Abstract
The application of mercaptosuccinic acid-capped gold nanoparticles as a sensing probe for the colorimetric detection of Fe(III) is reported. The well-dispersed gold nanoparticles (AuNPs) with a diameter of around 20 nm were obtained by a one-step reaction of tetrachloroauratic acid with mercaptosuccinic acid (MSA) as a reducing and capping agent, respectively. Fe(III) reportedly causes the aggregation of prepared MSA-capped AuNPs followed by a change in color and a shift to long wavelengths in the absorbance spectra. The resulting method allows for a visual and spectrophotometric Fe(III) determination with detection limits of 30 ng/mL and 23 ng/mL, respectively. MSA-capped AuNPs have been used as sensing probes for the detection of Fe(III) in drinking water samples with a detection limit that is much lower than the maximum permissible level of Fe(III) specified by official regulations (300 ng/mL).
1. Introduction
Currently, the control of the quality and composition of consumed drinking water has become extremely important. One of the main problems with a centralized water supply is the almost ubiquitous increased concentration of heavy metals in water, the main concentration of which is iron. This is primarily due to the widespread occurrence of this chemical element in various soils [1]; however, significant amounts of Fe(III) can also come from wastewater from the metallurgical, metalworking, textile, paint, and varnish industries as well as from agricultural wastewater. Considering that a high consumption of Fe(III) can cause toxic effects, the determination of Fe(III) content in water resources is of great importance for human life.
Various analytical methods, such as atomic absorption spectrometry [2], inductively couple plasma mass spectrometry [3], liquid chromatography [4], and spectrophotometric [5] and fluorescent [6] methods, are successfully applied for Fe(III) determination. Despite the high sensitivity of these methods, they are complex and time-consuming, and usually require expensive equipment operated by skilled personnel. On the contrary, colorimetric systems offer a promising approach for the detection of various metal ions largely due to their simplicity and rapidity as well as due to the opportunity to estimate results visually. To date, a number of colorimetric sensors, based on the aggregation of nanomaterials induced by metal ions through registration of color changes (visually), and a shift of the plasmon resonance peak (spectrophotometrically) have been reported [7]. A widespread approach used to increase the selectivity of these techniques is the functionalization of the surface of nanomaterials with various ligands [8]. Among these, pyrophosphate [9], hydroxamic acid [10], oxamic and p-aminobenzoic acids [11], casein [12], and (glycol)chitosan [13,14] have been employed for the colorimetric detection of Fe(III) in various environmental and biological samples.
A colorimetric system based on gold nanoparticles (AuNPs) functionalized with mercaptosuccinic acid (MSA) for the simple, rapid, selective, and cost-effective detection of trace Fe(III) ions in water was developed. The choice of the functionalizing agent was determined by previously described binding properties of mercaptosuccinic acid [15,16,17]. Moreover, the one-step preparation and functionalization of the proposed AuNPs greatly simplifies and accelerates the preparation of the sensor system. To the best of our knowledge, this is the first report of using mercaptosuccinic acid-functionalized AuNPs as a colorimetric sensor for the detection of trace levels of Fe(III) in aqueous media.
2. Experimental Design
2.1. Chemicals and Materials
An aqueous solution of Fe(III) (1 g/L) was obtained from the Center of Standardization of Samples and High-Purity Substances (St. Petersburg, Russia). Salts of Cd2+, As3+, Cu2+, Zn2+, Pb2+, Sn2+, and Cr3+ were also obtained from the Center of Standardization of Samples and High-Purity Substances. 2-Mercaptosuccinic acid (MSA) and tetrachloroauric acid (HAuCl4) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Milli-Q-purified water was obtained using a Milli-Q Simplicity system from Millipore (Bedford, MA, USA) and used to prepare all aqueous solutions.
2.2. Synthesis of MSA-Functionalized AuNPs
Gold nanoparticles were synthesized through the reduction of HAuCl4 by mercaptosuccinic acid [18]. First, 100 mL of the 0.01% HAuCl4 solution was heated to boiling temperature and stirred using a magnet stirrer. After that, 12.5 mL of the 1 mM aqueous solution of MSA was added to the reaction mixture. The MSA solution was preliminarily neutralized with a sodium hydroxide in a stoichiometric ratio of 1:2. Thereafter, the reaction mixture was incubated with continuous stirring for 15 min and cooled to room temperature. The synthesized MSA-functionalized AuNPs were concentrated 10 times by centrifugation, resuspended in Milli-Q water with an adjustment to pH 3–4, and stored at 4–6 ° C until analysis.
