The Aminometylation of 4-(Alkylthio)-6-amino-2-oxo(thioxo)-1,2-dihydropyridine-3,5-dicarbonitriles †
Abstract
:1. Introduction
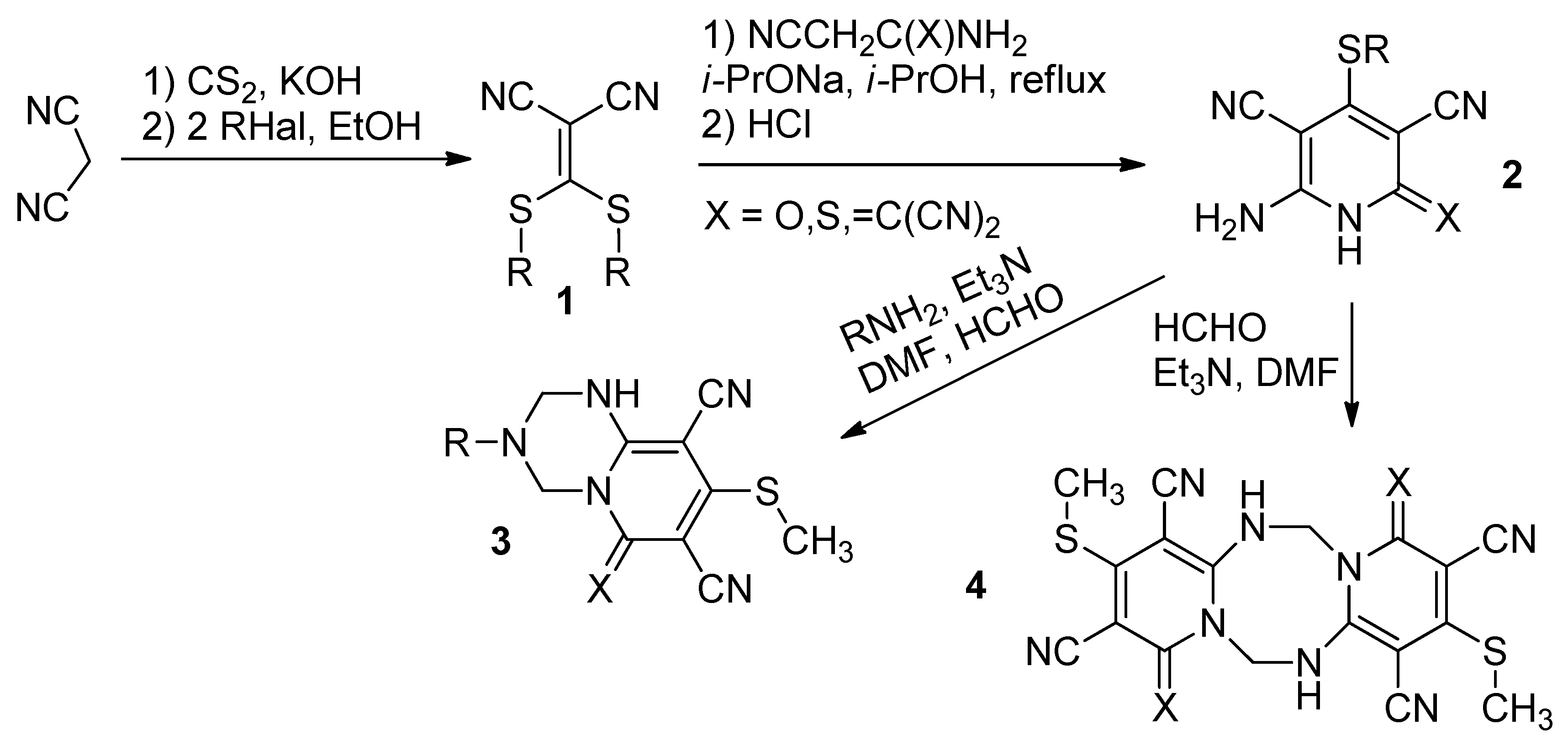
2. Results and Discussion
3. Experimental
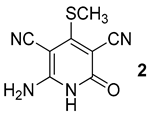
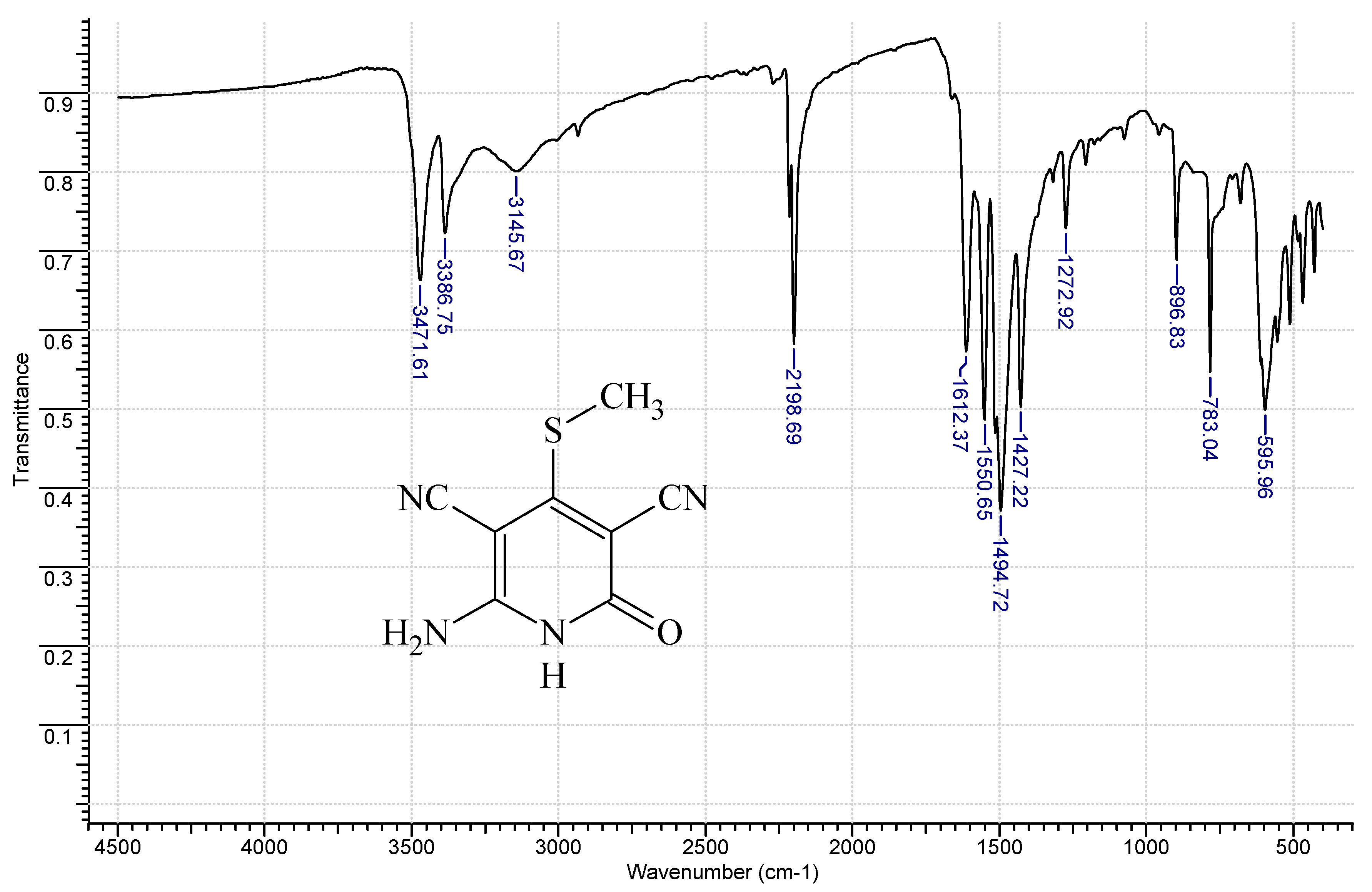
3.1. 6-Amino-4-(methylthio)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile (2)
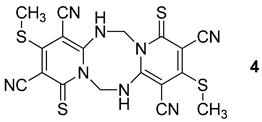
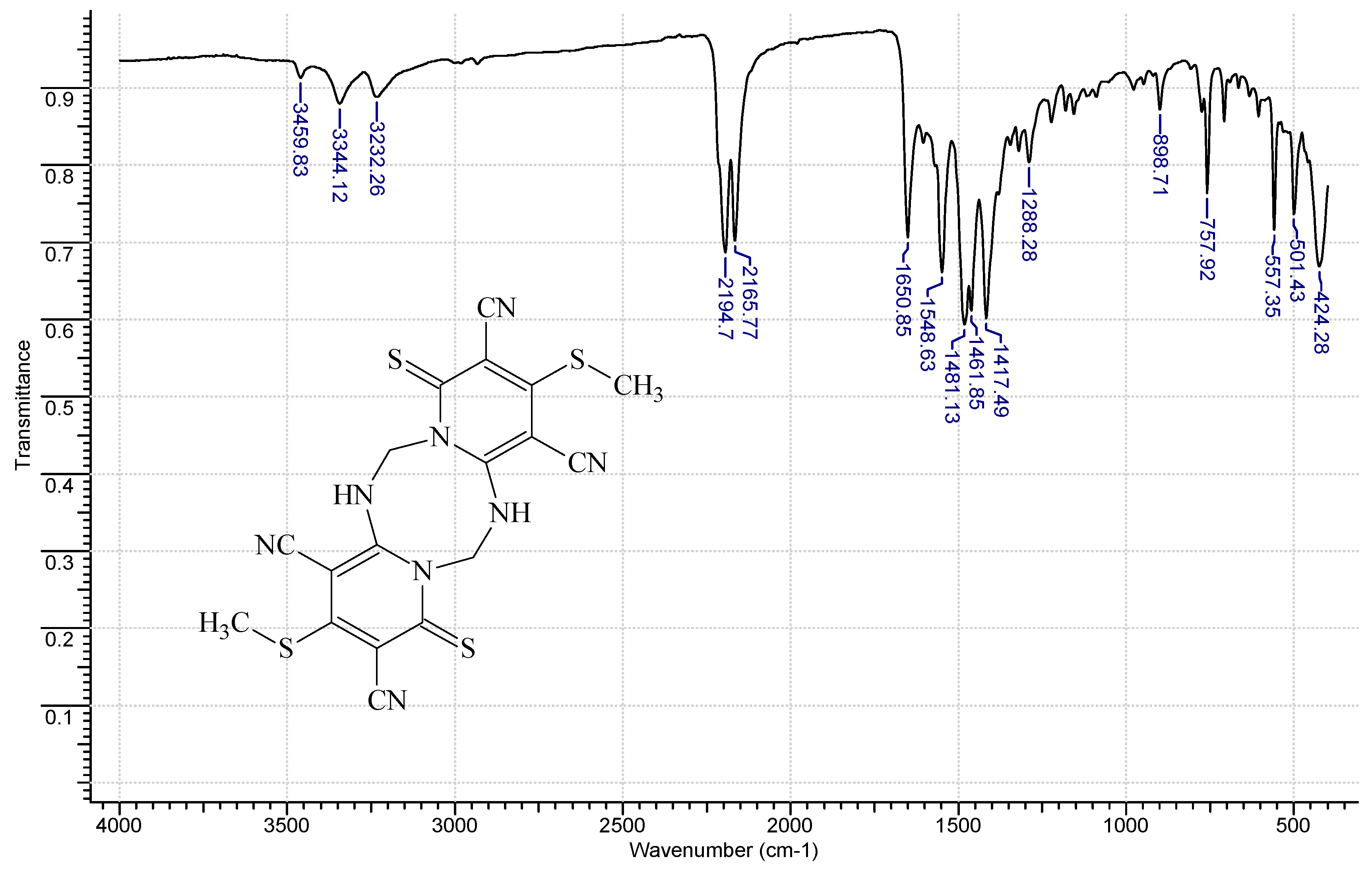
3.2. 3.,10-Bis(methylthio)-1,8-dithioxo-5,6,12,13-tetrahydro-1H,8H-dipyrido[1,2-a;1′,2′-e][1,3,5,7]tetrazocine-2,4,9,11-tetracarbonitrile (4, R = CH3, X = S)
Author Contributions
Funding
Conflicts of Interest
References
- Huang, L.; Wu, J.; Hu, J.; Bi, Y.; Huang, D. Ketene dithioacetals in organic synthesis. Tetrahedron Lett. 2020, 61, 151363. [Google Scholar] [CrossRef]
- Pan, L.; Bi, X.; Liu, Q. Recent developments of ketene dithioacetal chemistry. Chem. Soc. Rev. 2013, 42, 1251–1286. [Google Scholar] [CrossRef] [PubMed]
