New Reactions of 5-Amino-3-(Cyanomethyl)-1H-Pyrazole-4-Carbonitrile †
Abstract
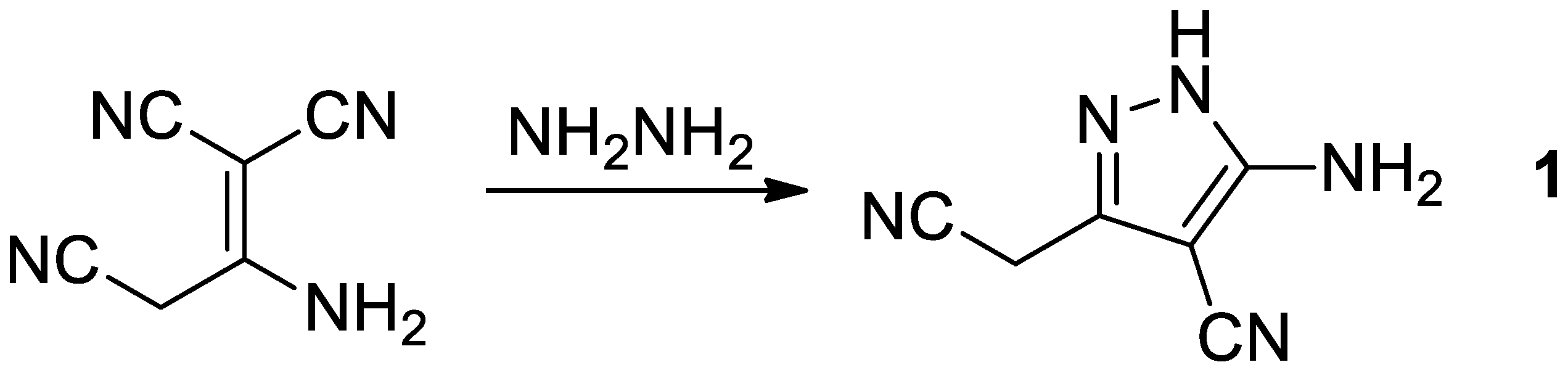
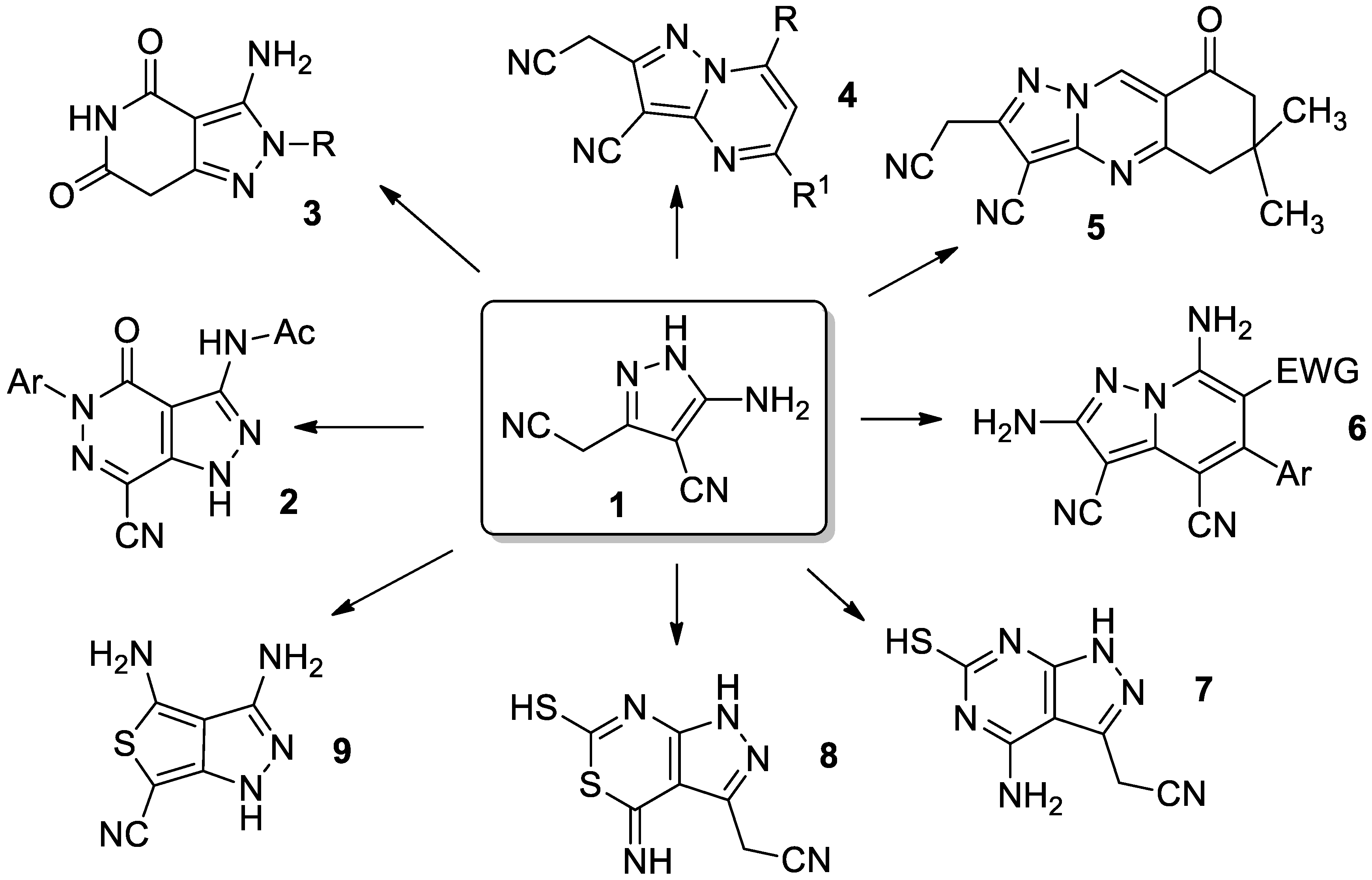
:1. Introduction
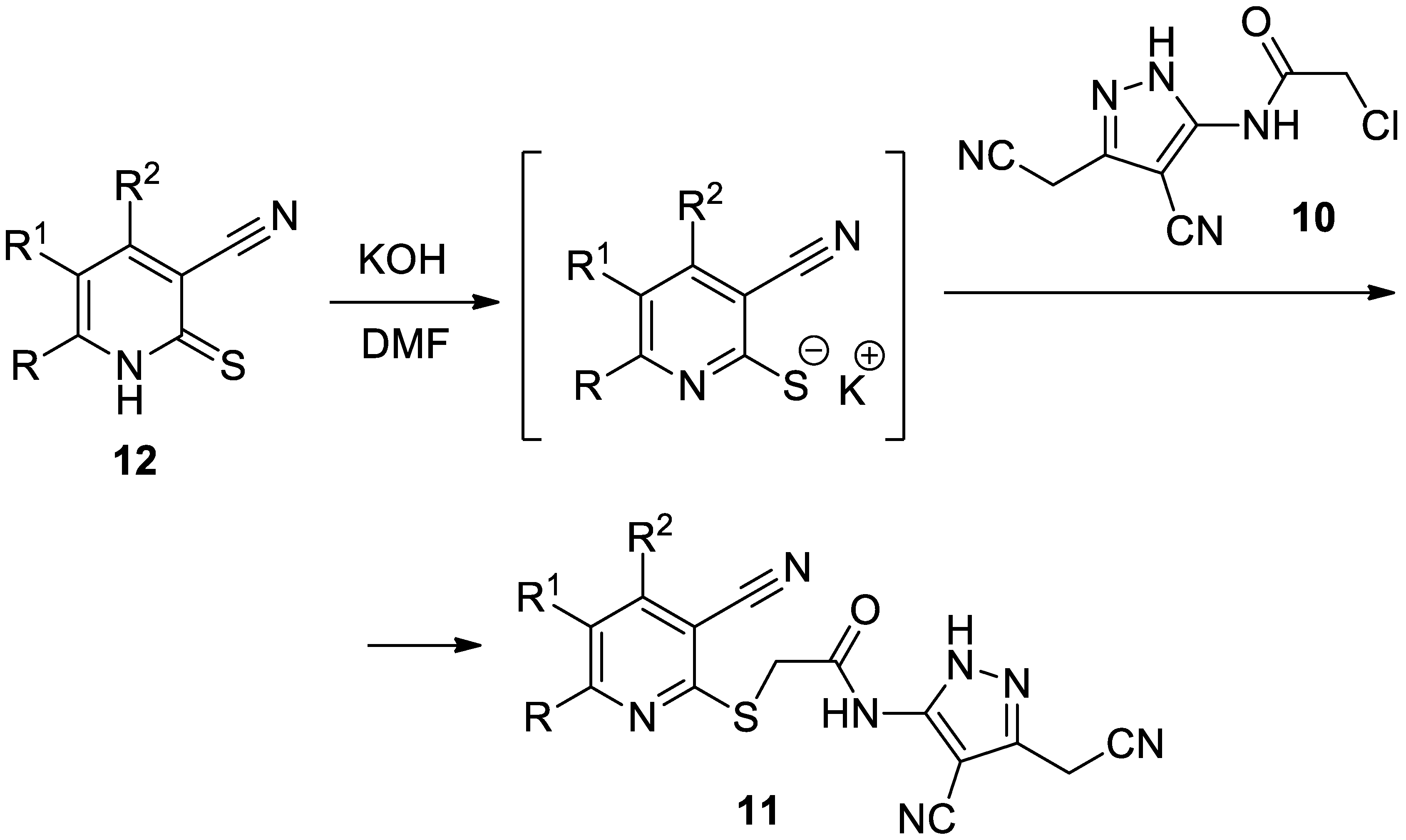
2. Results and Discussion
3. Experimental
3.1. Preparation of 2-Chloro-N-(4-Cyano-3-(Cyanomethyl)-1H-Pyrazol-5-yl)Acetamide 10
3.2. Preparation of Compound 11
Funding
References
- Aggarwal, R.; Kumar, S. 5-Aminopyrazole as precursor in design and synthesis of fused pyrazoloazines. Beilstein J. Org. Chem. 2018, 14, 203–242. [Google Scholar] [CrossRef] [PubMed]
- Abu Elmaati, T.M.; El-Taweel, F.M. New trends in the chemistry of 5-aminopyrazoles. J. Heterocycl. Chem. 2004, 41, 109–134. [Google Scholar] [CrossRef]
- Shaabani, A.; Nazeri, M.T.; Afshari, R. 5-Amino-pyrazoles: Potent reagents in organic and medicinal synthesis. Mol. Divers. 2019, 23, 751–807. [Google Scholar] [CrossRef] [PubMed]
- Anwar, H.F.; Elnagdi, M.H. Recent developments in aminopyrazole chemistry. ARKIVOC 2009, 198–250. [Google Scholar] [CrossRef]
- Taylor, E.C.; Hartke, K.S. The Reaction of Malononitrile with Hydrazine. J. Am. Chem. Soc. 1959, 81, 2452–2455. [Google Scholar] [CrossRef]
- Elkholy, A.; Al-Qalaf, F.; Elnagdi, M.H. Regio-orientation in condensation of aminopyrazoles with 1, 3-difunctional reagents: Synthesis of new pyrazolo[1,5-a]pyrimidines; pyrazolo[3,4-d]pyridazines and 2, 4-dihydropyrano[2,3-c]pyrazoles. Arkivoc 2008, 14, 124–131. [Google Scholar] [CrossRef]
