Abstract
Copper-based sulfide-rich nanomaterials have recently attracted attention in various applications due to the lack of expense, abundance, and non-toxicity of their constituent elements. As the members of this family, Cu4SnS4 and CuCo2S4 have exhibited good capability in a stable manner in solar cells, energy storage electrodes, batteries, etc. In this work, through a facile and rapid combustion reaction accelerated by microwave irradiation, Cu4SnS4/CuCo2S4 nanoparticles were prepared. Thiourea was used as a sulphur source and also organic driving agent. The features of the synthesized product were studied using FT-IR, XRD, SEM, and EDX techniques. The FT-IR and XRD results showed the formation of a multi-components structure containing orthorhombic crystalline phase of Cu4SnS4 alongside carrollite CuCo2S4 phase.
1. Introduction
In recent years, copper-based sulfide rich nanomaterials have attracted lots of attention because of having more abundant, low-cost, easily available, and non-toxic constituents compared to cadmium and lead-based compounds [1]. Usually, sulfide-rich materials have a higher activity and conductivity, as well as a lower electronegativity and band gap, than metal oxides. These compounds have significant features such as layered structures and suitable band gaps for light absorption, which enable them to be semiconductor materials in various applications [2].
Copper-based ternary sulfides, such as Cu4SnS4 and CuCo2S4 (as the members of this large family), have been studied in variety of photovoltaic, energy storage, solar cells, and catalytic applications, among others [3,4,5]. The synthesis of hetero-structured building blocks consisting of these compounds to improve multifunctional features and create highly efficient products is notable. Amongst reported synthesis methods, such as the solvothermal/hydrothermal, hot-injection, and sol–gel approaches, microwave-assisted technique can be introduced as a simple and rapid method to prepare nanomaterials [6,7,8]. As a matter of fact, the synthesis of Cu4SnS4/CuCo2S4 heterostructure nanoparticles using a solvent-free microwave-assisted procedure has not been yet reported.
2. Experimental Section
2.1. Materials
All initial reactants were provided from valid Co. and used without further purification.
2.2. Synthesis Method
The proper stoichiometric amounts of metal sources in the presence of thiourea as a sulphur source and driving agent were mixed with each other, put into a microwave oven, and treated with microwave irradiation at a power of 900 W for 20 minutes. The resulting black powder was washed, dried overnight, and characterized by FT-IR, XRD, and SEM analyses.
2.3. Characterizations
XRD patterns were recorded by a DRON-8 powder diffractometer using Cu Kα radiation (λ = 1.54060 Å). FT-IR spectra were obtained by a Shimadzu-8400S spectrometer in the range of 400–4000 cm−1 using KBr pellets. SEM and energy-dispersive X-ray images were taken on a VEGA\\TESCAN S360 with gold coating.
3. Results and Discussion
Figure 1 indicates the FT-IR spectrum of the prepared sample. The observed strong peaks at 522, 569, and 664 cm−1 could be assigned to vibration frequencies of the Co-O, Sn-S, CoS, and Cu-S bands from the prepared heterostructure molecule [9,10]. The peaks at 2361 and 3403 cm−1 could have been related to the vibrational frequencies of the H-O and C-O-C functional groups from the adsorbed H2O and CO2 molecules on the product surface.
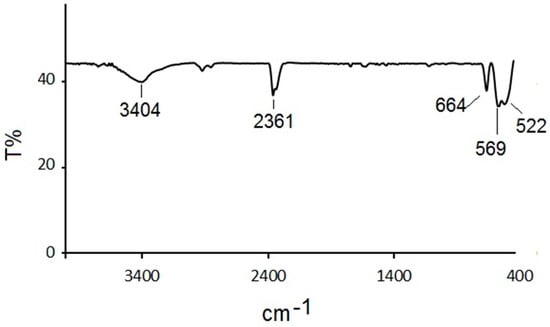
Figure 1.
FT-IR spectrum of the synthesized Cu4SnS4/CuCo2S4.
The recorded XRD pattern of Cu4SnS4/CuCo2S4 nanoparticles is shown in Figure 2. Based on this pattern, it can be said that the diffractions peaks were related to the formation of orthorhombic phase of Cu4SnS4 structure (JCPDS card No. 27-0196). In addition, the diffraction peaks at 26.59°, 31.26°, 32.70°, 38.88°, 44.88°, 48.84°, 51.89°, 54.73°, 58.29°, 61.74°, 64.79°, 66.11°, 68.14°, 71.39°, 75.36°, and 78.91°—in close accordance with the 220; 311,222; 400; 331; 422; 333,440; 531; 620; 533; 622,444; 711; 642; and 731 crystal planes—were attributed to the cubic phase of the carrollite CuCo2S4 structure (JCPDS card No. 75-1570). The slight shift at the 2 theta positions could have been a result of being the sandwich and crystallization of the carrollite CuCo2S4 phase between the Cu4SnS4 layers.
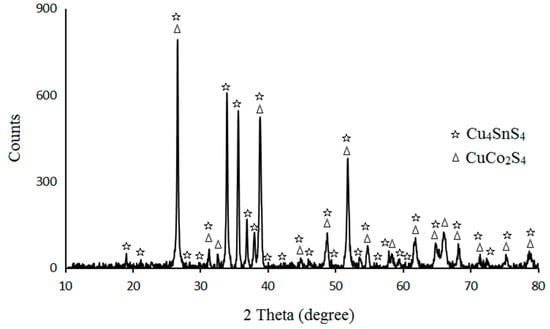
Figure 2.
