Abstract
Upon treatment with chloroacetyl chloride, 3-aminothieno[2,3-b]pyridines gave the corresponding chloroacetamides. The latter readily react with sodium thiosulphate to afford water soluble S-alkylthiosulphates with pharmacophoric heterocyclic units.
1. Introduction
Bunte salts (Figure 1) are easily available and handy reagents that are useful for the introduction of sulfur-containing fragments into a molecule [1]. They are practically odorless crystalline solids that appear to be water soluble even if they contain highly lipophilic organic fragments [1]. Bunte salts are useful in organic synthesis as “surrogates of sulfur” [2,3,4], in the synthesis of metal nanoparticles [5,6,7,8,9] and as complexing agents. In addition, some of these compounds reveal interesting biological activity [10]. It is known that Bunte salts are stable in aqueous media, whereas the corresponding alkylthiosulfuric acids, RSSO3H, are usually unstable in aqueous solution [11]. A survey of the literature revealed a couple of methods to prepare Bunte salts [12,13]. The most common approach is based on the reaction of alkyl halides with readily available and cheap sodium thiosulfate [13]. Here, we propose a new approach to the synthesis of Bunte salts bearing a pharmacophoric aminothieno[2,3-b]pyridine (Figure 1) core. 3-Aminothieno[2,3-b]pyridines are known to exhibit a broad spectrum of biological activity and they have been recognized as valuable reagents for heterocyclic synthesis [14,15,16,17].
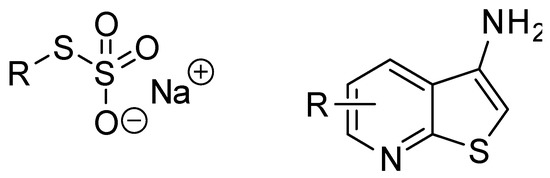
Figure 1.
Structure of Bunte salts (left) and 3-aminothieno[2,3-b]pyridines (right).
2. Results and Discussion
3-Aminothienopyridines 1 can easily be N-acylated with ClCH2C(O)Cl to give corresponding chloroacetamides 2 [18]. The yields are given in Table 1. The structure of several compounds 2 was proven by means of IR and NMR spectroscopy, including 2D NMR experiments (1H-13C HSQC, 1H-13C HMBC, 1H-15N HMBC) (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8). When chloroacetamides 2 were treated with Na2S2O3 in aq. EtOH, the corresponding Bunte salts 3 were isolated with good yields (Scheme 1). Compounds 3 are colorless solids soluble in alcohol and water, and due to their solubility, they are useful in agrochemistry and pharmacy as prospective bioactive molecules.

Table 1.
The prepared α-chloro-N-(thienopyridine-3-yl)acetamides 2.
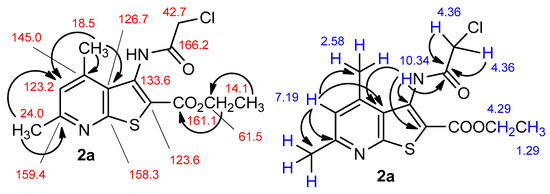
Figure 2.
Complete assignments, chemical shifts and key correlations in HSQC and HMBC 2D NMR spectra of the representative compound 2a.
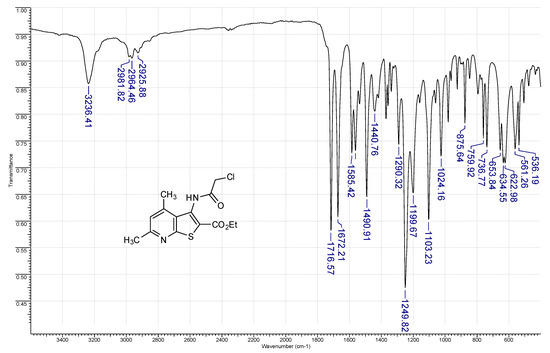
Figure 3.
