalization [4].
Starting from the advantages of the mesoporous silica supports, innovative drug delivery systems can be developed in order to obtain controlled, targeted drug delivery systems that are able to maintain the therapeutic needs of the BAAs (Figure 1). In this work, several examples of drug delivery systems based on mesoporous silica and different polyphenols will be discussed, highlighting the potential of their use in the treatment of different diseases, and especially, in the treatment of dysbiosis.
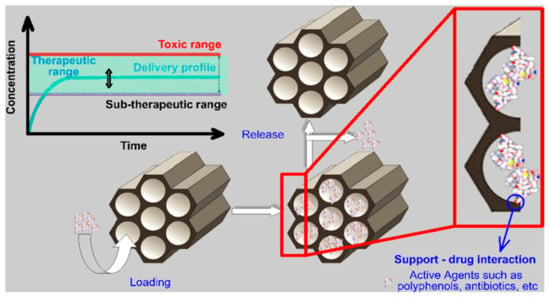
Figure 1.
Mesoporous silica-based drug delivery system for the treatment of dysbiosis.
Author Contributions
Conceptualization, D.F., I.F., and A.F.; Methodology, D.F., L.M. and G.P.; Writing—Original Draft Preparation, L.M. and G.P.; Writing—Review & Editing, R.C.F., A.F. and C.B.; Supervision, R.C.F., A.F. and C.B.; Project Administration, D.F., I.F. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Romanian National Authority for Scientific Research and Innovation, CNCS/CCCDI—UEFISCDI, project No. PN-III-P2-2.1-PED-2019-4018, contract 524PED/2020, within PNCDI III. Additionally, UPB is part of the COST action CA_20126: Network for research, innovation and product development on porous semiconductors and oxides 220/2020, and project number PN-III-P2-2.1-PED-2019-3166, contract 299PED/2020, within PNCDI III.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sonmez, M.; Ficai, D.; Ficai, A.; Alexandrescu, L.; Georgescu, M.; Trusca, R.; Gurau, G.; Titu, M.A.; Andronescu, E. Applications of mesoporous silica in biosensing and controlled release of insulin. Int. J. Pharmaceut. 2018, 549, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Popescu, S.; Ardelean, I.L.; Gudovan, D.; Radulescu, M.; Ficai, D.; Ficai, A.; Vasile, B.S.; Andronescu, E. Multifunctional materials such as MCM-41/Fe3O4/folic acid as drug delivery system. Rom. J. Morphol. Embryol. 2016, 57, 483–489. [Google Scholar] [PubMed]
- Gunduz, O.; Yetmez, M.; Sonmez, M.; Georgescu, M.; Alexandrescu, L.; Ficai, A.; Ficai, D.; Andronescu, E. Mesoporous Materials Used in Medicine and Environmental Applications. Curr. Top. Med. Chem. 2015, 15, 1501–1515. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).