Synthesis and Antioxidant Activity of 2-Amino-5-R-1,3,4-Oxadiazoles with Hindered Phenol Fragments †
Abstract
:1. Introduction
2. Results and Discussions
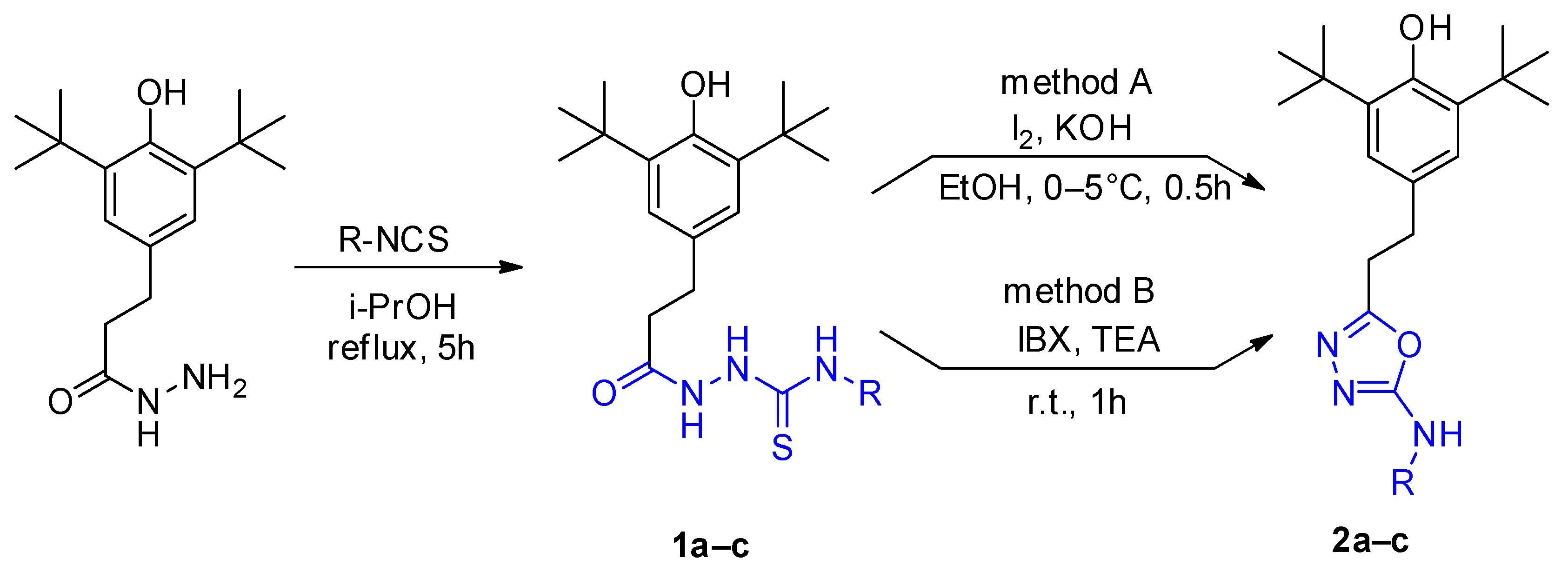
2.1. Synthesis
2.2. Antioxidant Properties
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Analytical Data of Preparated Compounds
3.2.1. Synthesis of Compounds 1a–c
3.2.2. Synthesis of Compounds 2a–c
3.3. Antioxidant Properties of Preparated Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, B.; Chen, Y.; Shi, J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxidative Med. Cell. Longev. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Mezeiova, E.; Spilovska, K.; Nepovimova, E.; Gorecki, L.; Soukup, O.; Dolezal, R.; Malinak, D.; Janockova, J.; Jun, D.; Kuca, K. Profiling donepezil template into multipotent hybrids with antioxidant properties. J. Enzym. Inhib. Med. Chem. 2018, 33, 583–606. [Google Scholar] [CrossRef] [PubMed]
- Hanikoglu, A.; Ozben, H.; Hanikoglu, F.; Ozben, T. Hybrid compounds & oxidative stress induced apoptosis in cancer therapy. Curr. Med. Chem. 2020, 27, 2118–2132. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Aguilar, T.A.F.; Navarro, B.C.H.; Perez, J.A.M. Endogenous antioxidants: A review of their role in oxidative stress. Master Regul. Oxidative Stress-Transcr. Factor Nrf2 2016, 1–20. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Kocyigit, A.; Selek, Ş. Exogenous Antioxidants are Double-edged Swords. Bezmialem Sci. 2016, 2, 70–75. [Google Scholar] [CrossRef]
- Dhahad, H.A.; Fayad, M.A. Role of different antioxidants additions to renewable fuels on NOX emissions reduction and smoke number in direct injection diesel engine. Fuel 2020, 279, 118384. [Google Scholar] [CrossRef]
- El-Ashry, E.S.; El-Rafey, M.; El-Nagdi, M.; Abou-Elnaga, H.; Bakry, W.; Boghdady, Y. Synthesis of benzotriazole derivatives as antioxidants for industrial lubricating oils. Lubr. Sci. 2006, 18, 109–118. [Google Scholar] [CrossRef]
- Cressier, D.; Prouillac, C.; Hernandez, P.; Amourette, C.; Diserbo, M.; Lion, C.; Rima, G. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorgan. Med. Chem. 2009, 17, 5275–5284. [Google Scholar] [CrossRef] [PubMed]
