Abstract
The high concentration of pesticide residues existing in vegetation, crops, and various edible products and the prolonged exposure to them can harm human life and contribute to the disappearance of honey bees, and several avian and animal species. The honey bees (Apis mellifera), which are efficient pollinators in addition to honey producers, are also considered important non-target test species for the terrestrial toxicity assessment of chemicals. In this context, using thiacloprid and acetamiprid as queries, we performed a 3D similarity search to select new potential products with less harmful effects against bees. For a similarity search, a small dataset of 302 compounds with pesticide activity, compiled from the literature, was used. The first 10 compounds were selected and structurally analyzed according to the TanimotoCombo metrics, and compared with each of these two queries, which is known to be effective, easily metabolized, and less toxic for bees. This approach came as a forward step in the research of pesticide ecotoxicological risk assessment for the evaluation of their potential impact on the pollinator insects and the environment.
1. Introduction
Neonicotinoids are the most commonly used insecticides for pest control. The main problems associated with the use of these insecticides, alone or in combination with other factors, are related to their negative impact against many species of insects including bees. Additionally, the application mode of insecticides (e.g., direct spray, soil and seed application, etc.) plays a key role in their impact against pollinators. Honeybees are considered the most successful and commercially valuable pollinators due to their pollination functions, maintenance of biodiversity in natural ecosystems as well as the commercial products delivered such as honey, propolis, etc. This negative neonicotinoid impact on bees has been extremely studied because the effect at different doses is still not fully understood [1]. Honeybees can be envisaged as very vulnerable to pesticides because their genome has fewer genes compared to other insects [2]. The exposure to neonicotinoids can influence the flying and the foraging ability, reproduction, and pollination for many useful insects including honeybees [3]. In particular, they affect bees by forgetting the locations of flowers or even hives, and also by increasing the fertility of queens or bumblebees [3]. In this context, the strategy of designing new neonicotinoids by modifying existing structures may be an effective way to overcome this harmful influence against pollinators.
Of the eight marketed neonicotinoids (https://www.reportbuyer.com/product/3952801/ (accessed on 26 July 2020)), thiacloprid and acetamiprid are considered to be less toxic for honeybees. These neonicotinoids are considered a group of neurotoxins, chemically similar to nicotine, which acts specifically as nicotinic acetylcholine receptor (nAChR) antagonists [4].
Theoretical methods (QSAR, linear and nonlinear regression techniques, molecular docking, 2D and 3D similarity search, etc.) [5,6,7] applied in cheminformatics to discover new drugs have also been successfully employed to predict novel insecticides and pesticides with less polluting and toxic effects to fill data gaps and to reduce toxicity testing on animals. In the current work, thiacloprids and acetamiprids were used as template molecules in the 3D similarity analysis accomplished with ROCS (Rapid Overlay of Chemical Structures) [8,9] from the OpenEye package. The main goal is to find novel compounds, similar to template molecules, which are easy to metabolize and less toxic for bees.
2. Methods
From the literature [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25], a dataset of 302 compounds with known pesticide activity was selected and used further for a 3D-similarity search. The conformational space of selected compounds was carried out with the Omega tool, OpenEye (OMEGA v.2.5.1.4, OpenEye Scientific Software, Santa Fe, NM, USA. www.eyesopen.com (accessed on 26 July 2020)) [26]. A maximum of 200 conformers for each compound was generated using default options.
The lowest energy conformers for thiacloprid and acetamiprid were also generated with Omega. The BIOVIA Discovery Studio facilities were used for structure visualization and picture delivery (Figure 1).
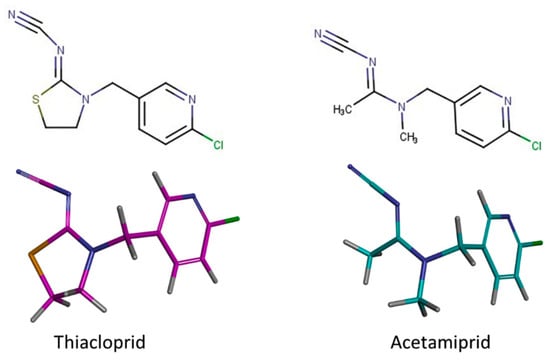
Figure 1.
The 2D and 3D structure of the query compounds.
A 3D similarity search was performed with ROCS (ROCS v. 3.2.1.4, OpenEye Scientific Software, Santa Fe, NM, USA [8,9]. ROCS, a shape comparison application, is faster and more useful in handling the large conformer databases. This tool offers alignment and scoring of a database engaging thirteen similarity coefficients. The resulted aligned molecules are ordered by a TanimotoCombo ranking score (as the default option).