- Elgemeie, G.H.; Elzanate, A.M.; Elghandour, A.H.; Ahmed, S.A. Novel intramolecular cyclization of pyrazolone ketene S, N-acetals for the construction of methylsulfanylpyrazolo[4,3-b]pyridines. Synth. Commun. 2002, 32, 3509–3517. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Suikov, S.Y.; Pekhtereva, T.M.; Krivokolysko, S.G. Mannich reaction in the synthesis of N, S-containing heterocycles. 16. Synthesis of derivatives of tetrahydropyrido[1,2-a][1,3,5]triazine and 5,6,12,13-tetrahydro-1H,8H-dipyrido[1,2-a:1′,2′-e][1,3,5,7] tetrazocine. Chem. Heterocycl. Compd. 2013, 49, 1009–1023. [Google Scholar] [CrossRef]
- Khrustaleva, A.N.; Frolov, K.A.; Dotsenko, V.V.; Krivokolysko, S.G. Aminomethylation of 5-substituted 6-amino-2-oxo-1,2-dihydropyridine-3-carbonitriles. Russ. J. Org. Chem. 2014, 50, 1804–1808. [Google Scholar] [CrossRef]
- Khrustaleva, A.N.; Frolov, K.A.; Dotsenko, V.V.; Dmitrienko, A.O.; Bushmarinov, I.S.; Krivokolysko, S.G. Synthesis of Pyrido[1,2-a][1,3,5]Triazine Derivatives by Aminomethylation of 6-Amino-4-Aryl-2-Oxo-1,2-Dihydropyridine-3,5-Dicarbonitriles. Chem. Heterocycl. Compd. 2014, 50, 46–52. [Google Scholar] [CrossRef]
- Litvinov, V.P. Cyanoacetamides and their thio- and selenocarbonyl analogues as promising reagents for fine organic synthesis. Russ. Chem. Rev. 1999, 68, 737–763. [Google Scholar] [CrossRef]
- Fadda, A.A.; Bondock, S.; Rabie, R.; Etman, H.A. Cyanoacetamide derivatives as synthons in heterocyclic synthesis. Turkish J. Chem. 2008, 32, 259–286. [Google Scholar]
- Ghozlan, S.A.; Abdelmoniem, A.M.; Ramadan, M.A.; Abdelwahab, H.M.; Abdelrahman, M.G.M.; Abdelhamid, I.A. Synthesis, and synthetic applications of cyanoacetamides. Org. Chem. 2020, 297–399. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Dyachenko, I.V.; Nenajdenko, V.G. Cyanothioacetamide: A polyfunctional reagent with broad synthetic utility. Russ. Chem. Rev. 2018, 87, 1. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khrapova, E.A.; Ryzhkova, N.A.; Dotsenko, V.V.; Aksenov, N.A. The Aminometylation of 4-(Alkylthio)-6-amino-2-oxo(thioxo)-1,2-dihydropyridine-3,5-dicarbonitriles. Chem. Proc. 2021, 3, 30. https://doi.org/10.3390/ecsoc-24-08401
Khrapova EA, Ryzhkova NA, Dotsenko VV, Aksenov NA. The Aminometylation of 4-(Alkylthio)-6-amino-2-oxo(thioxo)-1,2-dihydropyridine-3,5-dicarbonitriles. Chemistry Proceedings. 2021; 3(1):30. https://doi.org/10.3390/ecsoc-24-08401
Chicago/Turabian StyleKhrapova, Ekaterina A., Natalya A. Ryzhkova, Victor V. Dotsenko, and Nicolai A. Aksenov. 2021. "The Aminometylation of 4-(Alkylthio)-6-amino-2-oxo(thioxo)-1,2-dihydropyridine-3,5-dicarbonitriles" Chemistry Proceedings 3, no. 1: 30. https://doi.org/10.3390/ecsoc-24-08401
APA StyleKhrapova, E. A., Ryzhkova, N. A., Dotsenko, V. V., & Aksenov, N. A. (2021). The Aminometylation of 4-(Alkylthio)-6-amino-2-oxo(thioxo)-1,2-dihydropyridine-3,5-dicarbonitriles. Chemistry Proceedings, 3(1), 30. https://doi.org/10.3390/ecsoc-24-08401