- Vavříková, E.; Křen, V.; Jezova-Kalachova, L.; Biler, M.; Chantemargue, B.; Pyszkova, M.; Riva, S.; Kuzma, M.; Valentová, K.; Ulrichova, J. Novel flavonolignan hybrid antioxidants: From enzymatic preparation to molecular rationalization. Eur. J. Med. Chem. 2017, 127, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Osipova, V.; Polovinkina, M.; Telekova, L.; Velikorodov, A.; Stepkina, N.; Berberova, N. Synthesis and antioxidant activity of new hydroxy derivatives of chalcones. Russ. Chem. Bull. 2020, 69, 504–509. [Google Scholar] [CrossRef]
- Pojero, F.; Poma, P.; Spanò, V.; Montalbano, A.; Barraja, P.; Notarbartolo, M. Targeting multiple myeloma with natural polyphenols. Eur. J. Med. Chem. 2019, 180, 465–485. [Google Scholar] [CrossRef]
- Koshelev, V.; Kelarev, V.; Belov, N. Effect of azoles and sym-triazines with hindered phenol fragments on protective properties of turbine oils. Chem. Technol. Fuels Oils 1995, 31. [Google Scholar] [CrossRef]
- Latyuk, V.; Kelarev, V.; Koshelev, V.; Korenev, K. Sulfides of the sym—Triazine Series as Oil—Soluble Corrosion Inhibitors. Chem. Technol. Fuels Oils 2002, 38, 312–315. [Google Scholar] [CrossRef]
- Zhang, H.-Y. Structure-activity relationships and rational design strategies for radical-scavenging antioxidants. Curr. Comput. Aided Drug Des. 2005, 1, 257–273. [Google Scholar] [CrossRef]
- Perevozkina, M. Synergism of sulfur-containing phenol (SO-4) with mexidol, α-tocopherol, and phospholipids. Pharm. Chem. J. 2006, 40, 441–447. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, L.; Fang, Y.; Zhang, H.; Chen, D.; He, T.; Chen, M.; Xu, K. Catalytic Thioalkylation of Phenols Based on Mannich-Type Phenol. Synth. Commun. 2007, 37, 2609–2613. [Google Scholar] [CrossRef]
- Ivanović, N.; Jovanović, L.; Marković, Z.; Marković, V.; Joksović, M.D.; Milenković, D.; Djurdjević, P.T.; Ćirić, A.; Joksović, L. Potent 1, 2, 4-Triazole-3-thione Radical Scavengers Derived from Phenolic Acids: Synthesis, Electrochemistry, and Theoretical Study. ChemistrySelect 2016, 1, 3870–3878. [Google Scholar] [CrossRef]
- Dvornikova, I.; Buravlev, E.; Fedorova, I.; Shevchenko, O.; Chukicheva, I.Y.; Kutchin, A. Synthesis and antioxidant properties of benzimidazole derivatives with isobornylphenol fragments. Russ. Chem. Bull. 2019, 68, 1000–1005. [Google Scholar] [CrossRef]
- Yehye, W.A.; Abdul Rahman, N.; Saad, O.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M.; Matlob, A.A. Rational design and synthesis of new, high efficiency, multipotent Schiff base-1, 2, 4-triazole antioxidants bearing butylated hydroxytoluene moieties. Molecules 2016, 21, 847. [Google Scholar] [CrossRef] [PubMed]
