Abstract
Hybrid materials constructed from two or more constituents provide new features and distinctive applications which not found in the single-part material. Spinel ferrites with the general formula of AB2O4, where A denotes divalent ions and B represents trivalent ions, appeared as efficient catalysts for the synthesis of organic compounds. These magnetic nanoparticles have both the Lewis acid and the Lewis base sites in their structure. In the present study, ZnFe2O4 was prepared and modified with dimethylglyoxime to obtain a magnetic ZnFe2O4@dimethylglyoxime hybrid catalyst. Dimethylglyoxime is a dibasic acid substance that has been used as a chelating agent for divalent metal ions due to containing two nitrogen atoms and hydroxyl groups. Surface modification of zinc ferrite with this dibasic acid substance produced a bifunctional hybrid catalyst with increased catalytic active sites. In the next step, a prepared catalyst was applied in the synthesis of 2-amino-tetrahydro-4H-chromene-3-carbonitrile derivatives by condensation of aromatic aldehydes, malononitrile and dimedone in ethanol at room temperature. The present method offers advantages such as simple procedure, mild reaction condition, short reaction times and retrievable catalyst.
1. Introduction
Magnetic catalysts with applicable advantages such as reusability and easy separation from the reaction mixture have attracted great attention [1]. For the production of heterogeneous magnetic catalysts, iron is generally more suitable due to its properties such as abundance in nature, low toxicity and availability [2]. Chemists have found that hybrid materials made from two or more components exhibited different properties than each component and synergistic effects, and so therefore the design and fabrication of hybrid materials is an effective approach in catalyst chemistry [3]. Ferrites are polycrystalline materials and are made of a large number of tiny crystals with different orientations [4]. Zinc ferrite has been widely used in diverse applications due to its magnetization properties, simple synthesis, low toxicity and good chemical stability [3,4,5]. Dimethylglyoxime is a dibasic acid substance that has been utilized for divalent ions owning to its special structure and the presence of chelating elements of nitrogen and hydroxyl [6,7]. MCRs are a significant approach in organic chemistry to construct a variety of biologically active compounds [8,9,10]. The tetrahydro-4H-chromene and its derivatives are important biologically active compounds because of significant medicinal properties such as being antispasmodic, anticoagulant and also their effective therapeutic effects in neurological diseases and cancer [11]. Continuing our research on MCRS and the heterocyclic compound, in the present work the modified ZnFe2O4 was prepared by a co-precipitation procedure in the presence of dimethylglyoxime as a dibasic acid substance to obtain a hybrid catalyst with more active sites and enhanced catalytic efficiency. The prepared ZnFe2O4@dimethylglyoxime composite was then used as a hybrid catalyst for the synthesis of 2-amino-tetrahydro-4H-chromene-3-carbonitrile derivatives via one-pot condensation of various aromatic aldehydes, malononitrile and dimedone (Scheme 1).
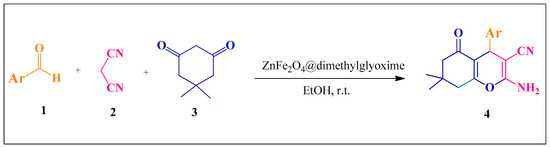
Scheme 1.
The synthesis of 2-amino-tetrahydro-4H-chromene-3-carbonitrile derivatives.
2. Experimental
2.1. General
All consumed chemicals and solvents were purchased from Sigma Aldrich and Merck companies. Fourier transform infrared (FT-IR) spectra were recorded on a Shimadzu 8400 S spectrometer using KBr pellets. Elemental analysis of a prepared sample was carried out by energy-dispersive X-ray (EDX) analysis recorded on Numerix JEOL-JDX 8030 (30 kV, 20 mA). X-ray diffraction (XRD) pattern of the prepared catalyst was recorded on an X-ray diffractometer (Bruker D8 Advance). Melting points were measured with an electrothermal 9100 apparatus.
