Abstract
Heterocyclic aldehydes show a variety of optical properties and the versatility of their reactivity allows them to yield a wide range of more complex compounds, with application in areas such as medicinal, materials and supramolecular chemistry. The biological and environmental relevance of certain molecules and ions turns them into targets for the design of molecular recognition systems. Recently, heterocyclic aldehydes have been reported in the literature as ion chemosensors. Following the group’s work on optical chemosensors, for the detection and quantification of ions and molecules with environmental and medicinal relevance, this work reports the synthesis and characterization of two heterocyclic aldehydes based on the thieno[3,2-b]thiophene core, by Suzuki coupling, as well as the synthesis of the corresponding precursors. Preliminary chemosensory studies for the synthesized heterocyclic aldehydes in the presence of selected cations were also performed, in solution, in order to determine their potential application as optical chemosensors.
1. Introduction
Aldehydes are one of the most recurrent functional groups in organic molecules. Aldehydes can be found in nature as flavouring agents, such as benzaldehyde and cinnamaldehyde, carbohydrates or steroid hormones. This group can be introduced into heterocyclic moieties through several synthetic methodologies, being the most used methods Vilsmeier–Haack formylation and metalation followed by addition of DMF (dimethylformamide) [1,2,3,4,5].
Vilsmeier–Haack formylation is a known synthetic method to introduce a formyl group into electron-rich aromatic compounds using the Vilsmeier reagent. This reagent is prepared by reacting a N,N-disubstituted formamide, normally DMF, and an acid chloride, generally POCl3. An electrophilic aromatic substitution of the substrate takes place, followed by hydrolysis to yield the heterocyclic carbaldehyde [3]. π-Conjugated heterocyclic aldehydes can also be prepared through a Suzuki cross-coupling reaction using the appropriate coupling components [6,7,8,9].
Due to its versatility, the carbaldehyde group, linked to heterocyclic moieties, can be converted to stronger electron-withdrawing groups, yielding π-conjugated push-pull systems with a wide variety of optical applications. Such applications include, for instance, non-linear optics (NLO) [10,11], photodynamic therapy (PDT) [12], dye-sensitized solar cells (DSSCs) [6,13,14], organic light emitting diodes (OLEDs) [15] or optical chemosensors for cations (Cu2+, Hg2+) or anions (F−) [7,16,17,18].
Carrying on the research group’s investigation on optical chemosensors [7,9,19,20,21,22], this work reports the synthesis and characterization of two heterocyclic aldehydes by Suzuki coupling of 5-bromothieno[3,2-b]thiophene-2-carbaldehyde with two different phenylboronic acids. The synthesis and the characterization of the thieno[3,2-b]thiophene precursors is also included.
Preliminary chemosensory studies for the synthesized heterocyclic aldehydes in the presence of selected cations were also performed, in solution, in order to determine their potential application as optical sensors.
2. Experimental Section
2.1. Methods and Materials
Nuclear magnetic resonance (NMR) spectra were obtained on a Bruker Avance III 400 at an operating frequency of 400 MHz for 1H and 100.6 MHz for 13C using the solvent peak as internal reference. The solvents (deuterated choloroform; CDCl3 and dimethyl sulfoxide-d6; DMSO-d6) are indicated in parenthesis before the chemical shifts values (δ relative to tetramethylsilane (TMS)). Peak assignments were supported by spin decoupling-double resonance and bidimensional heteronuclear correlation techniques. All reagents were purchased from Sigma-Aldrich, Acros and Fluka and used as received. Compounds 1, 2 and 3a–c were synthesized as previously reported [6,23,24]. TLC (thin layer chromatography) analysis were carried out on 0.25 mm thick precoated silica plates (Merck Fertigplatten Kieselgel 60F254) and the spots were observed under ultraviolet (UV) light. Chromatography on silica gel was carried out on Merck Kieselgel (230–400 mesh).