3. Results and Discussion
A 3D overlay with ROCS has a great advantage as it allows for optimal visualization of overlapping compounds, which leads to a better understanding of their similarity. The ROCS principles are based on the Gaussian function, which is widely used to represent shape and molecular volume.
In this light, several highly occupied regions corresponding to the pyridine ring and a yliden-cyanamide group of both template molecules were identified (Figure 2). These regions appear to have multiple hydrogen binding abilities. The pyridine ring of thiacloprids and acetamiprids and the thiazolidine ring of thiacloprid may be involved in п–п or п–σ hydrophobic bonds. As can be seen in Figure 2, the compounds prioritized by ROCS follow the same trend as the query compounds. This trend is in line with the high ShapeTanimoto similarity values (Figure 3), and implicitly the shapes (Figure 2) displayed by all the prioritized compounds.

Figure 2.
Molecular shapes of queries molecules. The Rapid Overlay of Chemical Structures (ROCS) overlapping of the top compounds ranked by TanimotoCombo against thiacloprid (a) and acetamiprid (b); the surface around queries is rendered.
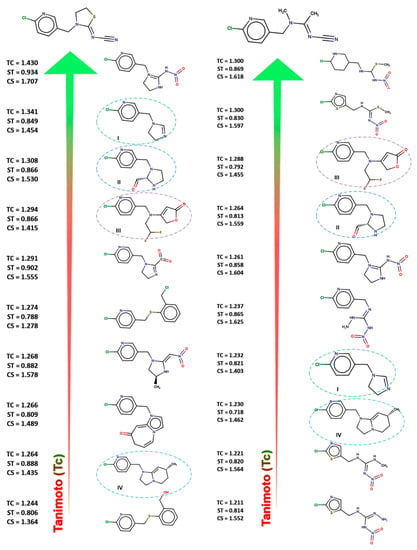
Figure 3.
The chemical structures of the top ten compounds ranked by TanimotoCombo; against thiacloprid (left) and acetamiprid (right).
As can be observed from Figure 3, the 3D coefficients calculated with ROCS for all ten prioritized compounds against each of the two queries showed values greater than 1.2 for TanimotoCombo, greater than 0.8 for ShapeTanimoto, and greater than 1.2 for ComboScore [27,28]. These high values indicate a very good similarity between the selected compounds and acetamiprid and thiacloprid, respectively. Four out of ten prioritized ROCS compounds, highlighted with circles in Figure 3, were considered to have a good profile through a comparison with each of the two queries.
2-Chloro-5-(4,5-dihydroimidazol-1-ylmethyl)pyridine, I,(green circles) was the second compound prioritized by thiacloprid and the seventh by acetamiprid. (2S)-1-[(6-Chloropyridin-3-yl)methyl]imidazolidine-2-carbaldehyde, II, (blue circles) was the third compound prioritized in accordance with thiacloprid, and the fourth by acetamiprid. 4-{[(6-Chloropyridin-3-yl)methyl](2,2-difluoroethyl)amino}-5H-furan-2-one, III, (purple circles) was the fourth compound prioritized toward thiacloprid and the third toward acetamiprid. 2-Chloro-5-{[(7R)-7-methyl-2H,3H,5H,6H,7H-imidazo [1,2-a]pyridin-1-yl]methyl}pyridine, IV, (cyan circles) was the third compound prioritized by thiacloprid and the eighth by acetamiprid. Based on the good qualities demonstrated by ROCS analysis, these four compounds will be subjected, in further studies, to molecular docking and molecular dynamic simulation.
The computed pharmacokinetic proprieties of the selected four compounds and the two queries are listed in Table 1. These were performed with a freely accessible web server pkCSM (http://biosig.unimelb.edu.au/pkcsm/ (accessed on 28 September 2020)). The pkCSM program affords a fast and easy method to the early assessment of compounds [29]. Regarding the CNS (central nervous system) permeability, it could be observed that all four selected compounds showed logPS values lower than −3, being considered unable to penetrate the CNS of insects.

Table 1.
Pharmacokinetic properties of thiacloprid, acetamiprid, and the selected compounds *.
4. Conclusions
In this study, thiacloprid and acetamiprid were used as queries in order to find new potential compounds with less harmful effects against bees. Four compounds (2-chloro-5-(4,5-dihydroimidazol-1-ylmethyl)pyridine, (2S)-1-[(6-chloropyridin-3-yl)methyl]imidazolidine-2-carbaldehyde, 4-{[(6-chloropyridin-3-yl)methyl](2,2-difluoroethyl)amino}-5H-furan-2-one, and 2-chloro-5-{[(7R)-7-methyl-2H,3H,5H,6H,7H-imidazo[1,2-a]pyridin-1-yl]methyl}pyridine) were selected as similar in shape and volume to both queries, which are known for their reduced toxic effect against bees [30,31]. This approach is a first attempt to find novel compounds with an enhanced safety profile against pollinator insects and the environment.