- Koshelev, V.N.; Primerova, O.V.; Vorobyev, S.V.; Ivanova, L.V. Synthesis, Redox Properties and Antibacterial Activity of Hindered Phenols Linked to Heterocycles. Molecules 2020, 25, 2370. [Google Scholar] [CrossRef]
- Vorobyev, S.V.; Primerova, O.V.; Ivanova, L.V.; Ryabov, V.D.; Koshelev, V.N. Facile synthesis of phenolic derivatives, containig lactamomethyl substituents. Izvestiya Vysshikh Uchebnykh Zavedenii Khimiya Khimicheskaya Tekhnologiya 2019, 62, 40–48. [Google Scholar] [CrossRef]
- Karakhanov, R.; Kelarev, V.; Koshelev, V.; Morozova, G.; Dibi, A. Synthesis and properties of furan derivatives. 4. Synthesis of 2, 5-disubstituted 1, 3, 4-oxadiazoles containing furan fragments. Chem. Heterocycl. Compd. 1995, 31, 208–218. [Google Scholar] [CrossRef]
- Prabhu, G.; Sureshbabu, V. Hypervalent iodine (V) mediated mild and convenient synthesis of substituted 2-amino-1, 3, 4-oxadiazoles. Tetrahedron Lett. 2012, 53, 4232–4234. [Google Scholar] [CrossRef]

| Methods | 2a | 2b | 2c |
|---|---|---|---|
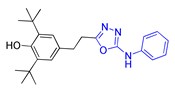 | 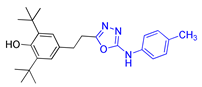 | 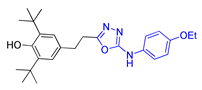 | |
| Method A | 58% | 56% | 48% |
| Method B | 74% | 80% | 75% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshelev, V.N.; Primerova, O.V.; Vorobyev, S.V.; Vakhromova, N.A. Synthesis and Antioxidant Activity of 2-Amino-5-R-1,3,4-Oxadiazoles with Hindered Phenol Fragments. Chem. Proc. 2021, 3, 69. https://doi.org/10.3390/ecsoc-24-08407
Koshelev VN, Primerova OV, Vorobyev SV, Vakhromova NA. Synthesis and Antioxidant Activity of 2-Amino-5-R-1,3,4-Oxadiazoles with Hindered Phenol Fragments. Chemistry Proceedings. 2021; 3(1):69. https://doi.org/10.3390/ecsoc-24-08407
Chicago/Turabian StyleKoshelev, Vladimir N., Olga V. Primerova, Stepan V. Vorobyev, and Natalya A. Vakhromova. 2021. "Synthesis and Antioxidant Activity of 2-Amino-5-R-1,3,4-Oxadiazoles with Hindered Phenol Fragments" Chemistry Proceedings 3, no. 1: 69. https://doi.org/10.3390/ecsoc-24-08407
APA StyleKoshelev, V. N., Primerova, O. V., Vorobyev, S. V., & Vakhromova, N. A. (2021). Synthesis and Antioxidant Activity of 2-Amino-5-R-1,3,4-Oxadiazoles with Hindered Phenol Fragments. Chemistry Proceedings, 3(1), 69. https://doi.org/10.3390/ecsoc-24-08407