- Kankanala, J.; Marchand, C.; Abdelmalak, M.; Aihara, H.; Pommier, Y.; Wang, Z. Isoquinoline-1, 3-diones as selective inhibitors of tyrosyl DNA phosphodiesterase II (TDP2). J. Med. Chem. 2016, 59, 2734–2746. [Google Scholar] [CrossRef] [PubMed]
- Metwally, N.H.; Abdallah, M.A.; Almabrook, S.A. Pyrazolo[1,5-a]pyrimidine derivative as precursor for some novel pyrazolo[1,5-a]pyrimidines and tetraheterocyclic compounds. J. Heterocycl. Chem. 2017, 54, 347–354. [Google Scholar] [CrossRef]
- Ragab, E.A.; Metwally, N.H.; Mohamed, M.S. Synthesis of some novel pyrazolo[1,5-a]quinazolines and their fused derivatives. Synth. Commun. 2017, 47, 148–158. [Google Scholar] [CrossRef]
- Naik, N.S.; Shastri, L.A.; Shastri, S.L.; Chougala, B.M.; Shaikh, F.; Madar, J.M.; Kulkarni, R.C.; Dodamani, S.; Jalalpure, S.; Joshi, S.D.; et al. Synthesis of polyfunctionalized fused pyrazolo-pyridines: Characterization, anticancer activity, protein binding and molecular docking studies. ChemistrySelect 2019, 4, 285–297. [Google Scholar] [CrossRef]
- Abdelmoniem, A.M.; Ghozlan, S.A.; Abdelmoniem, D.M.; Elwahy, A.H.; Abdelhamid, I.A. 3-Amino-5-cyanomethylpyrazole-4-carbonitrile: Versatile reagent for novel bis (pyrazolo[1,5-a]pyridine)derivatives via a multicomponent reaction. J. Heterocycl. Chem. 2018, 55, 2792–2798. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; El-Moghayar, M.R.; Fleita, D.H.; Hafez, E.A.; Fahmy, S.M. Pyrimidine derivatives and related compounds. 4. A route for the synthesis of pyrazolo[3,4-e]-as-triazines, pyrazolo[3,4-d]pyrimidines, and pyrazolo[1,5-c]-as-triazines. J. Org. Chem. 1976, 41, 3781–3784. [Google Scholar] [CrossRef] [PubMed]
- Bulychev, Y.N.; Korbukh, I.A.; Preobrazhenskaya, M.N. Synthesis of derivatives of pyrazolo[3, 4-d]pyrimidin-3-ylacetic acid and their nucleosides. Chem. Heterocycl. Compd. 1981, 17, 392–400. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; Erian, A.W. Studies on alkyl-substituted, heteroaromatic carbonitriles: Novel synthesis of thienoazines and benzoazines. Liebigs Ann. Chem. 1990, 1990, 1215–1219. [Google Scholar] [CrossRef]
- Semenova, A.M.; Oganesyan, R.V.; Dotsenko, V.V.; Chigorina, E.A.; Aksenov, N.A.; Aksenova, I.V.; Netrebae, E.E. Reaction of 5-Amino-3-(cyanomethyl)-1H-pyrazole-4-carbonitrile with Hydroxycyclohexanones. Russ. J. Gen. Chem. 2019, 89, 19–24. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Ismiev, A.I.; Khrustaleva, A.N.; Frolov, K.A.; Krivokolysko, S.G.; Chigorina, E.A.; Snizhko, A.P.; Gromenko, V.M.; Bushmarinov, I.S.; Askerov, R.K.; et al. Synthesis, structure, and reactions of (4-aryl-3-cyano-6-oxopiperidin-2-ylidene) malononitriles. Chem. Heterocycl. Compd. 