2.2. Preparation of the ZnFe2O4@dimethylglyoxime
The ZnFe2O4@dimethylglyoxime was prepared by co-precipitation of Zn2+ and Fe3+ aqueous solution in the presence of dimethylglyoxime under alkaline conditions. First, Zn(OAC)2·2H2O (1.6 mmol) and FeCl3·6H2O (3.2 mmol) were dissolved in 50 mL distilled water. Then dimethylglyoxime (4.5 mmol) was added to above solution and dispersed by ultrasonic at room temperature for 15 min. In the next step, NaOH (0.25 mmol) was dissolved in 50 mL of distilled water, then was added to the reaction mixture drop by drop. The reaction was completed at 90 °C after 1 hour. Finally, the resulting precipitate was separated with a magnet, washed with distilled water several times, and dried in an oven at 60 °C.
2.3. General Procedure for the Synthesis of 2-Amino-tetrahydro-4H-chromene-3-carbonitrile Derivatives Derivatives
The mixture of aromatic aldehyde (1.0 mmol), malononitrile (1.0 mmol), dimedone (1.0 mmol) and 0.01 g of ZnFe2O4@dimethylglyoxime catalyst was stirred in ethanol at room temperature. The completion of the reaction was monitored by thin layer chromatography. After completion of the reaction, the catalyst was separated with a magnet, washed with deionized water and dried. The pure product was then obtained by the crystallization of the crude precipitate in ethanol.
3. Results and Discussion
3.1. Characterization of Catalyst
The presence of constituent elements in the ZnFe2O4@dimethylglyoxime composition was studied by EDX analysis. The results of EDX analysis can be observed in Figure 1. The observed peaks are related to the presence of zinc, iron, nitrogen and oxygen, which indicate the existence of component elements in the prepared sample.
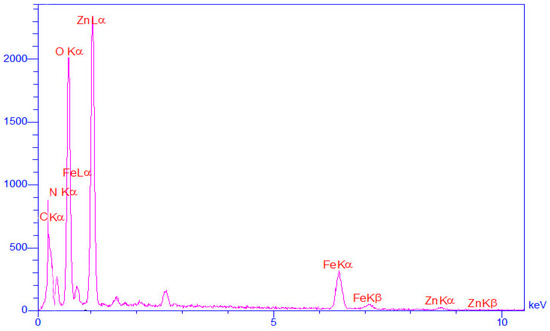
Figure 1.
Energy-dispersive X-ray (EDX) analysis of the ZnFe2O4@dimethylglyoxime catalyst.
The XRD pattern of ZnFe2O4@dimethylglyoxime is shown in Figure 2, which is compared to the standard pattern of ZnFe2O4. As is observed, the standard pattern of ZnFe2O4 with card no. JCPDS, 00-022-1020 have characteristic diffraction peaks at 2θ = 18.19°, 29.92°, 35.26°, 42.85°, 56.81° and 62.21°. The prepared catalyst pattern showed similar diffraction peaks at 2θ = 17.40°, 30.13°, 35.23°, 57.1° and 62.22°, confirming the existence of crystalline nanoparticles in the catalyst composition. The average crystallite size in the hybrid nanocatalyst was determined to be about 42 nm based on the information of the characteristic diffraction peaks and using the Debye-Scherer equation.
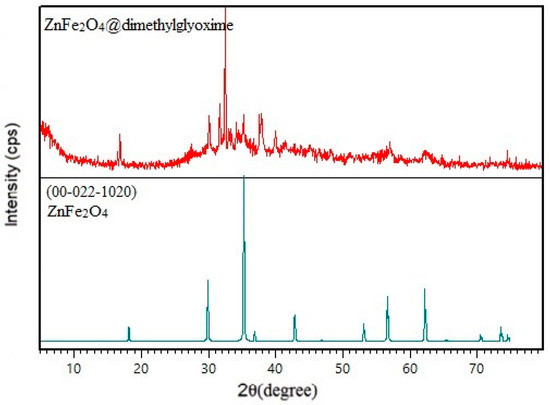
Figure 2.