FT-IR (ATR-mode) spectrum of compound 2a.
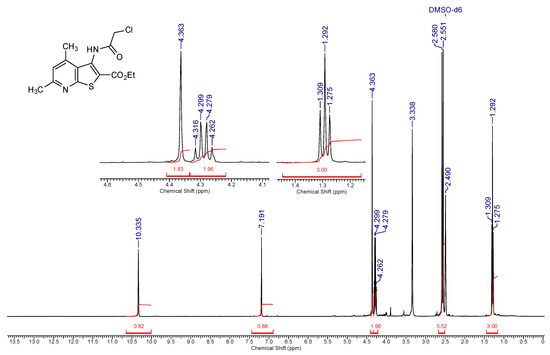
Figure 4.
1H-NMR spectrum of compound 2a (400 MHz, DMSO-d6).
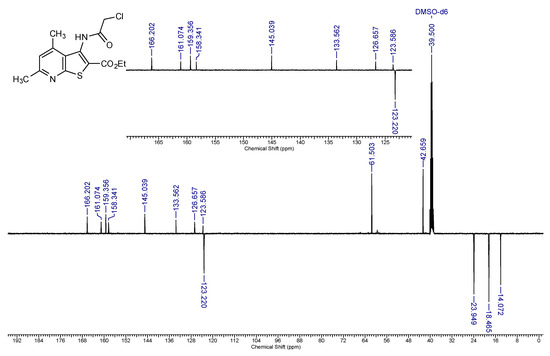
Figure 5.
13C-NMR DEPTQ spectrum of compound 2a (101 MHz, DMSO-d6).
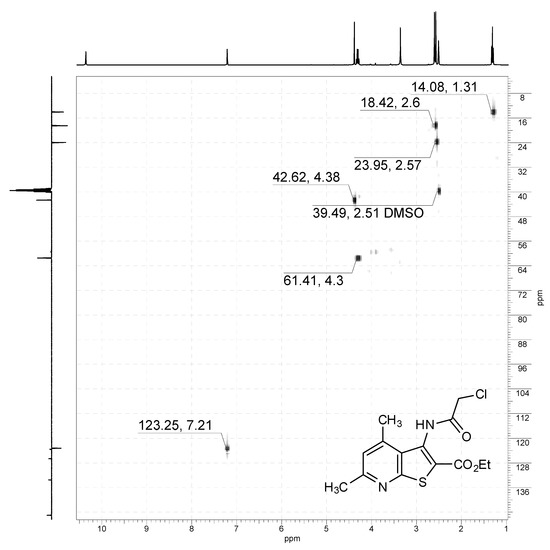
Figure 6.
1H-13C HSQC NMR spectrum of compound 2a (400/101 MHz, DMSO-d6).
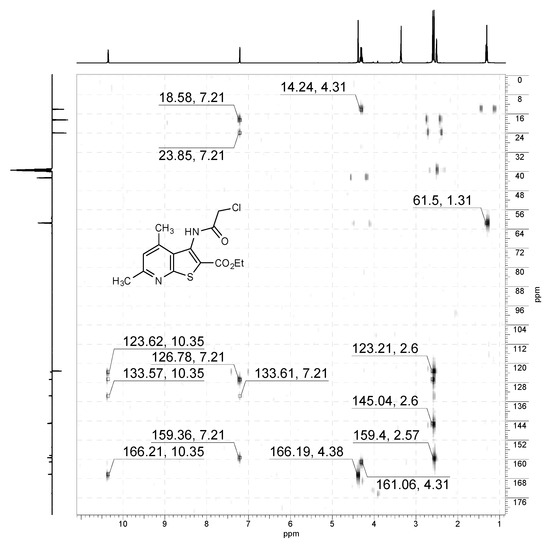
Figure 7.
1H-13C HMBC NMR spectrum of compound 2a (400/101 MHz, DMSO-d6).