2.2. Synthesis of Thieno[3,2-b]thiophene-2-Carbaldehyde (1)
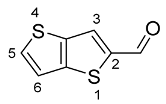
Thieno[3,2-b]thiophene (1.00 g, 7.13 mmol) was dissolved in DMF (5 mL) and cooled to 0 °C. A mixture of phosphorus oxychloride (1.96 mL, 21.39 mmol) and DMF (5 mL), cooled to 0 °C, was added dropwise with stirring. The reaction mixture was allowed to reach room temperature and was then stirred at 60 °C for 11 h. The resulting intermediate was poured over cold water and the pH was adjusted to 8–9 by adding saturated aqueous Na2CO3. The product was extracted with dichoromethane (DCM) (3 × 100 mL), the organic extract was washed with water (3 × 200 mL), dried with anhydrous MgSO4, filtered and the solvent was evaporated in vacuo. The crude product was purified by flash chromatography using DCM/hexane (1:1) as eluent. The obtained product was an orange solid (1.034 g, 88%). 1H NMR (400 MHz, CDCl3): δ = 7.35 (dd, 1H, J = 5.2 and 0.8 Hz, H-6), 7.71 (d, 1H, J = 5.2 Hz, H-5), 7.96 (d, 1H, J = 0.4 Hz, H-3), 9.99 (s, 1H, CHO) ppm.
2.3. Synthesis of 5-Bromothieno[3,2-b]thiophene-2-Carbaldehyde (2)
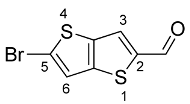
A solution of N-bromosuccinimide (NBS, 0.500 g, 3 mmol) in DMF (6 mL) was added dropwise to a solution of 1 (0.100 g, 3 mmol) in DMF (9 mL) in the dark at ambient temperature and the reaction was stirred for 4 h also in the dark. The reaction mixture was poured into water and the solid formed was filtered off and a white solid was obtained (0.57 g, 77%). 1H NMR (400 MHz, CDCl3): δ = 7.37 (d, 1H, J = 0.4 Hz, H-6), 7.85 (d, 1H, J = 0.8 Hz, H-3), 9.98 (s, 1H, CHO) ppm.
2.4. General Procedure for the Synthesis of Heterocyclic Aldehydes by Suzuki Cross-Coupling Reaction 3a–b
Precursor 2 (0.100 g, 0.4 mmol) was coupled with different phenylboronic acids (0.5 mmol) in a mixture of 1,2 dimethoxyethane (DME, 6 mL), aqueous Na2CO3 2 M (0.4 mL) and Pd(PPh3)4 (6 mol %) at 80 °C under nitrogen. The reactions were monitored by TLC, which determined reaction times. After cooling, the mixtures were filtered, and the solid was washed with an organic solvent and a saturated solution of NaCl. After phase separation, the organic phase was washed with water (3 × 10 mL) and a solution of 10% NaOH (1 × 10 mL). The organic phase obtained was dried with anhydrous MgSO4, filtered, and the solvent removed under vacuum.
2.4.1. Synthesis of 4-N,N-Dimethylaminophenylthieno[3,2-b]thiophene-2-Carbaldehyde (3a)
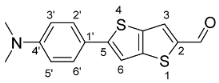
After extraction, the compound 3a was obtained pure as an orange solid (0.113 g, 98%). 1H NMR (400 MHz, DMSO-d6): δ = 2.97 (s, 6H, N(CH3)2), 6.78 (d, 2H, J = 8.8 Hz, H-3′ and H-5′), 7.57 (d, 2H, J = 8.8 Hz, H-2′ and H-6′), 7.76 (s, 1H, H-6), 8.32 (s, 1H, H-3), 9.91 (s, 1H, CHO) ppm.
2.4.2. Synthesis of 5-Phenylthieno[3,2-b]thiophene-2-Carbaldehyde (3b)
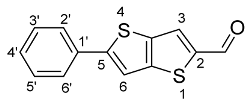
The crude product, obtained from extraction, was submitted to column chromatography on silica gel with petroleum ether/ethyl acetate (5:1) as eluent. Compound 3b was obtained as a yellow solid (0.063 g, 65%). 1H NMR (400 MHz, CDCl3): δ = 7.40–7.48 (m, 3H, H-3′, H-4′ and H-5′), 7.55 (s, 1H, H-6), 7.67 (br d, 2H, J = 6.8 Hz, H-2′ and H-6′), 7.94 (s, 1H, H-3), 9.97 (s, 1H, CHO) ppm.