XRD pattern of ZnFe2O4@dimethylglyoxime catalyst.
The FT-IR analysis was used to identify the functional groups of the ZnFe2O4@dimethylglyoxime catalyst. Figure 3 showed the IR spectrum of ZnFe2O4@dimethylglyoxime hybrid catalysts in the range of 400–4400 cm−1. The absorption at 415 cm−1 is related to the stretching vibration of Zn-O, and the appearing absorption in the range of 520–550 cm−1 is related to the stretching vibration of Fe-O [12]. The absorption bands at 1122 and 1050 cm−1 are attributed to the symmetrical and asymmetrical stretching vibration of an N-O bond, respectively. A weak band in 1572 cm−1 is ascribed to the starching vibration of C=N bond. The absorption band at 2936 cm−1 is related to stretching vibration of the C–H bond, the adsorption related to the stretching vibrations of the hydroxyl groups in the dimethylglycoxime structure and the ZnFe2O4 surface appeared as an intensive band in the range of 3400–3600 cm−1.
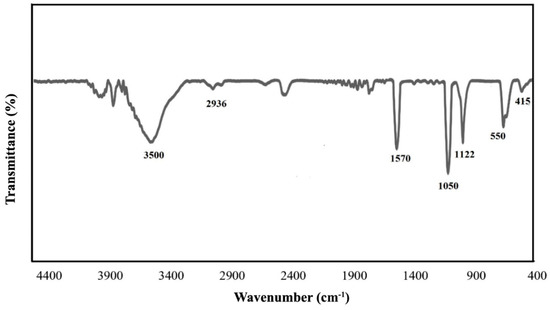
Figure 3.
FTIR analysis of the ZnFe2O4@dimethylglyoxime catalyst.
3.2. Catalytic Application of ZnFe2O4@dimethylglyoxime in the Synthesis of 2-Amino-tetrahydro-4H-chromene-3-carbonitrile Derivatives
Synthesis of 2-amino-tetrahydro-4H-chromene-3-carbonitrile derivatives using some aromatic aldehydes was selected under optimal conditions to evaluate the efficiency of the prepared ZnFe2O4@dimethylglyoxime catalyst. As shown in Table 1, all tested aromatic aldehydes with electron drawing and electron donating groups produced high yield products in short reaction times.

Table 1.
Synthesis of 2-amino-tetrahydro-4H-chromene-3-carbonitrile derivatives.
4. Conclusions
ZnFe2O4@dimethylglyoxime is a recyclable, efficient and cost-effective hybrid catalyst which applied in the synthesis of 2-amino-tetrahydro-4H-chromene-3-carbonitrile derivatives. This procedure provides advantages such as high yield products in a short time, an easy workup for purification of a product and the reusability of the catalyst.
Funding
This research received no external funding.