Author Contributions
L.C. and A.B. (Alina Bora) conceived of the presented idea, designed, and accomplished the computational framework including editing; A.B. (Ana Borota) performed some computational determinations; S.F.-T. selected and provided the pesticide dataset. All authors contributed to the writing of the paper and approved the content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank ChemAxon Ltd., OpenEye Ltd., and BIOVIA software Inc. (Discovery Studio Visualizer) for providing an academic license. The authors wish to thank Schrödinger Inc. for providing an academic trial license to complete the calculations for this paper. Project No 1.1 and No. 1.2 of the “Coriolan Dragulescu” Institute of Chemistry, Timisoara, financially supported the current work.
Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- Hassani, A.K.; Dacher, M.; Gary, V.; Lambin, M.; Gauthier, M.; Armengaud, C. Effects of Sublethal Doses of Acetamiprid and Thiamethoxam on the Behavior of the Honeybee (Apis mellifera). Arch. Environ. Contam. Toxicol. 2008, 54, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Claudianos, C.; Ranson, H.; Johnson, R.M.; Biswas, S.; Schuler, M.A.; Berenbaum, M.R.; Feyereisen, R.; Oakeshott, J.G. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 2006, 15, 615–636. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Bukowska, M.; Ligor, M.; Staneczko-Baranowska, I. A holistic study of neonicotinoids neuroactive insecticides—Properties, applications, occurrence, and analysis. Environ. Sci. Pollut. Res. 2019, 26, 34723–34740. [Google Scholar] [CrossRef]
- Blacquière, T.; Smagghe, G.; van Gestel, C.A.M.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef] [PubMed]
- Petric, M.; Crisan, L.; Crisan, M.; Micle, A.; Maranescu, B.; Ilia, G. Synthesis and QSRR Study for a Series of Phosphoramidic Acid Derivatives. Heteroatom. Chem. 2013, 24, 138–145. [Google Scholar] [CrossRef]
- Goodarzi, M.; Bora, A.; Borota, A.; Funar-Timofei, S.; Avram, S.; Heyden, Y.V. Modeling of 2-pyridin-3-yl-benzo[d][1,3]oxazin-4-one derivatives by several conformational searching tools and molecular docking. Curr. Pharm Des. 2013, 19, 2194–2203. [Google Scholar] [CrossRef]
- Funar-Timofei, S.; Borota, A.; Crisan, L. Combined molecular docking and QSAR study of fused heterocyclic herbicide inhibitors of D1 protein in photosystem II of plants. Mol. Divers. 2017, 21, 437–454. [Google Scholar] [CrossRef]
- Hawkins, P.C.D.; Skillman, A.G.; Nicholls, A. Comparison of Shape-Matching and Docking as Virtual Screening Tools. J. Med. Chem. 2007, 50, 74–82. [Google Scholar] [CrossRef]
- Rush, T.S.; Grant, J.A.; Mosyak, L.; Nicholls, A. A shape-based 3-D scaffold hopping method and its application to a bacterial protein—Protein interaction. J. Med. Chem. 2005, 48, 1489–1495. [Google Scholar] [CrossRef]
- Hou, S.; Zhuang, Y.; Deng, Y.; Xu, X. Photostability study of cis-configuration neonicotinoid insecticide cycloxaprid in water. J. Environ. Sci. Health B 2017, 52, 525–537. [Google Scholar] [CrossRef]
- Zou, M.; Tian, X.; Chen, N.; Shao, X. Nematicidal Activity of Sprio and Bridged Heterocyclic Neonicotinoid Analogues against Meloidogyne incognita. Lett. Drug Des. Discov. 2015, 12, 439–445. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, J.; Xu, X.; Wang, H.; Wang, W.; Ye, Q.; Li, Z. Diastereoselective Metabolism of a Novel Cis-Nitromethylene Neonicotinoid Paichongding in Aerobic Soils. Environ. Sci. Technol. 2013, 47, 10389–10396. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, X.; Zhang, Q.; Fan, J.; Liu, L.; Liu, M.; Zhang, H.; Li, J.; Guo, Y. Iodine-mediated oxidative cyclization for one pot synthesis of new 8- hydroxyquinaldine derivatives containing a N-phenylpyrazole moiety as pesticidal agents. Bioorg. Med. Chem. Lett. 2018, 28, 3376–3380. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Bao, Y.; Ma, Q.; Xu, H. Synthesis and biological activities of novel pyrazolomatrine derivatives. Bioorg. Med. Chem. Lett. 2018, 28, 3338–3341. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Gu, H.; Bao, N.; Dai, H. Design, Synthesis, and Biological Activities of Novel Pyrazole Oxime Compounds Containing a Substituted Pyridyl Moiety. Molecules 2017, 22, 878. [Google Scholar] [CrossRef]