2.5. Preliminary Chemosensing Studies of Heterocyclic Aldehydes 3a–b
Evaluation of heterocyclic aldehydes 3a–b as chemosensors was carried out in the presence of several cations (Ag+, K+, Li+, Pb2+, Mn2+, Cd2+, Cu2+, Co2+, Pd2+, Ni2+, Ca2+, Hg2+, Zn2+, Fe2+, Fe3+ and Al3+) with environmental and biomedical relevance. Solutions of the compound (1.0 × 10−5 M) and solutions of the cations under study (1.0 × 10−2 M) were prepared in acetonitrile. A preliminary study was carried out by addition of up to 50 equivalents of each cation to the solution of compounds 3a–b in acetonitrile. The solutions were analyzed on a Vilber Lourmat CN6 viewing cabinet under an ultraviolet (UV) lamp at 365 nm.
3. Results and Discussion
3.1. Synthesis of Intermediates 1 and 2 and Heterocyclic Aldehydes 3a–b
The synthesis of thieno[3,2-b]thiophene-2-carbaldehyde (1) was performed through Vilsmeier–Haack formylation of thieno[3,2-b]thiophene precursor [23]. The intermediate 1 was obtained as an orange solid with a yield of 88%, after purification by flash chromatography using a mixture of DCM/hexane (1:1) as eluent. The structure was confirmed by 1H NMR, with the typical carbaldehyde singlet at 9.99 ppm.
Precursor 1 was then used in the synthesis of 5-bromothieno[3,2-b]thiophene-2-carbaldehyde (2), by bromination with N-bromosuccinimide (NBS), in DMF, in the dark [24]. The brominated precursor 2 obtained was a white solid with a yield of 77%. 1H NMR confirmed the disappearance of the H-5 signal at 7.71 ppm.
Heterocyclic aldehydes 3a–b were synthesized by Suzuki coupling [6] using as couplings components precursor 2 and the appropriate aryl boronic acids, in a mixture of 1,2 dimethoxyethane (DME), aqueous Na2CO3 and Pd(PPh3)4 at 80 °C, under nitrogen atmosphere (see Scheme 1).
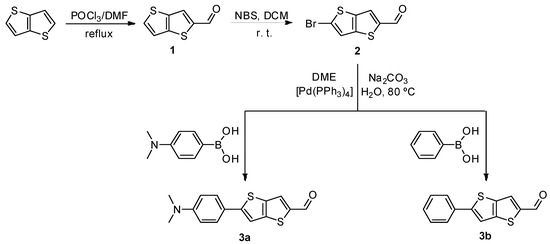
Scheme 1.
Synthesis of thieno[3,2-b]thiophene precursors 1 and 2, and heterocylic aldehydes 3a–b.
Thieno[3,2-b]thiophene 3a was obtained pure, as an orange solid with a yield of 98%, without needing purification by chromatography. 1H NMR confirmed the purity of the compound with the characteristic aliphatic singlet at 2.97 ppm due to the (N(CH3)2) protons and two duplets at 6.78 and 7.57 ppm due to four aromatic protons of the di-substituted phenyl group besides the singlets concerning the thieno[3,2-b]thiophene protons at 7.76 and 8.32 due to H-6 and H-3, respectively. Compound 3b was obtained as a yellow solid with a yield of 65%, after column chromatography on silica gel. In this case, 1H NMR showed the typical thieno[3,2-b]thiophene-2-carbaldehyde signals and two other aromatic signals from the five protons of the introduced phenyl moiety, at 7.40–7.48 and 7.67 ppm.
For the synthesis of 3a, different reaction conditions for Suzuki coupling, with toluene as solvent and K2CO3 instead of Na2CO3, were also used, since previous reports for other coupling products showed enhanced yields and shorter reaction times [6]. However, in this case, a considerably lower yield was obtained (14%) (Table 1).

Table 1.
Synthesis of heterocyclic aldehydes 3a–b by Suzuki coupling.
3.2. Preliminary Chemosensing Studies of Heterocyclic Aldehydes 3a–b
A preliminary colorimetric and fluorimetric chemosensing study was performed for heterocylic aldehydes 3a–b. Evaluation of the two compounds as chemosensors was performed in acetonitrile solutions, in the presence of 50 equivalents of different cations. It was observed that only 4-N,N-dimethylaminophenylthieno[3,2-b]thiophene-2-carbaldehyde 3a exhibited fluorescence quenching in the presence of Hg2+, Fe2+, Cu2+, Pd2+, Fe3+, and Al3+ cations (see Figure 1). Color changes were not observed for any interaction with the ions.

Figure 1.