2.3. Transmission Electron Microscopy
The prepared MSA-functionalized AuNPs were applied to 300 mesh grids (Pelco International, Redding, CA, USA) coated with a support film of polyvinyl formal deposited from chloroform. A JEM CX-100 electron microscope (JEOL, Tokyo, Japan) operating at 80 kV was used to obtain the images. The digital images were analyzed using Image Tool software (University of Texas Health Science Center, San Antonio, TX, USA).
2.4. Detection of Fe(III) Ions
To determine the Fe(III) ions, 5 μL of a concentrated MSA-AuNP colloidal solution was added to an aqueous solution (pH 5) containing various amounts of analyte. After 5 min, the absorption spectra were measured by the EnSpire Multi-mode Plate Reader (PerkinElmer Inc., Waltham, MA, USA). When applying this technique for real water samples, a preliminary dilution (2–15 times depending on the water source) of the samples was used. To test the selectivity of the developed technique and the interference from other heavy metal ions, the solutions containing Hg2+, Cd2+, As3+, Cu2+, Zn2+, Pb2+, Sn2+, or Cr3+ (100 ng/mL) were examined.
3. Results and Discussion
3.1. Synthesis and Characterization of MSA-Capped AuNPs
Mercaptosuccinic acid was chosen as a reducing agent due to the presence of two carboxyl groups providing functionalization by a chelate structure (Figure 1). The procedure of the MSA-capped AuNPs’ synthesis includes mixing a HAuCl4 and MSA solution at an optimized molar ratio of 2:1, which ensures a spherical form of particles with a size distribution within 20–25 nm [18]. Figure 2a,b shows the TEM image of MSA-functionalized AuNPs and the size distribution of the nanoparticles. The obtained nanoparticles have a spherical morphology with an average diameter of 19.9 ± 7.1 nm (number of particles was 195). In addition, the protection layer on the gold particles was observed in a TEM image. The obtained absorbance spectra of the MSA-AuNPs in the presence and absence of Fe(III) are given at Figure 2c. A strong peak at 530 nm was observed for MSA-AuNPs.
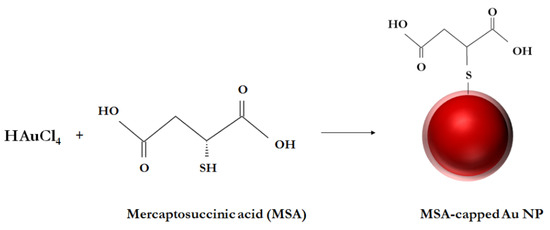
Figure 1.
Scheme of MSA-functionalized AuNPs’ synthesis.
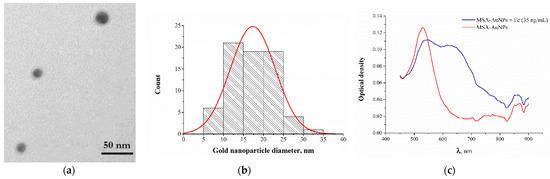
Figure 2.
(a) TEM image of MSA-capped AuNPS. (b) Histogram of MSA-AuNPs particles’ diameter distribution. (c) Absorption spectrum of MSA-AuNPs before (red) and after (blue) the addition of Fe(III) ions.
3.2. Colorimetric Determination of Fe(III) Ions
The proposed assay scheme is illustrated in Figure 3. In the presence of Fe(III), the coordination of MSA on the particle surface induced the critical convergence and loss of stability of the colloidal solution, resulting in a color change from red to blue. When the Fe(III) concentration reached 20 ng/mL, particle aggregation occurred, as registered by a decrease in the absorption peak at 530 nm and an increase in the peak at 660 nm. Consequently, the A530/A660 ratio was selected as an analytical signal.
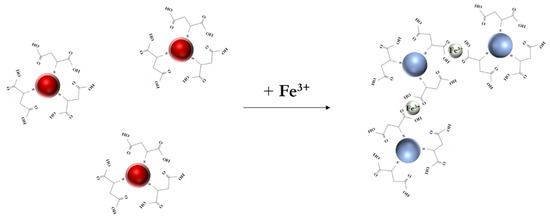
Figure 3.
Scheme of the colorimetric detection of Fe(III) ions using MSA-capped gold nanoparticles.
To find the optimal conditions for the determination of Fe(III) ions, we studied some factors affecting the analytical signal, such as the pH of the medium and the volume ratio of the reacting components (MSA-AuNPs and Fe(III)). The effect of pH on the changes in the analytical signal in the presence of Fe(III) ions was studied; the maximum detection sensitivity was achieved at pH 4.5.
To investigate the optimum volume ratio of the reaction components (MSA-AuNPs and Fe(III) solution), various ratios of the volumes of MSA-AuNPs and Fe(III) ion containing solutions (1:1, 1:3, and 1:30) were tested. The best sensitivity of the analysis was achieved with the volume ratio of MSA-AuNPs/Fe(III) ion solutions equal to 1:30.