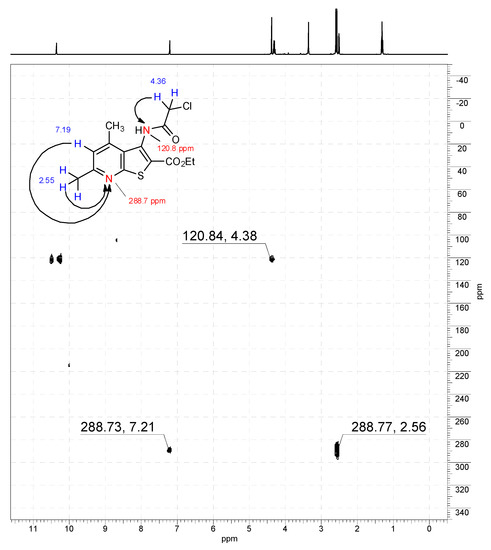
Figure 8.
1H-15N HMBC NMR spectrum of compound 11a (400/41 MHz, DMSO-d6).
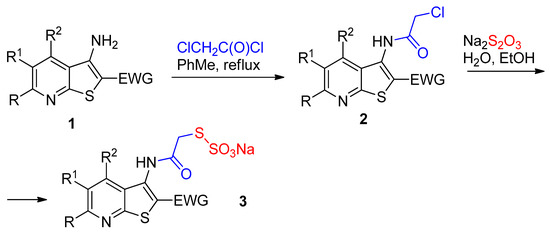
Scheme 1.
The synthetic pathway to Bunte salts 3. EWG = COOEt, CONHR, C(O)Ar, etc.
3. Experimental Methods
Solvents and starting reagents were purified according to common procedures. Melting points were determined using a Stuart SMP30 device. IR spectra were recorded on a Bruker Vertex 70 instrument in ATR (attenuated total reflection) mode. 1H, 13C DEPTQ, 1H–13C HSQC, 1H–13C HMBC, 1H–15N HSQC, 1H–15N HMBC NMR spectra were recorded on a Bruker Avance III HD spectrometer (400.17 MHz for 1H, 100.63 MHz for 13C, and 40.55 MHz for 15N) in DMSO-d6 using Me4Si (δ = 0.0 ppm) as an internal standard for 1H and 13C and nitromethane as a standard for 15N (δ = +380.23 ppm). Chemical shifts are given in parts per million (ppm), coupling constants are given in Hz, multiplicities are given as s (singlet), d (doublet), dd (doublet of doublets), m (multiplet) and br (broad). The purity of the compounds was checked by TLC (Sorbfil A plates) with hexane:AcOEt (1:1) or hexane:acetone (1:1) mixtures as eluents. The spots were visualized with iodine vapors, KMnO4–H2SO4 solution or UV-light. Chloroacetyl chloride is commercially available (ACROS).
3.1. General Procedure for the Synthesis of α-Chloro-N-(thienopyridine-3-yl)acetamides 2
A round-bottom 100 cm3 flask was charged with the corresponding 3-aminothienopyridine 1 (8–10 mmol) and dry toluene (20–40 cm3). The mixture was warmed until the starting material had dissolved, and chloroacetyl chloride (1.1 eq., 8.8–11 mmol) was added dropwise. The reaction mixture was refluxed until the evolution of HCl had ceased and the starting amine was consumed (TLC control, 3–8 h). Then toluene was evaporated in vacuo, and the crude product was recrystallized or purified by boiling with an appropriate solvent.