2016, 52, 473–483. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Semenova, A.M. Heterocyclization reactions using malononitrile dimer (2-aminopropene-1,1,3-tricarbonitrile). Chem. Heterocycl. Compd. 2018, 54, 989–1019. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Chigorina, E.A.; Krivokolysko, S.G. Synthesis of derivatives of a novel heterocyclic system 7-thia-1,4,6,8-tetraazabenzo [de] anthracene. Chem. Heterocycl. Compd. 2017, 53, 626–628. [Google Scholar] [CrossRef]
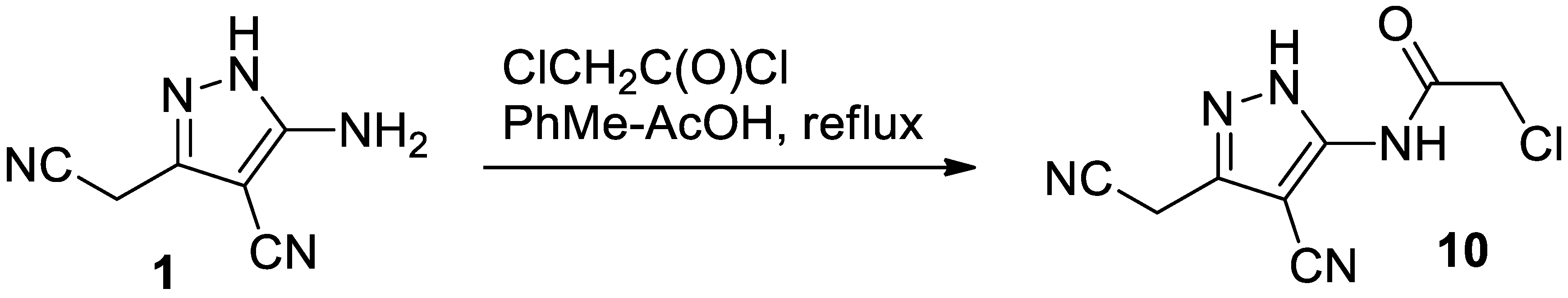
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotsenko, V.V.; Semenova, A.M.; Aksenov, N.A. New Reactions of 5-Amino-3-(Cyanomethyl)-1H-Pyrazole-4-Carbonitrile. Chem. Proc. 2021, 3, 23. https://doi.org/10.3390/ecsoc-24-08398
Dotsenko VV, Semenova AM, Aksenov NA. New Reactions of 5-Amino-3-(Cyanomethyl)-1H-Pyrazole-4-Carbonitrile. Chemistry Proceedings. 2021; 3(1):23. https://doi.org/10.3390/ecsoc-24-08398
Chicago/Turabian StyleDotsenko, Victor V., Aminat M. Semenova, and Nicolai A. Aksenov. 2021. "New Reactions of 5-Amino-3-(Cyanomethyl)-1H-Pyrazole-4-Carbonitrile" Chemistry Proceedings 3, no. 1: 23. https://doi.org/10.3390/ecsoc-24-08398
APA StyleDotsenko, V. V., Semenova, A. M., & Aksenov, N. A. (2021). New Reactions of 5-Amino-3-(Cyanomethyl)-1H-Pyrazole-4-Carbonitrile. Chemistry Proceedings, 3(1), 23. https://doi.org/10.3390/ecsoc-24-08398