The aggregation of MSA-AuNPs in the presence of Fe(III) was proceeded by complexation with the chelating ligands of mercaptosuccinic acid, which was accompanied by a blue shift in the absorption spectrum. The detection limit using MSA-AuNPs as the sensing probe was 23 ng/mL, at least 10 times lower than the maximum permissible concentration of Fe(III) for drinking water, which is 300 ng/mL by official regulations. The linear range of the calibration curve was 20–30 ng/mL, with an approximation factor of R2 0.98 (Figure 4). In addition, the developed sensing system allowed for the detection of Fe(III) by the naked eye at a concentration of 30 ng/mL, which corresponded to the blue color of the reaction mixture. The effectiveness of the developed system for the analysis of real samples was confirmed by the determination of Fe(III) ions in water samples without a matrix influence.
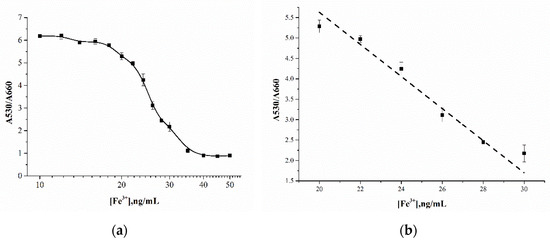
Figure 4.
(a) Calibration curve for Fe(III) ion detection using AuNP-MSA. (b) Linear range of the calibration curve.
3.3. Selectivity of Fe(III) Ions’ Detection
To check for the possible influence of other metal ions on the analytical signals for the detection of Fe(III) ions, solutions of Hg(II), Cd(II), As(II), Cu(II), Zn(II), Pb(II), Sn(II), and Cr(II) were tested under the chosen optimal conditions (Figure 5). It was found that the tested ions did not influence the analytical signal; the color change was achieved only for Fe(III) ions. The experimental data showed that the analytical signal (A530/A660) induced by Fe(III) significantly exceeded the signals for other metal ions.
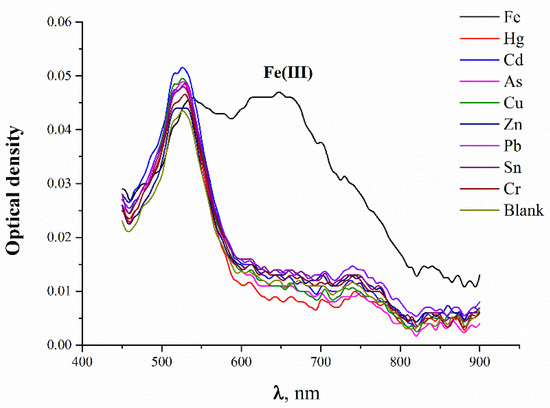
Figure 5.
Absorbance spectra of MSA-capped AuNPs after the addition of 100 ng/mL of metal ions.
4. Conclusion
A highly sensitive and selective colorimetric system for Fe(III) ion detection based on mercaptosuccinic acid-functionalized gold nanoparticles was developed. The sensing mechanism is the aggregation of gold nanoparticles due to the selective complexation of mercaptosuccinic acid with Fe(III), resulting in a change in color and in the absorbance spectrum. Under optimal conditions, this system showed a good linear correlation between Fe(III) concentrations and the colorimetric signal, with visual and instrumental detection limits of 30 and 23 ng/mL, respectively. The system demonstrated high selectivity against other metal ions. The assay combined simple fabrication and operation with the possibility of sensitive on-site monitoring. The effectiveness of the developed system was confirmed by the determination of Fe(III) ions in the water samples without a matrix influence.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/CSAC2021-10624/s1.