Preliminary chemosensing study of heterocyclic aldehyde 3a, in acetonitrile solutions and in the presence of 50 equivalents of each cation, on a viewing cabinet under an ultraviolet (UV) lamp at 365 nm.
4. Conclusions
Two heterocyclic aldehydes 3a–b were successfully synthesized by Suzuki coupling, with yields of 98% (3a) and 65% (3b). The synthesis of 3a under different reaction conditions (Method B; solvent and base) resulted in a significantly lower yield (14%). The thieno[3,2-b]thiophene precursors 1–2 were also synthesized, with yields of 88% and 77%, respectively. Preliminary chemosensing studies of heterocyclic aldehyde 3a showed fluorescence quenching in the presence of Hg2+, Fe2+, Cu2+, Pd2+, Fe3+, and Al3+. In the near future, further studies will be performed to assess the coordination ratio and limits of detection.
Funding
This research was funded by Fundação para a Ciência e Tecnologia (FCT) and FEDER (European Fund for Regional Development)-COMPETE-QRENEU for financial support through the Chemistry Research Centre of the University of Minho (Ref. CQ/UM (UID/QUI/00686/2019 and UID/QUI/00686/2020), project “SolSensors—Development of Advanced Fiber Optic Sensors for Monitoring the Durability of Concrete Structures”, reference POCI-01-0145-FEDER-031220, and a PhD grant to R.P.C.L.S. (SFRH/BD/145639/2019). The NMR spectrometer Bruker Avance III 400 is part of the National NMR Network (PTNMR) and are partially supported by Infrastructure Project No 022161 (co-financed by FEDER through COMPETE 2020, POCI and PORL and FCT through PIDDAC).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Meth-Cohn, O.; Stanforth, S.P. The Vilsmeier–Haack reaction. In Comprehensive Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 1991; pp. 777–794. [Google Scholar] [CrossRef]
- Bird, C.W. Comprehensive Heterocyclic Chemistry II; Pergamon: Oxford, UK, 1996. [Google Scholar]
- Kurti, L.; Czako, B. Strategic Applications of Named Reactions in Organic Synthesis; Elsevier: London, UK, 2005. [Google Scholar]
- Rajput, S. The Synthesis of Heterocycles: Synthesis and Formylation of Heterocycles Using Vilsmeier-Haack Reaction; LAP Lambert Academic Publishing: Saarbrücken, Germany, 2017. [Google Scholar]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Raposo, M.M.; Herbivo, C.; Hugues, V.; Clermont, G.; Castro, M.C.R.; Comel, A.; Blanchard-Desce, M. Synthesis, fluorescence, and two-photon absorption properties of push-pull 5-arylthieno[3,2-b]thiophene derivatives. Eur. J. Org. Chem. 2016, 31, 5263–5273. [Google Scholar] [CrossRef]
- Okda, H.E.; El Sayed, S.; Ferreira, R.C.M.; Gonçalves, R.C.R.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. N,N-Diphenylanilino-heterocyclic aldehyde-based chemosensors for UV-vis/NIR and fluorescence Cu(II) detection. New J. Chem. 2019, 43, 7393–7402. [Google Scholar] [CrossRef]
- Fernandes, S.S.M.; Castro, M.C.R.; Mesquita, I.; Andrade, L.; Mendes, A.; Raposo, M.M.M. Synthesis and characterization of novel thieno[3,2-b]thiophene based metal-free organic dyes with different heteroaromatic donor moieties as sensitizers for dye-sensitized solar cells. Dyes Pigments 2017, 136, 46–53. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Batista, R.M.F.; Raposo, M.M.M.; Costa, S.P.G. Novel functionalized imidazo-benzocrown ethers bearing a thiophene spacer as fluorimetric chemosensors for metal ion detection. Dyes Pigments 2016, 135, 134–142. [Google Scholar] [CrossRef]
- Raposo, M.M.M.; Sousa, A.M.R.C.; Fonseca, A.M.C.; Kirsch, G. Synthesis of formyl-thienylpyrroles: versatile building blocks for NLO materials. Tetrahedron 2006, 62, 3493–3501. [Google Scholar] [CrossRef][Green Version]
- He, Y.P.; Su, G.B.; Shi, J.Q.; Yiu, G.M.; Jang, R.H. New organic nonlinear optical crystals of indole-3-aldehyde. J. Cryst. Growth 1993, 130, 444–446. [Google Scholar] [CrossRef]