XRD pattern of the prepared Cu4SnS4/CuCo2S4 nanoparticles.
The elemental EDX analysis (Figure 3) revealed the presence of Cu, Sn, Co, and S elements, thus confirming the mentioned structural data of FT-IR and XRD analyses.
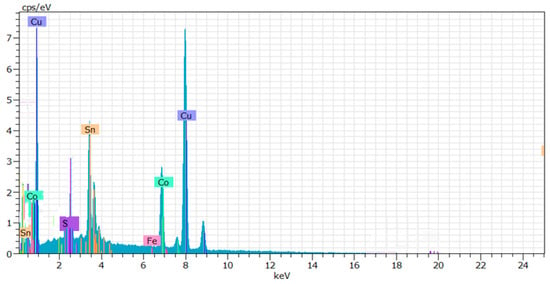
Figure 3.
EDX analysis of the resulting product.
The recorded SEM images of the prepared product depicted aggregations of crystalline flake-like morphology with an average thickness of 40 nm and a width of 210 nm (Figure 4). This morphology could have originated from the role of thiourea as a driving agent, alongside being sulfide source. A particulate morphology with an average particle size of 60 nm was also observed.
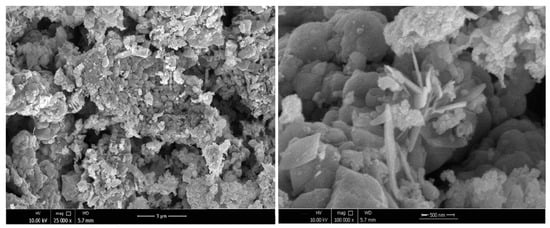
Figure 4.
SEM images of the synthesized Cu4SnS4/CuCo2S4.
4. Conclusions
Cu4SnS4/CuCo2S4 nanoparticles were well-synthesized by a microwave-assisted method. Amongst various chemical methods, the advantages of this synthesis technique include its simplicity, high speed, low energy consumption, and solvent-free reaction. In an effort to decrease the growth time, microwave heating treatment can be introduced as a capable technique to produce nanomaterials in the short reaction time compared to the conventional procedures with a long reaction time.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The necessary data has been given and this study does not report more data.
Acknowledgments
We gratefully acknowledge IUST for providing materials and facilities.
References
- Perin, G.; Jacob, R.G.; Dutra, L.G.; de Azambuja, F.; dos Santos, G.F.; Lenardao, E.J. Addition of chalcogenolate anions to terminal alkynes using microwave and solvent-free conditions: Easy access to bis-organochalcogen alkenes. Tetrahedron Lett. 2006, 47, 935–938. [Google Scholar] [CrossRef]
- Kanatzidis, M.G. Discovery-synthesis, design, and prediction of chalcogenide phases. Inorg. Chem. 2017, 56, 3158–3173. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lim, J.M.; Youn, D.H.; Liu, Y.; Cai, Y.; Kawashima, K.; Kim, J.H.; Peng, D.L.; Guo, H.; Henkelman, G.; et al. Cu4SnS4-Rich Nanomaterials for Thin-Film Lithium Batteries with Enhanced Conversion Reaction. ACS Nano 2019, 13, 10671–10681. [Google Scholar] [CrossRef]
- Xu, J.-M.; Wang, X.-C.; Cheng, J.-P. Supercapacitive performances of ternary CuCo2S4 sulfides. ACS Omega 2020, 5, 1305–1311. [Google Scholar] [CrossRef]
- Lokhande, A.C.; Gurav, K.V.; Jo, E.; He, M.; Lokhande, C.D.; Kim, J.H. Towards cost effective metal precursor sources for future photovoltaic material synthesis: CTS nanoparticles. Opt. Mater. 2016, 54, 207–216. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Wang, Y.; Shen, L.; Gupta, A.; Bao, N. Simple one-pot synthesis of Cu 4 SnS 4 nanoplates and temperature-induced phase transformation mechanism. CrystEngComm 2020, 22, 1220–1229. [Google Scholar] [CrossRef]
- Balalaie, S.; Arabanian, A. One-pot synthesis of tetrasubstituted imidazoles catalyzed by zeolite HY and silica gel under microwave irradiation. Green Chem. 2000, 2, 274–276. [Google Scholar] [CrossRef]
- Kent, R.D.; Vikesland, P.J. Dissolution and persistence of copper-based nanomaterials in undersaturated solutions with respect to cupric solid phases. Environ. Sci. Technol. 2016, 50, 6772–6781. [Google Scholar] [CrossRef] [PubMed]
- Oeba, D.A. Electrical and optical characterization of Cu4SnS4 and CdS: B Thin films for photovoltaic applications; Kenyatta University: Nairobi, Kenya, 2018. [Google Scholar]
- Vani, V.; Reddy, M.V.; Reddy, K. Thickness-dependent physical properties of coevaporated Cu4SnS4 Films. ISRN Condens. Matter Phys. 2013, 2013. [Google Scholar] [CrossRef]
Publisher†s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).