Author Contributions
Conceptualization, A.N.B. and A.V.Z.; methodology, A.N.B., N.S.K. and K.V.S.; software, N.S.K.; validation, N.S.K., K.V.S. and A.N.B.; formal analysis, N.S.K., K.V.S. and S.M.P.; investigation, A.N.B. and K.V.S.; resources, S.M.P.; data curation, A.V.Z. and B.B.D.; writing—original draft preparation, N.S.K., K.V.S. and A.N.B.; writing—review and editing, A.V.Z. and B.B.D.; visualization, N.S.K.; supervision, A.V.Z. and B.B.D.; project administration, A.V.Z.; funding acquisition, A.N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Russian Science Foundation (project number 19-44-02020, assay development) and the Ministry of Science and Higher Education of the Russian Federation (study of assay selectivity).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
This work was financially supported by the Russian Science Foundation (project number 19-44-02020, assay development) and the Ministry of Science and Higher Education of the Russian Federation (study of assay selectivity).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, T.T.; Yang, J.X.; Song, J.M.; Chen, J.S.; Niu, H.L.; Mao, C.J.; Zhang, S.Y.; Shen, Y.H. Synthesis of high fluorescence graphene quantum dots and their selective detection for Fe3+ in aqueous solution. Sens. Actuators B Chem. 2017, 243, 863–872. [Google Scholar] [CrossRef] [Green Version]
- Bagheri, H.; Afkhami, A.; Saber-Tehrani, M.; Khoshsafar, H. Preparation and characterization of magnetic nanocomposite of Schiff base/silica/magnetite as a preconcentration phase for the trace determination of heavy metal ions in water, food and biological samples using atomic absorption spectrometry. Talanta 2012, 97, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Boyle, E.A. Determination of iron in seawater by high-resolution isotope dilution inductively coupled plasma mass spectrometry after Mg(OH)2 coprecipitation. Anal. Chim. Acta 1998, 367, 183–191. [Google Scholar] [CrossRef]
- Ariga, T.; Ito, K.; Imura, Y.; Yoshimura, E. High-performance liquid chromatography method for ferric iron chelators using a post-column reaction with Calcein Blue. J. Chromatogr. B 2015, 985, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Kuljanin, J.; Janković, I.; Nedeljković, J.; Prstojević, D.; Marinković, V. Spectrophotometric determination of alendronate in pharmaceutical formulations via complex formation with Fe(III) ions. J. Pharm. Biomed. Anal. 2002, 28, 1215–1220. [Google Scholar] [CrossRef]
- Hirayama, T. Fluorescent probes for the detection of catalytic Fe(II) ion. Free Radic. Biol. Med. 2019, 133, 38–45. [Google Scholar] [CrossRef]
- Piriya, V.S.A.; Joseph, P.; Daniel, S.C.G.K.; Lakshmanan, S.; Kinoshita, T.; Muthusamy, S. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar] [CrossRef]
- Alex, S.; Tiwari, A. Functionalized Gold Nanoparticles: Synthesis, Properties and Applications. A Review. J. Nanosci. Nanotechnol. 2015, 15, 1869–1894. [Google Scholar] [CrossRef]
- Wu, S.P.; Chen, Y.P.; Sung, Y.M. Colorimetric detection of Fe3+ ions using pyrophosphate functionalized gold nanoparticles. Analyst 2011, 136, 1887–1891. [Google Scholar] [CrossRef] [PubMed]
- Karami, C.; Alizadeh, A.; Taher, M.A.; Hamidi, Z.; Bahrami, B. UV-Visible Spectroscopy Detection of Iron(III) Ion on Modified Gold Nanoparticles With a Hydroxamic Acid. J. Appl. Spectrosc. 2016, 83, 687–693. [Google Scholar] [CrossRef]
- Buduru, P.; Reddy, B.C.S.R. Oxamic acid and p-aminobenzoic acid functionalized gold nanoparticles as a probe for colorimetric detection of Fe3+ ion. Sens. Actuators B Chem. 2016, 237, 935–943. [Google Scholar] [CrossRef]
- Kim, D.Y.; Shinde, S.; Saratale, R.; Syed, A.; Ameen, F.; Ghodake, G. Spectrophotometric determination of Fe(III) by using casein-functionalized gold nanoparticles. Microchim. Acta 2017, 184, 4695–4704. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Huo, D.; Hou, C.; Fa, H.; Yang, M.; Zhang, L. Colorimetric measurement of Fe3+ using a functional paper-based sensor based on catalytic oxidation of gold nanoparticles. Sens. Actuators B Chem. 2017, 242, 1265–1271. [Google Scholar] [CrossRef]
- Kim, K.; Nam, Y.S.; Lee, Y.; Le, K.B. Highly Sensitive Colorimetric Assay for Determining Fe3+ Based on Gold Nanoparticles Conjugated with Glycol Chitosan. J. Anal. Methods Chem. 2017, 2017, 3648564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheney, G.E.; Fernando, Q.; Freiser, H. Some Metal Chelates of Mercaptosuccinic Acid. J. Phys. Chem. 1959, 63, 2055–2057. [Google Scholar] [CrossRef]
- Larkworthy, L.F.; Sattari, D. Some complexes of thiomalate with bivalent transition metal ions and gold (I). J. Inorg. Nucl. Chem. 1980, 42, 551–559. [Google Scholar] [CrossRef]
- Pawelec, M.; Stochel, G.; Eldik, R. Mechanistic information on the copper-catalysed autoxidation of mercaptosuccinic acid in aqueous solution. Dalton Trans. 2004, 2, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, K.; Zhu, T.; Glasser, G.; Knoll, W.; Kreiter, M. Preparation of gold nanoparticles in an aqueous medium using 2-mercaptosuccinic acid as both reduction and capping agent. J. Nanosci. Nanotechnol. 2008, 8, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).