3.2. Ethyl 3-(2-Chloroacetamido)-4,6-dimethylthieno[2,3-b]pyridine-2-carboxylate (2a) (EWG = COOEt, R = R2 = Me, R1 = H)
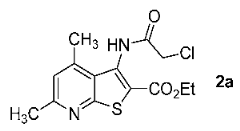
Off-white solid, soluble in hot EtOH, yield 93%, m.p. 210 °C; FTIR (ATR): ν = 3236 (N–H), 1716 (C=Oester), 1672 (C=Oamide) cm−1; 1H-NMR (400 MHz, DMSO-d6): δ 1.29 (t, 3H, 3J = 6.9 Hz, OCH2CH3), 2.55 (s, 3H, C(6)CH3), 2.58 (s, 3H, C(4)CH3), 4.29 (q, 2H, 3J = 6.9 Hz, OCH2CH3), 4.36 (s, 2H, CH2Cl), 7.19 (s, 1H, H-5), 10.34 (s, 1H, CONH) ppm; 13C DEPTQ NMR (101 MHz, DMSO-d6): δ 14.1* (CH2CH3), 18.5* (C(4)CH3), 24.0* (C(6)CH3), 42.7 (CH2Cl), 61.5 (OCH2), 123.2* (C-5), 123.6 (C-2), 126.7 (C-3a), 133.6 (C-3), 145.0 (C-4), 158.3 (C-7a), 159.4 (C-6), 161.1 (C=Oester), 166.2 (C=Oamide) ppm. *Anti-phase signals. 15N NMR (41 MHz, DMSO-d6): δ 120.8 (NH), 288.7 (N-7) ppm.
3.3. Preparation of Compound 3a (EWG = COOEt, R = R2 = Me, R1 = H)
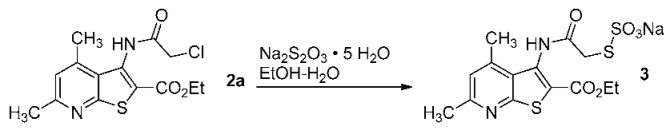
The starting ethyl 3-(chloroacetamido)-4,6-dimethylthieno[2,3-b]pyridine-2-carboxylate (1) (0.01 mol) was dissolved in hot EtOH (20 mL), and a solution of an excess sodium thiosulfate (0.02 mol) in water (10 mL) was added. Then, the reaction mixture was boiled under reflux for 5 h (checked for completion by TLC). To isolate the product, the solvent was distilled off, resulting in the precipitation of a white crystalline solid. The resulting crude product 3 was filtered off and washed with a small amount of acetone to give pure Bunte salt 3 as colorless crystals; the yield was 30%. The compound is readily soluble in water and aq. EtOH, and slightly soluble in acetone and diethyl ether. The IR spectrum of compound 3a is given below (Figure 9).
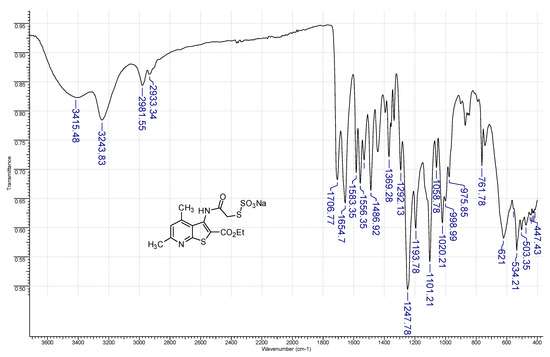
Figure 9.
FT-IR (ATR-mode) spectrum of compound 3a.
Funding
The reported study was funded by RFBR and Krasnodar region according to the research project № 19-43-230007.
