Iminosugars are sugar mimetics present in plants and microorganisms that were first reported in the 1960s [1,2]. They can formally result from the replacement of the endocyclic oxygen atoms monosaccharides by a basic nitrogen and the lack of the anomeric hydroxy group (Figure 1). Taking into account their ability to inhibit carbohydrate-processing enzymes such as glucosidases, they have been proposed as a source for therapeutic agents [3]. Thus, two derivatives of the natural iminosugar deoxynojirimycin (DNJ) are now marketed drugs: miglitol for the treatment of type 2 diabetes and miglustat for the treatment of the lysosomal storage disorders Gaucher’s and Nieman–Pick type 2 diseases.
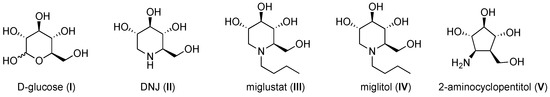
Figure 1.
Selection of six membered iminosugars and aminocyclopentitols.
Aminocyclopentitols are also inhibitors of glucosidases that can be considered as carbohydrate mimics resulting from the lack of the endocyclic oxygen atom of furanoses and that carry an exocyclic amino group [4]. Similar to the deoxynojirimycins, they are mimicking either the protonated glycoside [5,6] or the intermediate glycosyl (oxycarbenium) cation [7].
As part of our continued interest in glycosidase inhibitors, we report here an improved stereoselective synthesis of aminocyclopentitol V.
As shown in Scheme 1, protection of the OH group of the starting diaceton-D-glucose as OBn was followed by the selective deprotection of the exocyclic acetonide group of the resulting compound 3 [8]. Treatment of this compound with Björn Classon conditions provided sugar olefin 4, in good yield [9]. Reaction of this compound with a 2:1 TFA/H2O mixture resulted in the removal of the acetonide-protecting group, a process that allows one to obtain compound 5 with its anomeric position free, as required for further transformations.
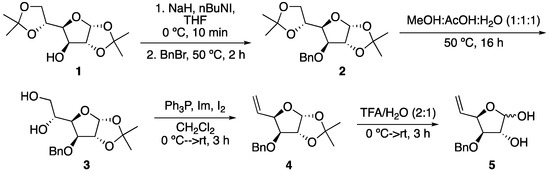
Scheme 1.
Synthesis of sugar olefin 5.
When compound 5 was reacted with N-benzylhydroxylamine hydrochloride and triethylamine under argon, it directly provided an 81% yield of an 89:11 mixture of cis-fused isoxazolidines 7 and 8, which were easily isolated by column chromatography (Scheme 2). Alternatively, when this reaction was carried out using a 4:1 EtOH/H2O mixture, compound 7 was selectively obtained, although in a 44% yield only. The structure of this compound was unequivocally confirmed by an X-ray experiment.
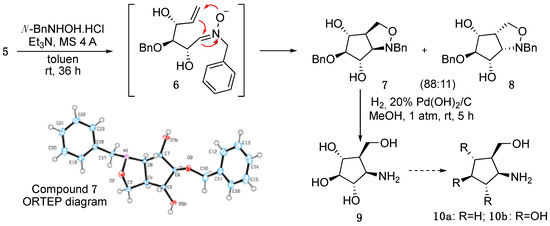
Scheme 2.
Synthesis of aminocyclopentitol 10b.
Formation of compounds 7 and 8 can be explained assuming that sugar olefin 5 provided nitrone 6, which spontaneously undertook a nitrone–alkene cycloaddition leading to mixture 7+8 [10,11].
Finally, catalytic hydrogenation of isoxazolidine 7, using Pd(OH)2/C as the catalyst, directly provided (1R,2S,3S,4R,5R)-4-amino-5-(hydroxymethyl)-cyclopentane-1,2,3-triol (9) as a result of the opening of the heterocyclic moiety and the hydrogenolysis of the benzyloxy group [12].
Molecular modelling studies carried out allowed us to explain the 88:11 ratio for compounds 7 and 8. Calculations using the MM2 software by Allinger [13,14] provided a value of 19.9563 Kcal/mol for compound 7 and 22.1753 for compound 8 (Figure 2). The difference of 2.219 kcal/mol is small enough to justify that 88:11 ratio. This suggests that this reaction could be subjected to a kinetic control.
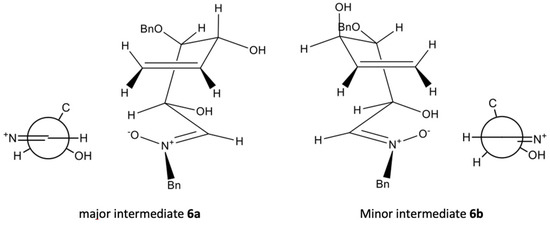
Figure 2.
Transitions states leading to compounds 7 and 8.
This suggests that this reaction could be subjected to kinetic control. Two parallel reactions take place, of which the fastest is the one through the most stable intermediate, 6a. Its Newman projection through the C-1–C-2 bond shows that the conformation of its transition state is energetically more favorable than that corresponding to 6b. The eclipsed arrangement of the nitrate and hydrogen at the C-2 positions present a lower steric hindrance than in the case of the conformation of intermediate 6b, in which the nitrate and the l hydroxy group in the C-2 position are eclipsed.
As a whole, here we report a modified route for the synthesis of aminocyclopentitol 9 [15], a known glycosidase inhibitor [16].