- Karges, J.; Heinemann, F.; Maschietto, F.; Patra, M.; Blacque, O.; Ciofini, I.; Spingler, B.; Gasser, G. A Ru(II) polypyridyl complex bearing aldehyde functions as a versatile synthetic precursor for long-wavelength absorbing photodynamic therapy photosensitizers. Bioorganic Med. Chem. 2019, 27, 2666–2675. [Google Scholar] [CrossRef] [PubMed]
- Ooyama, Y.; Hagiwara, Y.; Oda, Y.; Mizumo, T.; Harima, Y.; Ohshita, J. Dye-sensitized solar cells based on a functionally separated D-π-A fluorescent dye with an aldehyde as an electron-accepting group. New J. Chem. 2013, 37, 2336–2340. [Google Scholar] [CrossRef]
- Tang, J.; Qu, S.; Hu, J.; Wu, W.; Hua, J. A new organic dye bearing aldehyde electron-withdrawing group for dye-sensitized solar cell. Sol. Energy 2012, 86, 2306–2311. [Google Scholar] [CrossRef]
- Guo, K.; Gao, Z.; Cheng, J.; Shao, Y.; Lu, X.; Wang, H. Linear thiophene-containing π-conjugated aldehydes with aggregation-induced emission for building solid red luminophors. Dyes Pigments 2015, 115, 166–171. [Google Scholar] [CrossRef]
- Chebrolu, L.D.; Thurakkal, S.; Balaraman, H.S.; Danaboyina, R. Selective and dual naked eye detection of Cu2+ and Hg2+ ions using a simple quinoline-carbaldehyde chemosensor. Sens. Actuators B Chem. 2014, 204, 480–488. [Google Scholar] [CrossRef]
- Chakraborty, N.; Bhuiya, S.; Chakraborty, A.; Mandal, D.; Das, S. Synthesis and photophysical investigation of 2-hydroxyquinoline-3-carbaldehyde: AIEE phenomenon, fluoride optical sensing and BSA interaction study. J. Photochem. Photobiol. A Chem. 2018, 359, 53–63. [Google Scholar] [CrossRef]
- Barszcz, B.; Glowiak, T.; Jezierska, J. Crystal and molecular structures of eight-coordinate (CuN4O4) and six-coordinate (CuN4O2) Cu(II) complexes with 4-methyl-5-imidazole-carboxaldehyde or 1-benzyl-2-hydroxymethylimidazole, respectively : spectroscopic and potentiometric studies. Polyhedron 1999, 18, 3713–3721. [Google Scholar] [CrossRef]
- Lo Presti, M.; Martínez-Máñez, R.; Ros-Lis, J.V.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.; Sancenón, F. A dual channel sulphur-containing a macrocycle functionalized BODIPY probe for the detection of Hg(II) in a mixed aqueous solution. New J. Chem. 2018, 42, 7863–7868. [Google Scholar] [CrossRef]
- Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. Novel alanines bearing a heteroaromatic side chain: synthesis and studies on fluorescent chemosensing of metal cations with biological relevance. Amino Acids 2018, 50, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Okda, H.E.; El Sayed, S.; Otri, I.; Ferreira, R.C.M.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. 2,4,5-Triaryl imidazole probes for the selective chromo-fluorogenic detection of Cu(II). Prospective use of the Cu(II) complexes for the optical recognition of biothiols. Polyhedron 2019, 170, 388–394. [Google Scholar] [CrossRef]
- Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. Heterocyclic amino acids as fluorescent reporters for transition metals: Synthesis and evaluation of novel furyl-benzoxazol-5-yl-l-alanines. New J. Chem. 2018, 42, 3483–3492. [Google Scholar] [CrossRef]
- Podlesný, J.; Pytela, O.; Klikar, M.; Jelínková, V.; Kityk, I.V.; Ozga, K.; Jedryka, J.; Rudysh, M.; Bureš, F. Small isomeric push-pull chromophores based on thienothiophenes with tunable optical (non)linearities. Org. Biomol. Chem. 2019, 17, 3623–3634. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Tian, G.; Li, X.; Su, J.; Tian, H. Efficient and stable DSSC sensitizers based on substituted dihydroindolo[2,3-b]carbazole donors with high molar extinction coefficients. J. Mater. Chem. A 2013, 1, 11295–11305. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).