References
- Jiang, X.; Li, Y.; Wang, M. Recent Advances in Sulfuration Chemistry Enabled by Bunte Salts. Aldrichimica Acta J. 2020, 53, 19–25. [Google Scholar]
- Westlake, H.E.; Dougherty, G. The Use of Bunte Salts in Synthesis. I. The Preparation of Mercaptals. J. Am. Chem. Soc. 1941, 63, 658–659. [Google Scholar] [CrossRef]
- Reeves, J.T.; Camara, K.; Han, Z.S.; Xu, Y.; Lee, H.; Busacca, C.A.; Senanayake, C.H. The Reaction of Grignard Reagents with Bunte Salts: A Thiol-Free Synthesis of Sulfides. Org. Lett. 2014, 16, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Lu, G.P.; Cai, C.; Yi, W.B. An odorless thia-Michael addition using Bunte salts as thiol surrogates. RSC Adv. 2015, 5, 27107–27111. [Google Scholar] [CrossRef]
- Lukkari, J.; Meretoja, M.; Kartio, I.; Laajalehto, K.; Rajamäki, M.; Lindström, M.; Kankare, J. Organic Thiosulfates (Bunte Salts): Novel Surface-Active Sulfur Compounds for the Preparation of Self-Assembled Monolayers on Gold. Langmuir 1999, 15, 3529–3537. [Google Scholar] [CrossRef]
- Shon, Y.; Cutler, E. Aqueous Synthesis of Alkanethiolate-Protected Ag Nanoparticles Using Bunte Salts. Langmuir 2004, 20, 6626–6630. [Google Scholar] [CrossRef] [PubMed]
- Lohse, S.E.; Dahl, J.A.; Hutchison, J.E. Direct Synthesis of Large Water-Soluble Functionalized Gold Nanoparticles Using Bunte Salts as Ligand Precursors. Langmuir 2010, 26, 7504–7511. [Google Scholar] [CrossRef] [PubMed]
- Shon, Y.S.; Gross, S.M.; Dawson, B.; Porter, M.; Murray, R.W. Alkanethiolate-Protected Gold Clusters Generated from Sodium S-Dodecylthiosulfate (Bunte Salts). Langmuir 2000, 16, 6555–6561. [Google Scholar] [CrossRef]
- Shon, Y.S.; Wuelfing, W.P.; Murray, R.W. Water-Soluble, Sulfonic Acid-Functionalized, Monolayer-Protected Nanoparticles and an Ionically Conductive Molten Salt Containing Them. Langmuir 2001, 17, 1255–1261. [Google Scholar] [CrossRef]
- Stefańska, J.Z.; Starościak, B.J.; Orzeszko, A.; Kazimierczuk, Z. Antimicrobial activity of organic thiosulfates (Bunte salts). Die Pharm. 1998, 53, 190–192. [Google Scholar]
- Biswas, K.; Basu, B. Bunte Salts and Congeners as Efficient Sulfur Surrogates: Recent Advances. Curr. Organocatalysis 2018, 5, 182–195. [Google Scholar] [CrossRef]
- Bunte, H. Zur Constitution der unterschwefligen Säure. Ber. Deutsch. Chem. Ges. 1874, 7, 646–648. [Google Scholar] [CrossRef]
- Distler, H. The chemistry of Bunte salts. Angew. Chem. Int. Ed. 1967, 6, 544–553. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Dotsenko, V.V.; Krivokolysko, S.G. Thienopyridines: Synthesis, properties, and biological activity. Russ. Chem. Bull. 2005, 54, 864–904. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Dotsenko, V.V.; Krivokolysko, S.G. Chemistry of Thienopyridines and Related Systems; Russian Academy of Sciences: Moscow, Russia, 2006. (In Russian) [Google Scholar]
- Litvinov, V.P.; Dotsenko, V.V.; Krivokolysko, S.G. The chemistry of thienopyridines. Adv. Heterocycl. Chem. 2007, 93, 117. [Google Scholar]
- Dotsenko, V.V.; Buryi, D.S.; Lukina, D.Y.; Krivokolysko, S.G. Recent advances in the chemistry of thieno[2,3-b]pyridines 1. Methods of synthesis of thieno[2,3-b]pyridines. Russ. Chem. Bull. 2020, 69, 1829–1858. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Buryi, D.S.; Lukina, D.Y.; Stolyarova, A.N.; Aksenov, N.A.; Aksenova, I.V.; Strelkov, V.D.; Dyadyuchenko, L.V. Dyadyuchenko, Substituted N-(thieno[2,3-b]pyridine-3-yl)acetamides: Synthesis, reactions, and biological activity. Mon. Für Chem. 2019, 150, 1973–1985. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).