- Jeanmart, S.; Edmunds, A.J.F.; Lamberth, C.; Pouliot, M. Synthetic approaches to the 2010–2014 new agrochemicals. Bioorg. Med. Chem. 2016, 24, 317–341. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, Y.; Li, W.; Li, Q.; Luo, P.; Ye, Q. Nonstereoselective foliar absorption and translocation of cycloxaprid, a novel chiral neonicotinoid, in Chinese cabbage. Environ. Pollut. 2019, 252, 1593–1598. [Google Scholar] [CrossRef]
- Sun, C.W.; Wang, J.; Wu, Y.; Nan, S.B.; Zhang, W.G. Novel nitenpyram analogues with tetrahydropyridone fixed cis-configuration: Synthesis, insecticidal activities, and molecular docking studies. Heterocycles 2013, 87, 1865–1880. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Kuriyama, M.; Nagaoka, H.; Kato, A.; Akamatsu, M.; Yamauchi, S.; Shuto, Y. Synthesis of imidacloprid derivatives with a chiral alkylated imidazolidine ring and evaluation of their insecticidal activity and affinity to the nicotinic acetylcholine receptor. Bioorg. Med. Chem. 2012, 20, 6305–6312. [Google Scholar] [CrossRef]
- Jiang, D.; Zheng, X.; Shao, G.; Ling, Z.; Xu, H. Discovery of a Novel Series of Phenyl Pyrazole Inner Salts Based on Fipronil as Potential Dual-Target Insecticides. J. Agric. Food Chem. 2014, 62, 3577–3583. [Google Scholar] [CrossRef]
- Liu, S.H.; Peng, W.; Qu, Y.Y.; Xu, D.; Li, H.Y.; Song, D.L.; Duan, H.X.; Yang, X.L. Synthesis, insecticidal activity and molecular docking study of clothianidin analogues with hydrazide group. Chin. Chem. Lett. 2014, 25, 1017–1020. [Google Scholar] [CrossRef]
- Hua, X.; Mao, W.; Fan, Z.; Ji, X.; Li, F.; Zong, G.; Song, H.; Li, J.; Zhou, L.; Zhou, L.; et al. Novel Anthranilic Diamide Insecticides: Design, Synthesis, and Insecticidal Evaluation. Aust. J. Chem. 2014, 67, 1491–1503. [Google Scholar] [CrossRef]
- Shen, H.F.; Chen, X.; Liao, P.; Shao, X.S.; Li, Z.; Xu, X.Y. Design, synthesis, and insecticidal bioactivities evaluation of pyrrole- and dihydropyrrole-fused neonicotinoid analogs containing chlorothiazole ring. Chin. Chem. Lett. 2015, 3245, 1–4. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Hua, X.; Wan, Y.; Wei, W.; Song, H.; Yu, S.; Zhang, X.; Li, Z. Design, synthesis, antifungal activities and SARs of (R)-2-Aryl-4,5-dihydrothiazole-4-carboxylic acid derivatives. Chin. J. Chem. 2015, 33, 1269–1275. [Google Scholar] [CrossRef]
- He, Y.; Hu, D.; Lv, M.; Jin, L.; Wu, J.; Zeng, S.; Yang, S.; Song, B. Synthesis, insecticidal, and antibacterial activities of novel neonicotinoid analogs with dihydropyridine. Chem. Cent. J. 2013, 7, 76. [Google Scholar] [CrossRef]
- Hawkins, P.C.D.; Nicholls, A. Conformer generation with OMEGA: Learning from the data set and the analysis of failures. J. Chem. Inf. Model. 2012, 52, 2919–2936. [Google Scholar] [CrossRef]
- Muchmore, S.W.; Debe, D.A.; Metz, J.T.; Brown, S.P.; Martin, Y.C.; Hajduk, P.J. Application of Belief Theory to Similarity Data Fusion for Use in Analog Searching and Lead Hopping J. Chem. Inf. Model. 2008, 48, 941–948. [Google Scholar] [CrossRef]
- Bortolato, A.; Perruccio, F.; Moro, S. Successful Applications of In Silico Approaches for Lead/drug Discovery; Miteva, M.A., Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2011. [Google Scholar]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Laurino, D.; Porporato, M.; Patetta, A.; Manino, A. Toxicity of neonicotinoid insecticides to honey bees: Laboratory tests. Bull. Insectol. 2011, 64, 107–113. [Google Scholar]
- Brandt, A.; Hohnheiser, B.; Sgolastra, F.; Bosch, J.; Meixner, M.D.; Büchler, R. Immunosuppression response to the neonicotinoid insecticide thiacloprid in females and males of the red mason bee Osmia bicornis L. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).