Work is now in progress aimed at the transformation of 9 into its b-amino acid derivative 10b [17,18], a polyhydroxylated derivative of cispentacin (10a), a natural antifungal antibiotic [19].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
References
- Paulsen, H. Carbohydrates Containing Nitrogen or Sulfur in the “Hemiacetal” Ring. Angew. Chem. Int. Ed. Engl. 1966, 5, 495. [Google Scholar] [CrossRef]
- Inouye, S.; Tsuruoka, T.; Ito, T.; Niida, T. Structure and synthesis of nojirimycin. Tetrahedron 1968, 24, 2125. [Google Scholar] [CrossRef]
- Compain, P.; Martin, O.R. Iminosugars: From Synthesis to Therapeutic Applications; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Bøjstrup, M.; Lundt, I. Synthesis of aminocyclopentanols: α-d-galacto configured sugar mimics. Org. Biomol. Chem. 2005, 3, 1738. [Google Scholar] [CrossRef] [PubMed]
- Leroy, E.; Reymond, J.-L. Anomer-Selective Inhibition of Glycosidases Using Aminocyclopentanols. Org. Lett. 1999, 1, 775. [Google Scholar] [CrossRef] [PubMed]
- Boss, O.; Leroy, E.; Blaser, A.; Reymond, J.-L. Synthesis and Evaluation of Aminocyclopentitol Inhibitors of β-Glucosidases. Org. Lett. 2000, 2, 151. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Morikawa, T. Chemical Modification of the α-Mannosidase Inhibitor Mannostain A: Synthesis of a Potent Inhibitor 1L-(1,2,3,5/4)-5-Amino-4-O-methyl-1,2,3,4-cyclopentanetetrol. Eur. J. Org. Chem. 2005; 4065. [Google Scholar]
- Yua, H.; Cao, H.; Tiwari, V.K.; Lia, Y.; Chena, X. An improved stereoselective synthesis of (1R,2S,3S,4R,5R)-4-amino-5-(hydroxymethyl)-cyclopentane-1,2,3-triol Bioorg. Med. Chem. Lett. 2011, 21, 5037. [Google Scholar]
- Liu, Z.; Classon, B. A novel route to olefins from vicinal diols. J. Org. Chem. 1990, 55, 4273. [Google Scholar] [CrossRef]
- LeBel, N.A.; Whang, J.J. The addition of nitrones to Olefins. A New Route to Isoxazolidines. J. Am. Chem. Soc. 1959, 81, 6334. [Google Scholar] [CrossRef]
- Alcaide, B.; Sáez, E.A. Stereoselective Synthesis of 1,2,3-Trisubstituted 1,3-Dienes through Novel [3,3]-Sigmatropic Rearrangements in α-Allenic Methanesulfonates: Application to the Preparation of Fused Tricyclic Systems by Tandem Rearrangement/Diels−Alder Reaction. Eur. J. Org. Chem. 2005, 1680. [Google Scholar] [CrossRef]
- Das, N.B.; Torssell, K.B.G. Silyl nitronates, nitrile oxides, and derived 2-isoxazolines in organic synthesis. Functionalization of butadiene, a novel route to furans and 2-isoxazolines as an alternative to aldol-type condensations. Tetrahedron 1983, 39, 2247. [Google Scholar] [CrossRef]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127. [Google Scholar] [CrossRef]
- Burkert, U.; Allinger, N.L. Molecular Mechanics; American Chemical Society: Washington, DC, USA, 1982. [Google Scholar]
- Kleban, M.; Hilgers, P.; Greul, J.N.; Kugler, R.D.; Li, J.; Picasso, S.; Vogel, P.; Jager, V. Amino(hydroxymethyl)cyclopentanetriols, an Emerging Class of Potent Glycosidase Inhibitors—Part I: Synthesis and Evaluation of β-D-Pyranoside Analogues in the manno, gluco, galacto, and GlcNAc Series. ChemBioChem 2001, 2, 365. [Google Scholar] [CrossRef]
- Dickson, L.G.; Leroy, E.; Reymond, J.-L. Structure–activity relationships in aminocyclopentitol glycosidase inhibitors. Org. Biomol. Chem. 2004, 2, 1217. [Google Scholar] [CrossRef] [PubMed]
- Soengas, R.G.; Pampin, M.B.; Estevez, J.C.; Estevez, R.J. Stereocontrolled transformation of nitrohexofuranoses into cyclopentylamines via 2-oxabicyclo[2.2.1]heptanes. Part 2: Synthesis of (1S,2R,3S,4S,5R)-3,4,5-trihydroxy-2-aminocyclopentanecarboxylic acid. Tetrahedron Asymm. 2005, 16, 205. [Google Scholar] [CrossRef]
- Soengas, R.; Lorca, M.; Pampin, B.; Sanchez-Pedregal, V.M.; Estevez, R.J.; Estevez, J.C. New Morphiceptin Peptidomimetic Incorporating (1S,2R,3S,4S,5R)-2-Amino-3,4,5-trihydroxycyclopen-tane-1-carboxylic acid: Synthesis and Structural Study. Molecules 2020, 25, 2574. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Nishio, M.; Saitoh, K.; Miyaki, T.; Oki, T.; Kawaguchi, H. Cispentacin, a new antifungal antibiotic. J. Antibiot. 1989, 42, 1749. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).