References
- Aisida, S.O.; Ahmad, I.; Zhao, T.K.; Maaza, M.; Ezema, F.I. Calcination Effect on the Photoluminescence, Optical, Structural, and Magnetic Properties of Polyvinyl Alcohol Doped ZnFe2O4 Nanoparticles. J. Macromol. Sci. Part B 2020, 59, 295–308. [Google Scholar] [CrossRef]
- Kumar, A.S.; Reddy, M.A.; Knorn, M.; Reiser, O.; Sreedhar, B. Magnetically Recoverable CuFe2O4 Nanoparticles: Catalyzed Synthesis of Aryl Azides and 1,4-Diaryl-1,2,3-triazoles from Boronic Acids in Water. Eur. J. Org. Chem. 2013, 2013, 4674–4680. [Google Scholar] [CrossRef]
- Maleki, A.; Varzi, Z.; Hassanzadeh-Afruzi, F. Preparation and characterization of an eco-friendly ZnFe2O4@ alginic acid nanocomposite catalyst and its application in the synthesis of 2-amino-3-cyano-4H-pyran derivatives. Polyhedron 2019, 171, 193–202. [Google Scholar] [CrossRef]
- Andhare, D.; Jadhav, S.; Khedkar, M.; Somvanshi, S.B.; More, S.; Jadhav, K. Self-heating evaluation of superparamagnetic MnFe2O4 nanoparticles for magnetic fluid hyperthermia application towards cancer treatment. Ceram. Int. 2020, 46, 25576–25583. [Google Scholar]
- Chai, L.; Wang, Y.; Zhou, N.; Du, Y.; Zeng, X.; Zhou, S.; He, Q.; Wu, G. In-situ growth of core-shell ZnFe2O4@ porous hollow carbon microspheres as an efficient microwave absorber. J. Colloid Interface Sci. 2020, 581, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, W.S.; Dias, V.L.; Costa, W.M.; de Araujo Rodrigues, I.; Marques, E.P.; Sousa, A.G.; Boaventura, J.; Bezerra, C.W.; Song, C.; Zhang, J. Nickel-dimethylglyoxime complex modified graphite and carbon paste electrodes: Preparation and catalytic activity towards methanol/ethanol oxidation. J. Appl. Electrochem. 2009, 39, 55–64. [Google Scholar] [CrossRef]
- Hassanloie, N.; Noroozi Pesyan, N.; Sheykhaghaei, G. Anchored Ni-dimethylglyoxime complex on Fe3O4@ SiO2 core/shell nanoparticles for the clean catalytical synthesis of dicoumarols. Appl. Organomet. Chem. 2020, 34, e5242. [Google Scholar] [CrossRef]
- Hajizadeh, Z.; Hassanzadeh-Afruzi, F.; Jelodar, D.F.; Ahghari, M.R.; Maleki, A. Cu (ii) immobilized on Fe3O4@ HNTs–tetrazole (CFHT) nanocomposite: Synthesis, characterization, investigation of its catalytic role for the 1,3 dipolar cycloaddition reaction, and antibacterial activity. RSC Adv. 2020, 10, 26467–26478. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Hassanzadeh-Afruzi, F.; Maleki, A. Synthesis and characterization of a novel and green rod-like magnetic ZnS/CuFe2O4/agar organometallic hybrid catalyst for the synthesis of biologically-active 2-amino-tetrahydro-4H-chromene-3-carbonitrile derivatives. Appl. Organomet. Chem. 2020, 34, e5949. [Google Scholar] [CrossRef]
- Maleki, A.; Hassanzadeh-Afruzi, F.; Varzi, Z.; Esmaeili, M.S. Magnetic dextrin nanobiomaterial: An organic-inorganic hybrid catalyst for the synthesis of biologically active polyhydroquinoline derivatives by asymmetric Hantzsch reaction. Mater. Sci. Eng. C 2020, 109, 110502. [Google Scholar] [CrossRef] [PubMed]
- Paczkowski, I.M.; Guedes, E.P.; Mass, E.B.; de Meneses, E.W.; Marques, L.A.; Mantovani, M.S.; Russowsky, D. Synthesis of hybrid perillyl-4 H-pyrans. Cytotoxicity evaluation against hepatocellular carcinoma HepG2/C3A cell line. J. Heterocycl. Chem. 2020, 57, 2597–2614. [Google Scholar] [CrossRef]
- Liang, P.L.; Yuan, L.Y.; Deng, H.; Wang, X.C.; Wang, L.; Li, Z.J.; Shi, W.Q. Photocatalytic reduction of uranium (VI) by magnetic ZnFe2O4 under visible light. Appl. Catal. B Environ. 2020, 267, 118688. [Google Scholar] [CrossRef]
- Ponpandian, T.; Muthusubramanian, S. One-pot, catalyst-free synthesis of spirooxindole and 4 h-pyran derivatives. Synth. Commun. 2014, 44, 868–874. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).